Research Article
Preparation of Magnesium Oxide Nanoparticles and Study Its Loaded With Recombinant Human Erythropoietin alfa Drug
Fatin Fadhel Mohammed Al-Kazazz1, Shaimaa Hamed Jaber1, Asma Hadi Mohammed2, Amer Hasan Abdullah1, Mustafa M.Kadhim 3, Ameer Radhi Sultan1*
1Department of Chemistry, College of Science, Al-Mustansiriyah University, Iraq
2Department of physics, College of Science, Al-Mustansiriyah University, Iraq
3Department of Dentistry, Kut University College, Iraq
* Crossponding author. E-mail: ameer.radhi@yahoo.com
Received: Apr. 12, 2022; Accepted: Oct. 17, 2022; Published: Nov. 17, 2022
Citation: Fatin Fadhel Mohammed Al-Kazazz, Shaimaa Hamed Jaber, Asma Hadi Mohammed, Amer Hasan Abdullah, Mustafa M.Kadhim, and Ameer Radhi Sultan, Preparation of Magnesium Oxide Nanoparticles and Study Its Loaded With Recombinant Human Erythropoietin alfa Drug. Nano Biomed. Eng., 2022, 14(2): 186-191.
DOI: 10.5101/nbe.v14i2.p186-191
Abstract
Loading rHuEPO-alpha drug on the surface of magnesium oxide nanoparticles further improves the effect of the drug as a treatment for anemia. After preparation of magnesium oxide nanoparticles by precipitation method, it was diagnosed by X-ray diffraction and Transmission Electron Microscopy. the loaded was diagnosed by Docking program, UV-Visible and Transmission Electron Microscopy. The study of LD50 was for thirty male mice. Magnesium Oxide nanoparticles had an average crystallite size of 8.42 nm. oral LD50 test was 896 mg/kg. Docking results presented a high energy binding with force linkage due to the link of the oxygen atom of MgO NPs with three hydrogen bonds of amino acids in the rHuEPO-alpha structure. The UV-Visible result was certain that all rHuEPO-alpha drug was loaded on the surface of MgO NPs. TEM shows that the average particle size was 9.94 nm after loaded of the rHuEPO-alpha drug on MgO NPs while was 9 nm before the loaded. Magnesium oxide nanoparticles may exhibit an effect on increasing the efficacy of rHuEPO-alpha after the loaded, leading to elevated hemoglobin and reduced anemia disease.
Keywords: loaded rHuEPO-alpha drug on MgO NPs, Docking , XRD, TEM and UV-Visible
Introduction
Erythropoietin (EPO) is a glycoprotein hormone that is similar to cytokine composed of 165 amino acid residues that are made up of about 60% protein and 40% carbohydrate [1]. The primary structure of the EPO hormone which has asparagine at three positions (38, 24 and 83), for linked with oligosaccharide as an N-linked side, and one serine amino acid at position 126 that bind to short oligosaccharide for O-linked glycosylation[2], as shown in Fig. 1 . The drug epoetin alfa treatment was the first recombinant Human Erythropoietin (rHuEPO), it’s also called rHuEPO-alpha that approved by the US Food and Drug Administration for the treatment of anemia in patients with renal failure, myelodysplasia, and cancer chemotherapy[3]. Most patients with anemia have resistance to rHuEPO therapy and have been related to inflammation, oxidative stress, and iron deficiency[4]. Therefore, patients may use a high dose of rHuEPO, and this dose can cause adverse effects, such as hypertension, cardiac diseases, and thrombosis. Thus, safer and more effective strategies must be found to treat anemia[5]. Magnesium oxide nanoparticles (MgO NPs) are important in many applications due to high adsorption surface area, a high surface to volume ratio, chemical, specific magnetic, thermal, and electrical properties[6]. There has been extensive study of MgO NPs for drug delivery, diagnosis, inhibition of bacterial adverse, and anticancer[7]. Loading the drug with nanoparticles gives a feature such as reduced side effects, drug delivery to organ targeted, protecting the drug from degradation, and prolonged drug release[8]. Proteins are functionally significant biomolecules, and nanoparticle protein binding has found critical applications in catalysis and protein structural change perception[9]. By interacting directly with their receptors on cell membranes, most proteins promote nanoparticle internalization[10].
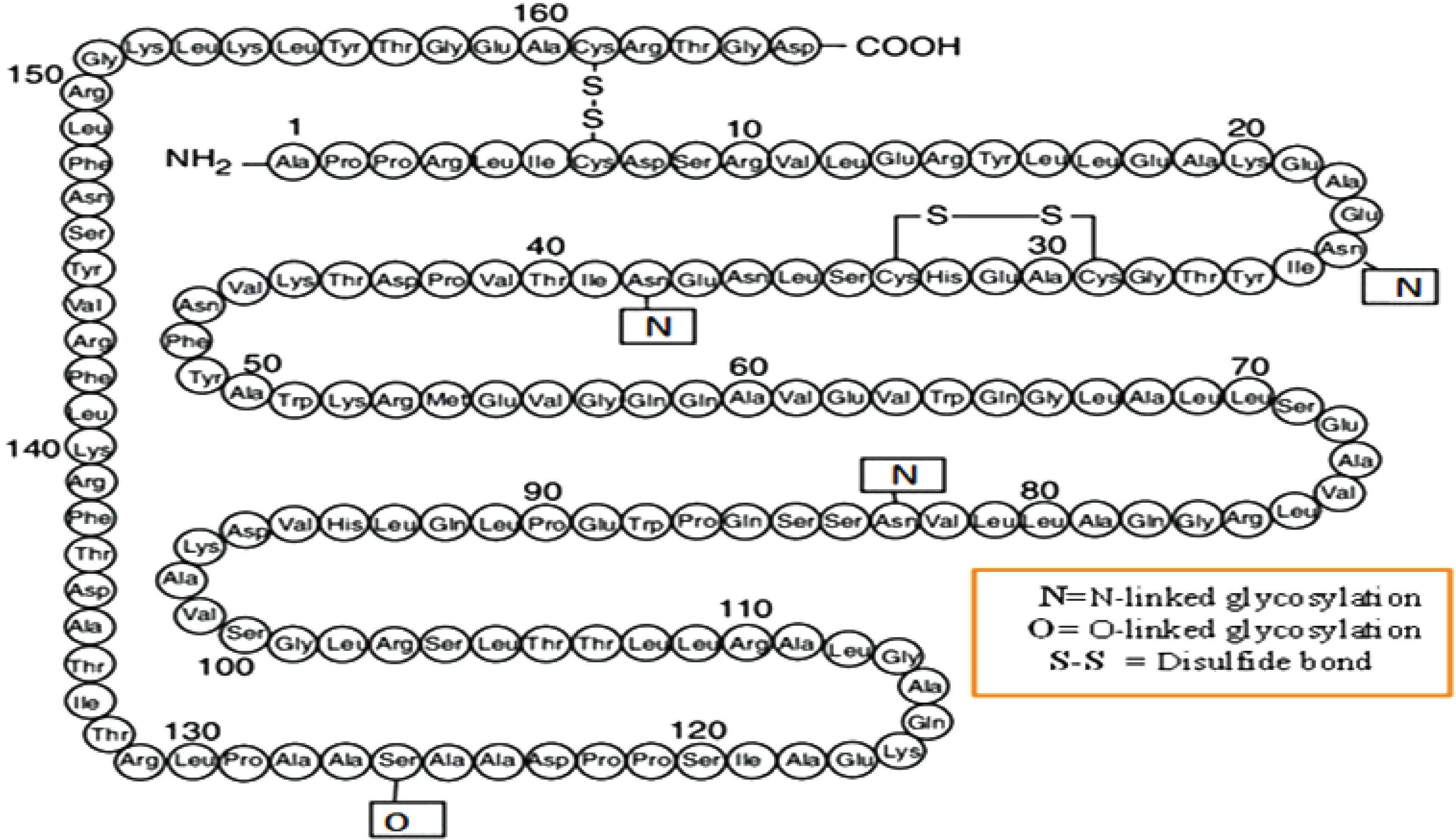
Fig.1 Primary structure of EPO hormone [2]
Experimental
Magnesium nitrate Mg(NO3)2(H2O)6, Sodium hydroxide (NaOH) and Polyvinyl pyrrolidone (PVP) (C6H9NO)n were provided from comapney of Sigma-Aldrich.
Preparation of MgO NPs
The preparation of MgO NPs was achieved by precipitation method. Mg(NO3)2(H2O)6, Polyvinylpyrrolidone(PVP) and NaOH were dissolved by deionized water (DIW). (0.01 M, 50 ml) of PVP was added to (1 M, 50 ml) of Mg(NO3)2(H2O)6 under ultrasonic sonication at 25 °C. The added 50 ml of 1 M NaOH under ultrasonic sonication for 2 h. The white precipitate of Mg (OH)2 was formed. The precipitate was centrifuged, washed several times with DIW and dried for 3 hours at 80°C. The calcination process was carried out using a muffle furnace for 3 hours at 600°C. The white powder of pure MgO NPs was obtained.
Determination the toxicity of MgO NPs using LD50 test
Thirty male mice of albino BALB/c were obtained from University of Mustansiriyah / Iraqi Center for Cancer Research and Medical Genetics. The weights of the mice were 25-30 grams, and were housed in plastic cages at a temperature between 21 to 25 oC. The mice were divided into five groups I to V, each group include six rats. The oral dose of MgO NPs for mice was 30 days:
Group I as a control that received DIW.
Groups II, III, IV and V were daily administered with of MgO NPs (500, 750, 1000 and 1250) mg/kg , respectively.
Loaded rHuEPO-alpha Drug with MgO NPs
The loaded rHuEPO-alpha drug with MgO NPs as a treatment of anemia was investigated by docking, UV-visible and TEM studies.
Docking Study
The downloaded of rHuEPO-alpha structure by protein data bank, has been used code of rHuEPO-alpha structure which are (1EER)[11]. A molecular docking of rHuEPO-alpha with MgO NPs was performed by Molecular Graphics Laboratory (MGL) with used auto dock with version 1.5.6.
UV-Visible Study
The activity unit of rHuEPO-alpha represented by IU can be converted to mass unit represented by microgram based on that each 120 IU of rHuEPO-alpha equal to 1 microgram [12], therefore 900 IU of rHuEPO-alpha equal to 7.5 μg. A 7.5 μg /ml of rHuEPO-alpha were prepared from the rHuEPO-alpha drug 4000 IU / 0.4 ml. The drug of rHuEPO-alpha was loaded on the surface of MgO nanoparticles accordingly; 0.01 gm of MgO NPs was added to 10 ml, 7.5 μg/ml of rHuEPO-alpha. Then, the mixed solution was shaking using water bath sonication at 25 °C for (0, 15, 30, 45) minutes. To monitor the percentage of loading, a UV-Vis spectrum was used in this study.
Results and Discussion
Characterization of MgO Nanoparticles
X-Ray Diffraction of MgO Nanoparticles
The structure of the MgO NPs has been investigated by XRD as shown in Fig. 2. Five major diffraction peaks appeared at 2θ= 36.92∘, 42.85∘, 62.18∘, 74.56∘, and 78.45∘ correspond to millers (111), (200), (220), (311) and (222) respectively, this result is matching with the card number of MgO (JCPDS 045-0946) cubic structure. In addition, no impure peaks in the XRD pattern are observed which indicates the purity of MgO NPs prepared, this result was in agreement with the result of Shah R et al[13].
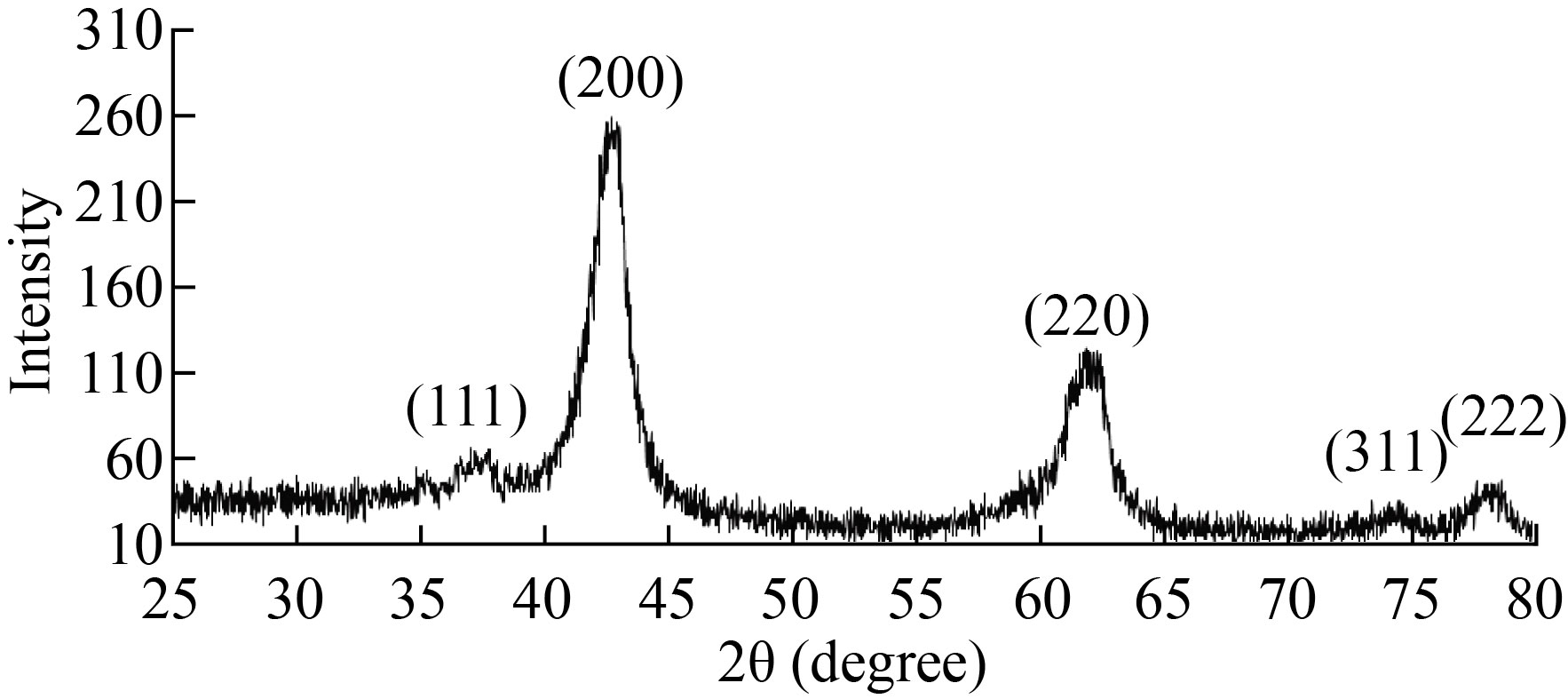
Fig.2 XRD of MgO NPs
The average crystallite size of MgO NPs was calculated by Debye Scherrer Equation 1[14]
D = kλ/βcosθ (1)
Where k =0. 9 which considered Scherrer constant, λ = 0.154 nm, is the wavelength of the Copper radiations, β is the full width at half maximum and θ is the angle of the Bragg diffraction peak. The mean crystallite size of MgO NPs was 8.42 nm. There is an inverse relationship between the width of the peaks of the XRD pattern and the size of nanoparticles, as the broadening peaks refer to a decrease in nanoparticles size[15]. In another study, it had observed that the average particle size decreased with the use of PVP and the intensity of the XRD peaks decreased with broad peak[16].
Transmission Electron Microscopy of MgO Nanoparticles
The morphology of MgO NPs was investigated using TEM. Figure 3 shows the TEM of sample prepared by co-precipitation method.
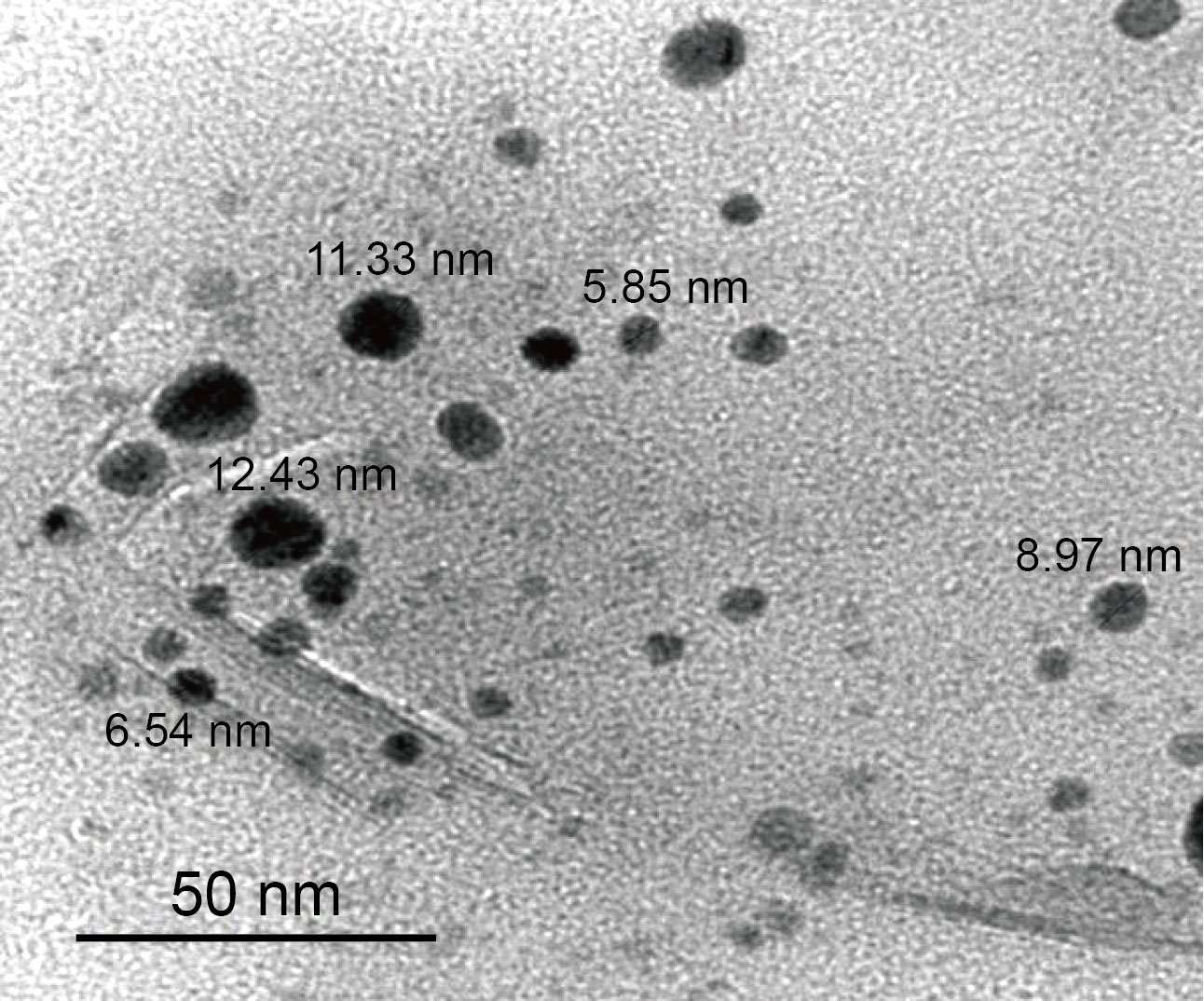
Fig. 3 TEM image of MgO NPs
These results of TEM showed that the particles of MgO NPs were spherical shape. The average particle size obtained was 9 nm. This result of average particle size was good agreement with XRD data. The results of the morphology of the prepared MgO NPs prepared are in agreement with the results of Jain U et al[17] . They had obtained the spherical shape of the MgO NPs that prepared by precipitation method using Mg (NO3)2 and NaOH. The homogenous morphology and particle size dispersion for nanoparticles is proportionated with using PVP. This due to the PVP that makes the particle size of nanoparticles became restricted and decreases accumulation, as long as the concentration of PVP is high sufficiently[18].
UV-Vis Spectroscopy of MgO Nanoparticles
UV-Vis absorption spectrum of MgO NPs has been recorded within the range from 200 to 400 nm. Figure 4 shows the maximum peak was 204 nm of MgO NPs, was in agreement with Nemade KR et al[19], they observed the broad peak of UV-Vis of MgO NPs was 203 nm while the average size was 9.2 nm.
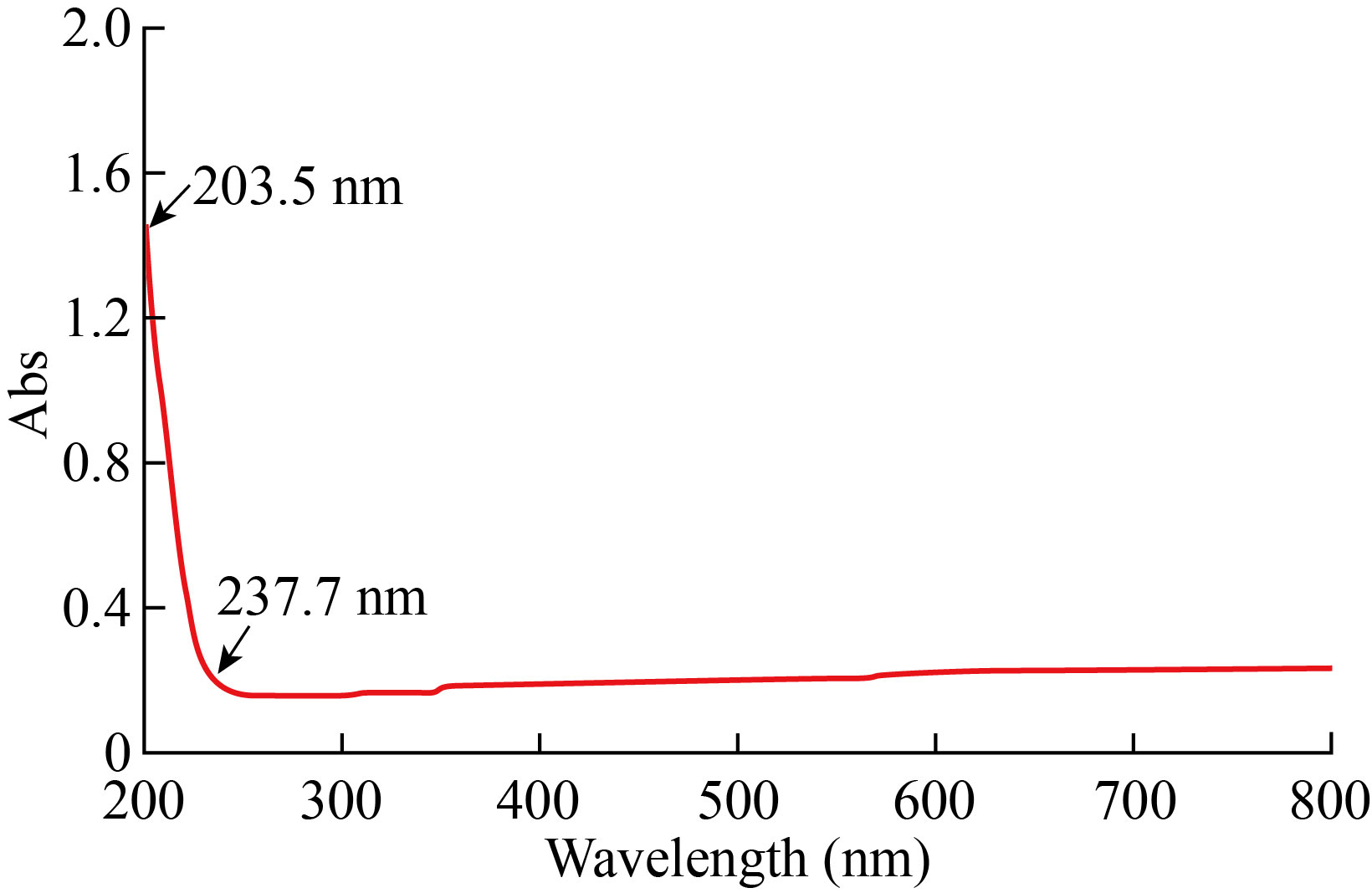
Fig. 4 UV-Vis image of MgO NPs
Evaluation LD50 of MgO Nanoparticles
The toxicity of MgO NPs on mice using oral LD50 test was applied depending on the Karber arithmetic [20], as shown in Equation 1. After 30 days of observing the number of dead mice in several groups, the LD50 was measured by Karber equation which was 896 mg/kg. The mortality of one mice belonging to the fourth group was observed at a concentration of 1000 mg/kg of oral MgO NPs, three rats from six rats belonging to the fifth group were observed mortality at a concentration of 1250 mg/kg of oral MgO NPs [21].
Table 1 Results the toxicity of MgO NPs for the determination of the LD50 after oral receive of male mice for 30 days
Groups | Number of mice |
| Number of mice dead | Dose difference (a) | Mean mortality (b) | Probity (ab) | |
I | 6 | 0 | 0 |
|
|
| |
II | 6 | 500 | 0 | 500 | 0 | 0 | |
III | 6 | 750 | 0 | 250 | 0 | 0 | |
IV | 6 | 1000 | 1 | 250 | 0.5 | 125 | |
V | 6 | 1250 | 3 | 250 | 2 | 500 |
LD50 =LD - sum (ab)/n (2)
Where (LD) is the lowest lethal dose in the group, (n) is the number of mice in each group, (a) is the dose difference and (b) is the mean mortality (number of mice dead in second group + number of mice dead in first group /2)
LD50 =1000-625/6 = 896 mg/kg
Loaded rHuEPO-alpha with MgO Nanoparticles
Molecular Docking
The molecular docking approach has been popularly used in the prediction of protein-ligand interactions. The measure of the affinity of protein-ligand defines as a binding energy [22]. The binding energy between rHuEPO-alpha and MgO NPs were -2.17kcal/mol and -3.14kcal/mol at the lowest and highest of binding energy respectively, according to the local of MgO in rHuEPO-alpha chains, as shown in Figs. 5 and 6.
Figure 5(A) shows the structure of rHuEPO-alpha with MgO expected site to interact with low binding energy -2.17kcal/mol. Figure 5(B) shows that the MgO NPs were surrounded by arginine, phenylalanine, leucine, alanine and tryptophan. In addition, MgO NPs were linked to serine by a hydrogen bond with oxygen atom of MgO NPs with bond length 2.152 Ao.
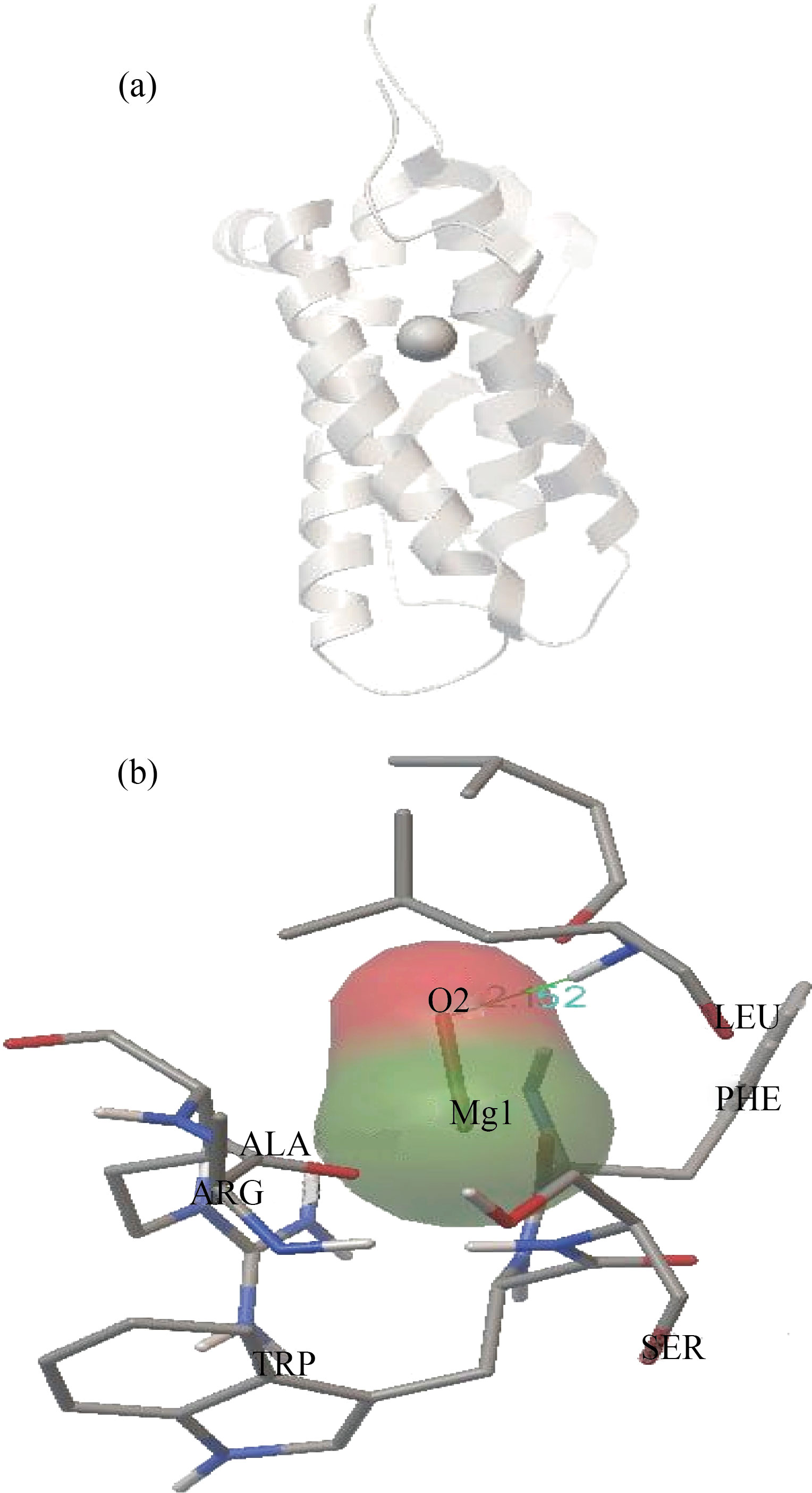
Fig. 5 (A) Docking between rHuEPO-alpha and MgO NPs. (B) Closest interacting of MgO NPs with some amino acids of rHuEPO-alpha structure. At binding energy -2.17kcal/mol
Figure 6(A) represents the expected site to interact with high binding energy -3.14 kcal/mol. Figure 6(B) shows that the MgO NPs were linked with tyrosine, phenylalanine, and serine by hydrogen bonds with oxygen atom of MgO NPs with bond lengths 1.681 Ao, 1.70 Ao and 2.07 Ao respectively. Thus, the high energy binding with force linkage due to the linked of the oxygen atom of MgO NPs with three hydrogen bonds of amino acids in rHuEPO-alpha structure. Behzadi E et al[23], observed there was linked between MgO NPs with albumin by docking studies. They demonstrated that the structure of albumin not altered or distorted due to the binding of MgO NPs with the albumin molecule.
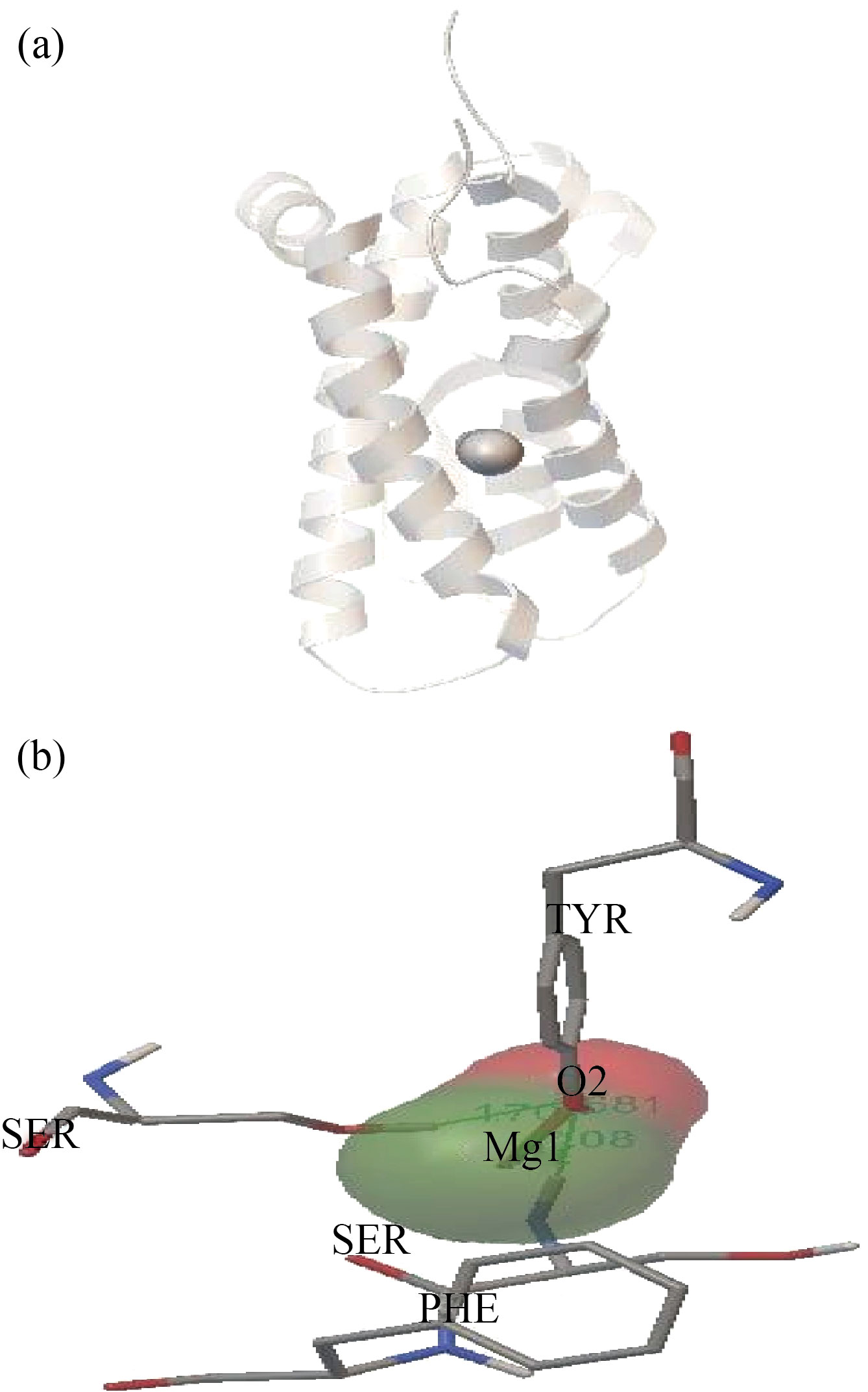
Fig. 6 (A) Docking between rHuEPO-alpha and MgO NPs,
(B) Closest interacting of MgO NPs with some amino acids of rHuEPO-alpha structure. At binding energy -3.14kcal/mol
UV-Visible Study
The period loaded of all rHuEPO-alpha on the surface of MgO NPs at 278 nm was 45 minutes at 25°C, as showing in Fig. 7. Thus it is possible to prepare a concentration of 7.5 μg/ml of rHuEPO-alpha loaded on MgO NPs as anemia treatment. Lu J et al[24], used 2000 IU/kg dose of rHuEPO-alpha and injected the subcutaneously of rats. According to the hematology results, the increased erythrocyte and hemoglobin levels occurred without side effects[24].
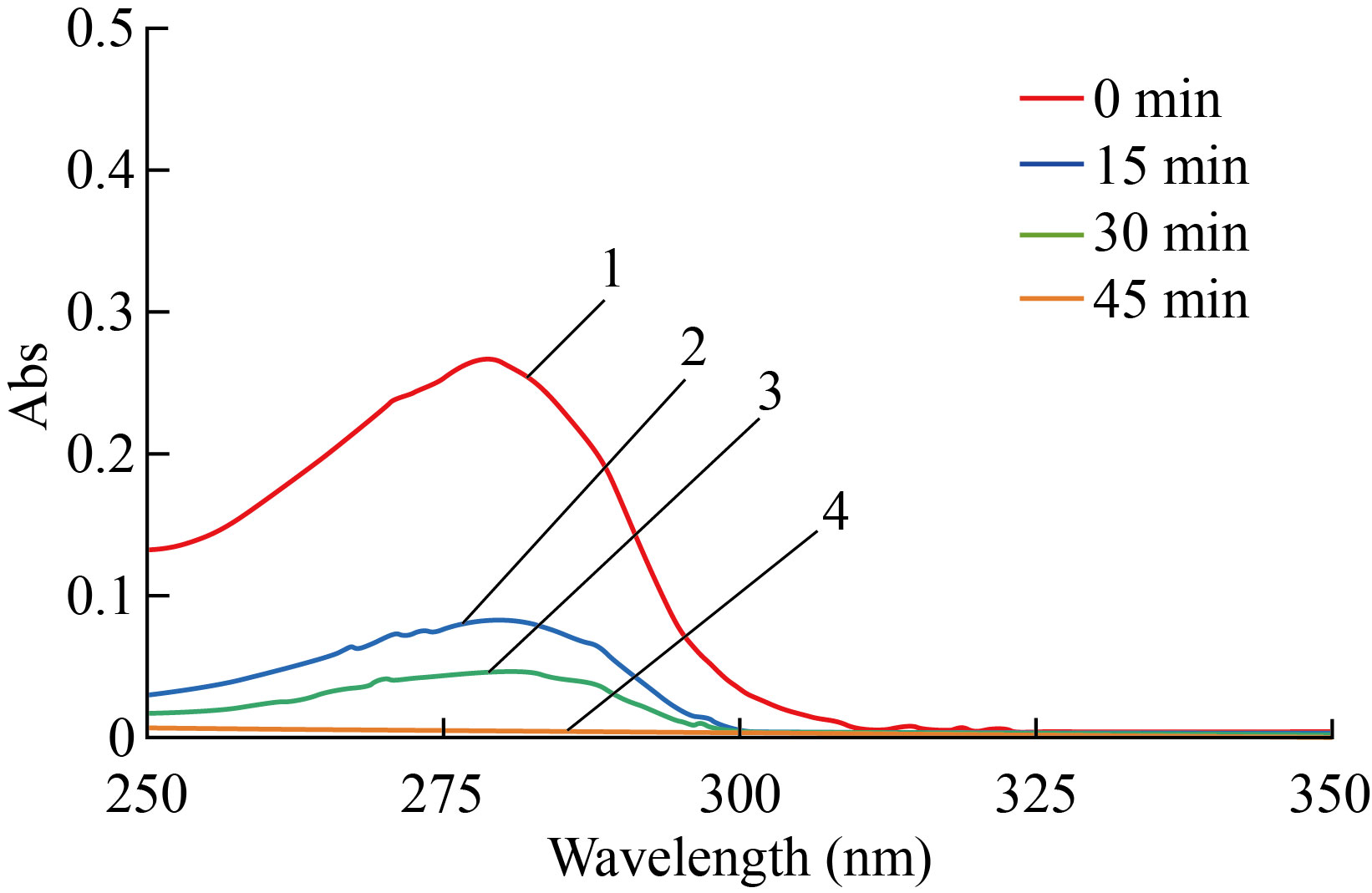
Fig. 7 Loaded of rHuEPO-alpha on MgO NPs surface at 278 nm (1) refer to rHuEPO-alpha without MgO NPs for zero min (2) refer to rHuEPO-alpha with MgO NPs for 15 min, (3) refer to rHuEPO-alpha with MgO NPs for 30 min and (4) refer to rHuEPO-alpha with MgO NPs for 45 min.
Transmission Electron Microscopy of MgO NPs Loaded with rHuEPO-alpha
Figure 8 of TEM shows the loaded of the rHuEPO-alpha drug on MgO NPs at 25°C for 45 minutes. The average particle size was 9.94 nm.
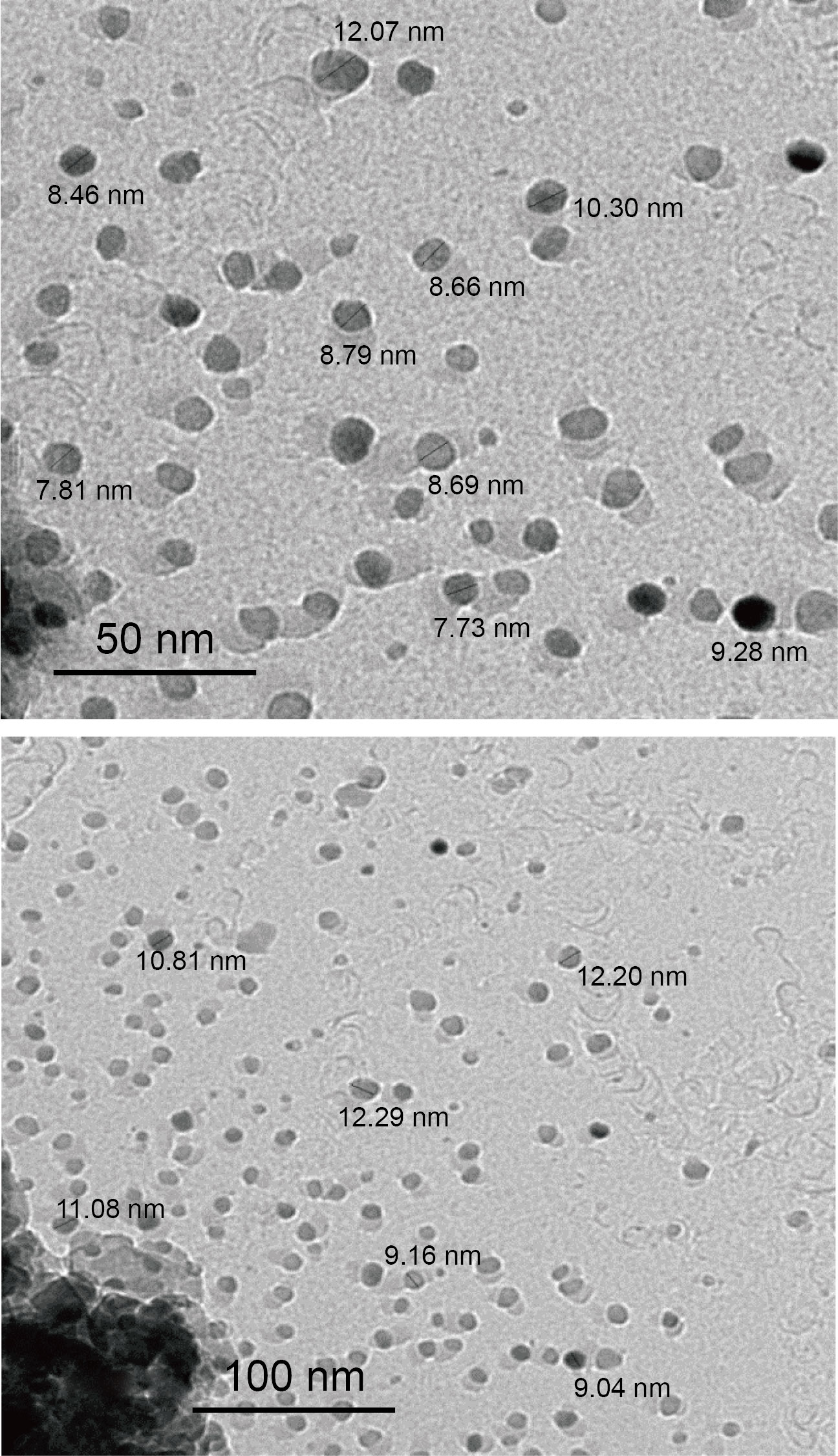
Fig. 8 TEM images of rHuEPO-alpha drug loaded with MgO NPs
Acknowledgements
The authors express their gratitude to B.h.D. Ahmed Mahdi Raheima for supported in this research
Conflict of Interests
The authors declare that no competing interest exists.
Refernces
1. Zubareva E V., Nadezhdin S V., Burda YE, Nadezhdina NA, Gashevskaya AS. Pleiotropic effects of Erythropoietin. Influence of Erythropoietin on processes of mesenchymal stem cells differentiation. Res Results Pharmacol. 2019;5(1):53–66.
2. Murakami M, Kiuchi T, Nishihara M, Tezuka K, Okamoto R, Izumi M, et al. Chemical synthesis of erythropoietin glycoforms for insights into the relationship between glycosylation pattern and bioactivity. Sci Adv. 2016;2(1):1–13.
3. Fishbane S, Spinowitz BS, Wisemandle WA, Martin NE. Randomized Controlled Trial of Subcutaneous Epoetin Alfa-epbx Versus Epoetin Alfa in End-Stage Kidney Disease. Kidney Int Reports. 2019;4(9):1235–1247.
4. Garrido P, Ribeiro S, Fernandes J, Vala H, Rocha-Pereira P, Bronze-da-Rocha E, et al. Resistance to recombinant human erythropoietin therapy in a rat model of chronic kidney disease associated anemia. Int J Mol Sci. 2016;17(28):1–22.
5. Wang K, Wu J, Xu J, Gu S, Li Q, Cao P, et al. Correction of Anemia in Chronic Kidney Disease With Angelica sinensis Polysaccharide via Restoring EPO Production and Improving Iron Availability. Front Pharmacol. 2018;9(7):1–17.
Rahmani-Nezhad S, Dianat S, Saeedi M, Hadjiakhoondi A. Synthesis, characterization and catalytic activity of plant-mediated MgO nanoparticles using Mucuna pruriens L. seed extract and their biological evaluation. J Nanoanalysis. 2017;4(4):290–298.
7. Ranathunge TA, Karunaratne DGGP, Rajapakse RMG, Watkins DL. Doxorubicin loaded magnesium oxide nanoflakes as ph dependent carriers for simultaneous treatment of cancer and hypomagnesemia. Nanomaterials. 2019;9(2):1–11.
8. Dhapake PR, Avari JG. Application of polymeric nanoparticles in oral delivery of recombinant human erythropoietin: A review. J Drug Deliv Ther. 2019;9(1-s):403–407.
9. Mukhopadhyay A, Basu S, Singha S, Patra HK. Inner-view of nanomaterial incited protein conformational changes: Insights into designable interaction. Research. 2018;2018(Article ID 9712832):1–15.
10. Cagliani R, Gatto F, Bardi G. Protein adsorption: A feasible method for nanoparticle functionalization? Materials (Basel). 2019;12(12):1–11.
11. Mittal A, Singh V, Chowdhary S, Moideen A, Kumar D, Maniar K, et al. The Effect of Recombinant Human Erythropoietin on Bacterial Growth: A Dual-Edged Sword. Kidney Dis. 2019;5(2):81–90.
12. Wang Y, Zhang ZG, Rhodes K, Renzi M, Zhang RL, Kapke A, et al. Post-ischemic treatment with erythropoietin or carbamylated erythropoietin reduces infarction and improves neurological outcome in a rat model of focal cerebral ischemia. Br J Pharmacol. 2007;151(8):1377–1384.
13. Shah R, Ashaq H S, Mohammad A S. Antibacterial activity of magnesium oxide nanostructures prepared by hydrothermal method. Asian J Nanosci Mater. 2019;2(4):425–430.
14. Ershadi M, Tavakkoli H, Ghaemi A, Azarkish A. A p p l ied C hemical R esearch Adsorbent for Rapid Removal of Trypan Blue Dye From Aqueous Media. 2018;12(1):8–15.
15. Vorokh AS. Scherrer formula: estimation of error in determining small nanoparticle size. Nanosyst Physics, Chem Math. 2018;9(3):364–369.
16. Gharibshahi L, Saion E, Gharibshahi E, Shaari AH, Matori KA. Influence of Poly(vinylpyrrolidone) concentration on properties of silver nanoparticles manufactured by modified thermal treatment method. PLoS One. 2017;12(10):1–17.
17. Jain U, Pundir CS, Gupta S, Chauhan N. A novel electrochemical comparative sensing interface of MgO nanoparticles synthesized by different methods. J Mech Eng Sci. 2017;6(28):1–10.
18. Al-Hada NM, Al-Ghaili AM, Kasim H, Saleh MA, Flaifel MH, Kamari HM, et al. The Effect of PVP Concentration on Particle Size, Morphological and Optical Properties of Cassiterite Nanoparticles. IEEE Access. 2020;8(5):93444–93454.
19. Nemade KR, Waghuley SA. Synthesis of MgO Nanoparticles by Solvent Mixed Spray Pyrolysis Technique for Optical Investigation. Int J Met. 2014;2014(4):1–4.
20. Yeo D, Bouagnon R, Djyh BN, Tuo C, N’guessan JD. Acute and subacute toxic study of aqueous leaf extract of Combretum molle. Trop J Pharm Res. 2012;11(2):217–223.
21. Mosaad RM, Samir A, Ibrahim HM. Median lethal dose (LD50) and cytotoxicity of Adriamycin in female albino mice. J Appl Pharm Sci. 2017;7(3):77–80.
22. Torres PHM, Sodero ACR, Jofily P, Silva-Jr FP. Key topics in molecular docking for drug design. Int J Mol Sci. 2019;20(18):1–29.
23. Behzadi E, Sarsharzadeh R, Nouri M, Attar F, Akhtari K, Shahpasand K, et al. Albumin binding and anticancer effect of magnesium oxide nanoparticles. Int J Nanomedicine. 2019;14:257–270.
24. Lu J, Yao Y yu, Dai Q ming, Ma G shan, Zhang S feng, Cao L, et al. Erythropoietin attenuates cardiac dysfunction by increasing myocardial angiogenesis and inhibiting interstitial fibrosis in diabetic rats. Cardiovasc Diabetol. 2012;11(105):1–11.
Copyright© Fatin Fadhel Mohammed Al-Kazazz, Shaimaa Hamed Jaber, Asma Hadi Mohammed, Amer Hasan Abdullah, Mustafa M.Kadhim, and Ameer Radhi Sultan. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.