Research Article
DFT-Quantum Chemical and Experimental Studies of a New 2-(Substituted Thio) Furan as a Corrosion Inhibitor in Acidic Media
Israa M. H. Al-mousawi *, Noor Ali Khudhair, Lama S. Ahmed
Department of Chemistry, College of Science, University of Baghdad.
* Corresponding author. E-mail: israamousawi@gmail.com
Received: Nov. 5, 2021; Accepted: Sep. 19, 2022; Published: Oct. 20, 2022
Citation: Israa M. H. Al-Mousawi, Noor Ali Khudhair, and Lama S. Ahmed, DFT-Quantum Chemical and Experimental Studies of a New 2-(Substituted Thio) Furan as a Corrosion Inhibitor in Acidic Media. Nano Biomed. Eng., 2022, 14(2): 136-148.
DOI: 10.5101/nbe.v14i2.p136-148.
Abstract
The corrosion inhibiting properties of the new furan derivative 5-(furan-2-ylmethylsulfonyl-4-phenyl-2,4- dihydro [1,2,4] triazole-3-thione in acidic solution (1.0 M HCl) were explored utilizing electrochemical, surface morphology (AFM), and quantum chemical calculations approaches. The novel furan derivative 5-(furan-2-ylmethylsulfonyl-4-phenyl-2,4- dihydro [1,2,4] triazole-3-thione shows with an inhibitory efficiency value of 99.4 percent at 150 ppm, carbon steel corrosion in acidic medium is effectively inhibited, according to the results. The influence of temperature on corrosion prevention was studied using adsorption parameters and activation thermodynamics. The novel furan derivative creates a protective layer over the metallic surface that separates the metal from harsh acid solution and thereby protects it from destructive disintegration, according to the AFM study. The experimental findings are supported by the theoretical method of density functional theory (DFT) at the B3LYP/6-311 ++G basis set for inhibitor.
Keywords: DFT, Corrosion inhibitors, Tafel polarization, Furan
Introduction
Corrosion is a chemical or electrochemical interaction that occurs between materials, usually a metal, and their environment, resulting in the materials' and their qualities deteriorating. Corrosion inhibitors are commonly used to decrease the amount of unwanted base metal dissolving caused by these processes. To preserve metallic components from corrosion, the hunt for new and effective corrosion inhibitors, particularly in hydrochloric acid solutions, has become a necessity [1-3]. Adsorption of organic inhibitor molecules (physically or chemically) is widespread across the metal surface, and the resulting adsorption layer serves as a corrosion barrier. This shows that the most important characteristics in selecting an inhibitor molecule are hydrophobicity, molecular structure, and electron density at donor-atoms, solubility, and dispersibility [4-6]. The majority of organic inhibitor compounds contain heteroatoms such as N, O, S and numerous Pi bonds in their molecules facilitate adsorption on the steel surface [7-8].
Thiocarbanilide was prevented electrochemical corrosion of high carbon steel in 1 M sulfuric acid and hydrochloric acid solutions, the thiocarbanilide efficiently inhibited carbon steel at all concentrations, with an average inhibition efficiency of roughly 70% in sulfuric acid and 80% in hydrochloric acid [9]. In addition, phenyltetrazole compounds were shown to be effective inhibitors of mild steel corrosion in a 5.0 M HCl solution, acting as cathodic type inhibitors [10]. While Tetrahydropyridine derivatives are excellent mild steel inhibitors in 1 M hydrochloric acid [11].
As well as the experimental studies, quantum chemical calculations are used to examine the correlation between electronic structure and corrosion inhibition capability [12]. Further, a theoretical study grants the pre-selection of compounds with the fundamental structural essence to act as organic corrosion inhibitors.
The purpose of this research is to look at the corrosion inhibitory properties of the new furan derivative 5-(furan-2-ylmethylsulfonyl-4-phenyl-2,4-dihydro[1,2,4] triazole-3-thione (FMSPhDHT) for carbon steel corrosion prevention in acidic solution (1 M HCl). Luma S. A. synthesized and characterized the FMSPhDHT (C14H13N3OS2) as shown in Fig.1[13]. To establish the molecule's electronic structure, we used the electrochemical (anodic and cathodic Tafel polarization) method and quantum chemical calculations utilizing the density function theory (DFT).
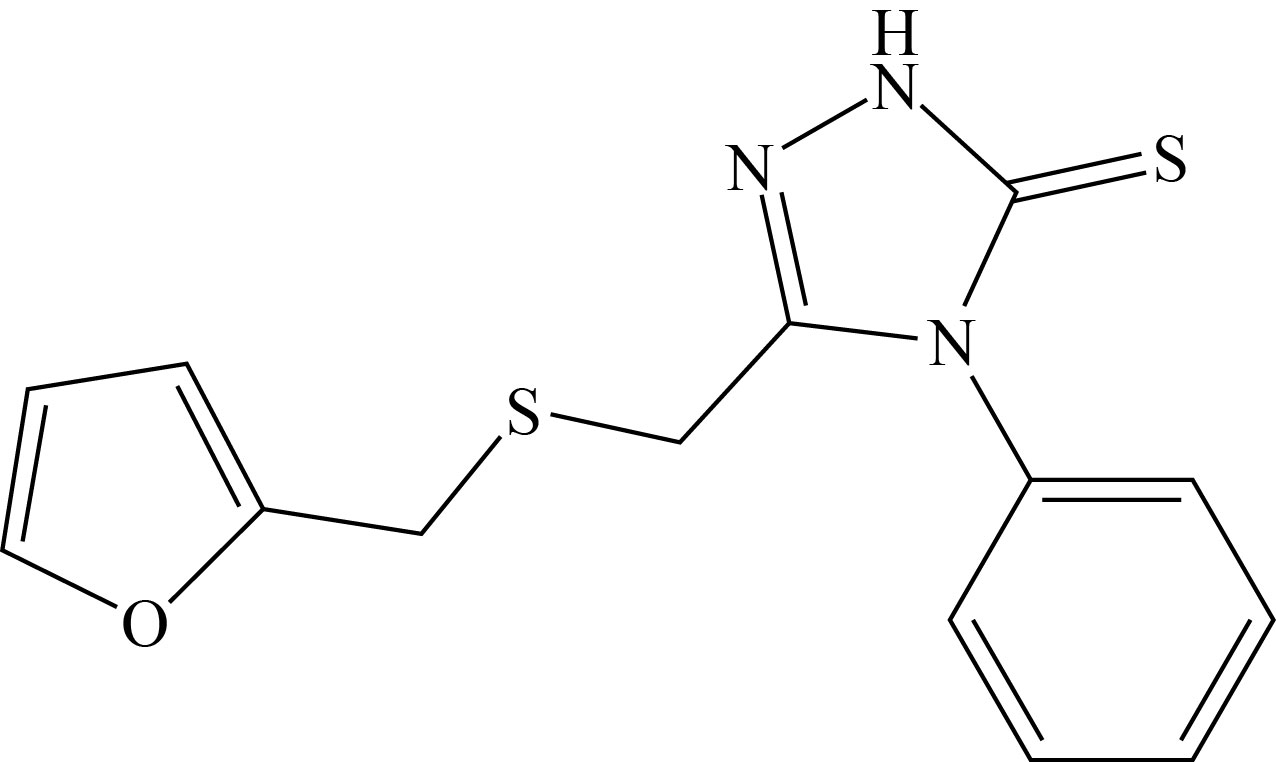
Fig.1 Structure of 5-(furan-2-ylmethylsulfonyl-4-phenyl-2,4- dihydro[1,2,4] triazole-3-thione.
Experimental
Chemicals
The chemical materials were used in this work are shown in Table (1).
Table 1 The chemical materials used in this study.
No. | Materials | Molecular formula | Suppliers |
1 | Hydrochloric acid | HCl | BDH
|
2 | dimethyl sulfoxide (DMSO) | (CH3)2SO | BDH
|
3 | FMSPhDHT | C14H13N3OS2 | locally created |
Preparation of carbon steel samples
Table 2 shows the metallic components in carbon steel reinforcing bar (CSRB) as a proportion of the overall composition.
Table 2 Chemical composition of carbon steel reinforcing bar(CSRB) which are used in this study.
Element | %C | %S | %Si | %N | %Cu | %Mn | %Ni | %Cr | %p |
percentage | 0.26 | 0.031 | 0.28 | 0.010 | 0.28 | 0.73 | 0.13 | 0.12 | 0.018 |
In order to learn more about the impacts of corrosion carbon steel was studied using samples in a circle with a diameter of 1.5 cm carbon steel samples were cleaned using one of the most well-known tools for preparation: immersion in hydrochloric acid to remove any oxide layer or impurities present on the metal's surface, followed by washing with distilled water. The samples were smoothed with silicon carbide smoothing papers ranging (80-2000). After that, the samples were washed thoroughly with distilled water until they were as glossy as a mirror and stored in a separate container.
Preparation of acid solution
In a volumetric flask with a volume of 1000 ml, the acid solution was made at a concentration of one molar, and the volume was completed to the mark with distilled water.
Corrosion Inhibitor FMSPHDHT Solution
FMSPHDHT powder was dissolved in dimethyl sulfoxide (DMSO) solvent and poured into a 10 mL volumetric flask to make three different concentrations (50, 100, and 150 ppm).
Corrosion Potential Measurements
The effect of the prepared compound FMSPHDHT in an acidic environment with one molar concentration was investigated using the electrical method (static stress polarization method) with circular carbon steel samples and a device (Advanced potentiostant winking MLab-200 (2007) [Bank Elektronik-Intelligent control GmbH]) as shown in Fig.2, with three electrodes of corrosion cell and accompanying accessories were used. The corrosion cell is shown in Fig.3, and the three electrodes were the first electrodes used as a reference, which was AgCl, Ag, and KCl (Ag/AgCl,3.0 M KCl) ) based on their potential. The second electrode is an auxiliary electrode made up of a platinum with a high purity rod, and the third is a working electrode (CS) with a 1.5 cm diameter that was installed on the working electrode load and the acidic solution was exposed via the hole. The steps of measurement are:
- The corrosion cell was filled with just blank solution (1000 ml).
- The working electrode was carbon steel, the reference electrode was in front of it, and the auxiliary electrode was beside it.
- After around 15 minutes, the response starts for the first time. The initial run was accompanied with a Tafel plot. The temperature was then increased to 50 degrees Celsius.
- The inhibitor was then added to the blank solution at 25 degrees Celsius to get the first run, Tafel plot, and corrosion rate, as well as the data reported in Table 3.
- The temperature is raised to 30, 40, and 50 degrees Celsius, respectively, and the process is repeated for all inhibitor doses.
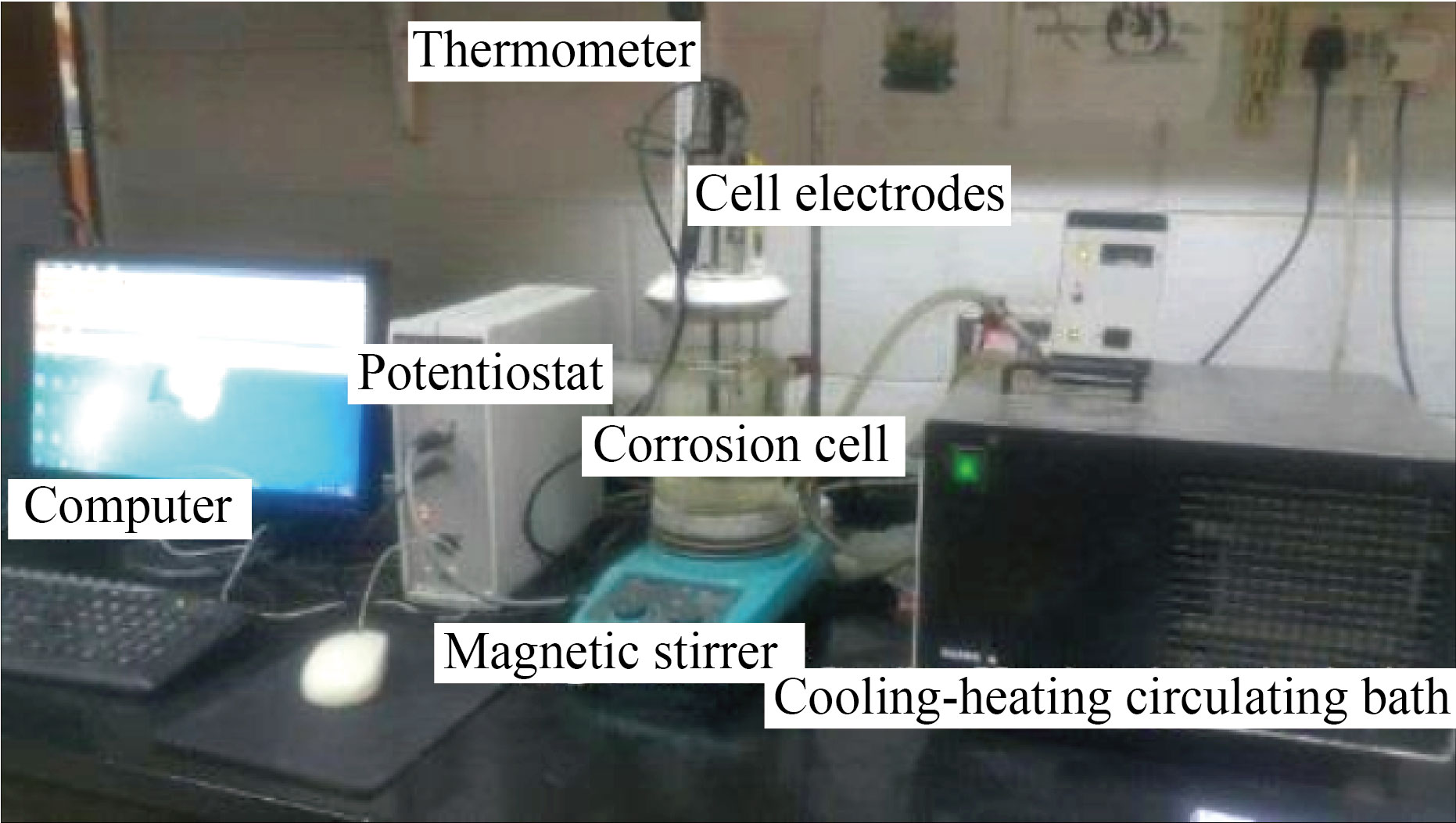
Fig.2 Electrochemical system.
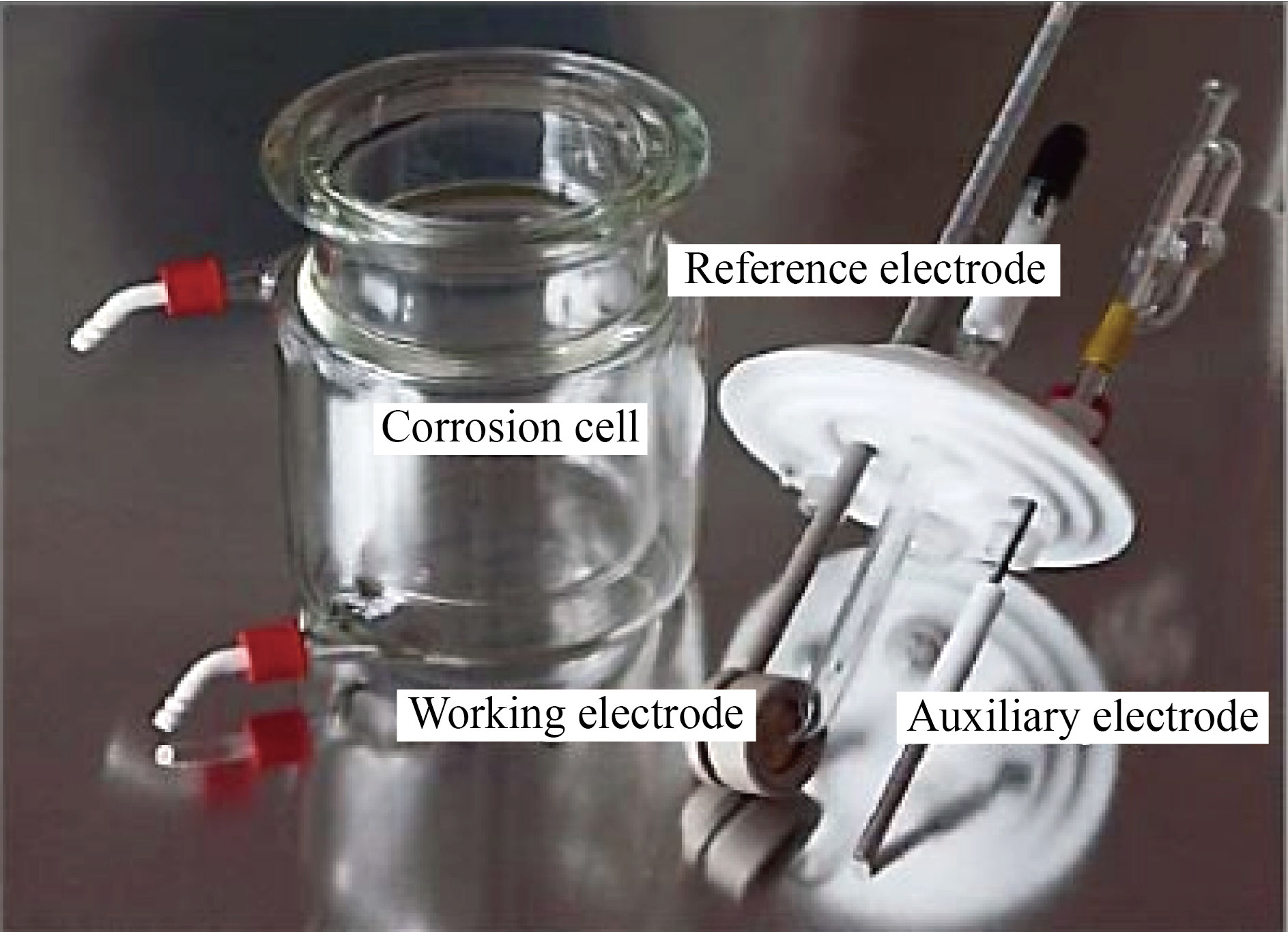
Fig.3 Corrosion cell.
Results and Discussion
Potentiodynamic polarization measurement
Polarization curves were extracted for the corrosion of carbon steel in the acidic solution of the new prepared inhibitor FMSPHDHT at concentrations (50, 100, 150) parts per million (ppm) and at four different temperatures (298-323) Kelvin in the absence and presence of the inhibitor FMSPHDHT as shown in the Fig.4.
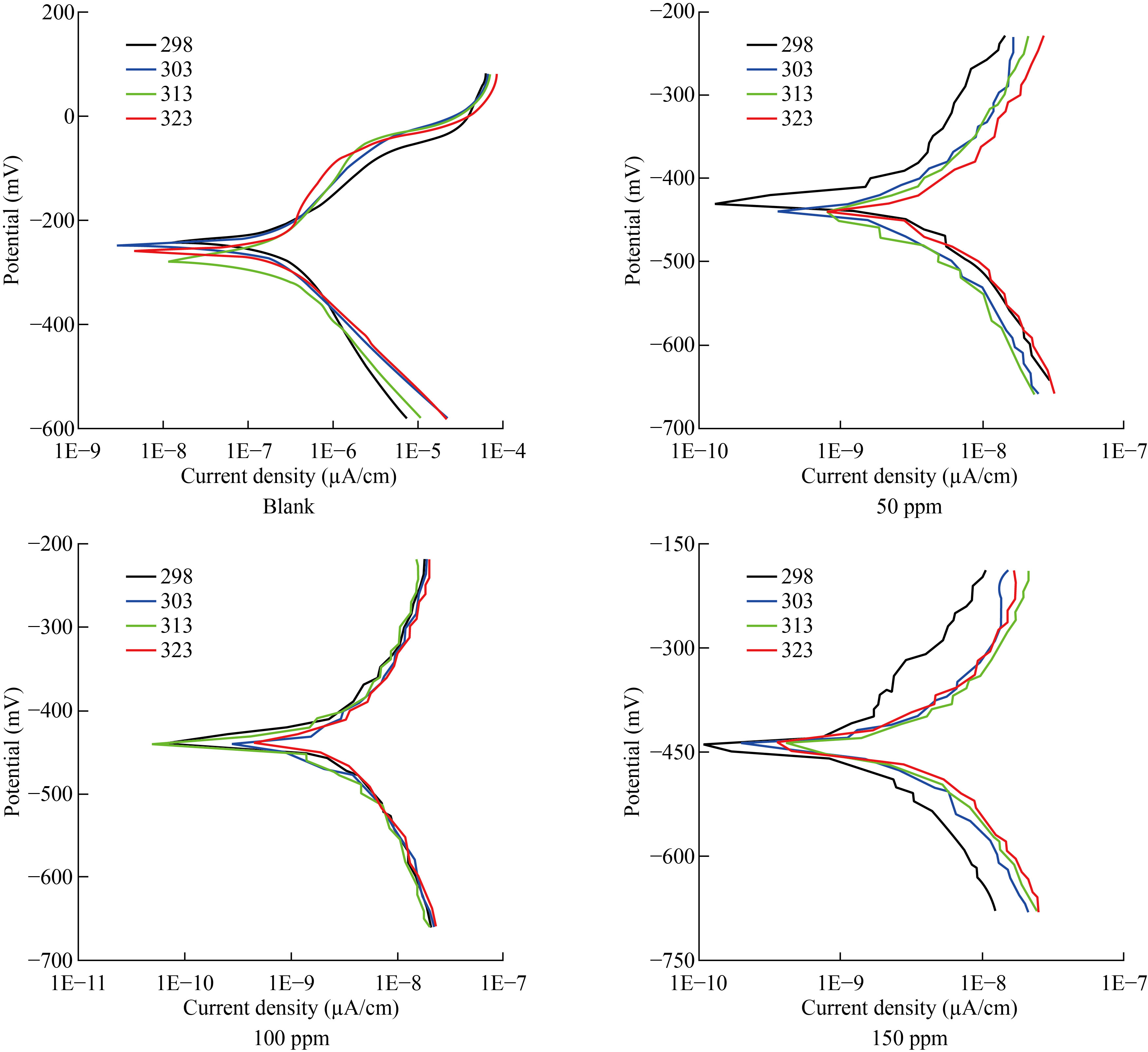
Fig.4 Potentioatatic Polarization curves for carbon steel with absence and present of FMSPHDHT compound in 1 M HCl at different concentration.
Table 3 shows the electrochemical characteristics for carbon steel acid corrosion in the presence of different concentrations of FMSPHDHT, such as corrosion potential (Ecorr), corrosion current density (Icorr), Tafel slopes (bc and ba), and inhibition efficiencies (IE).
Table 3 Polarization parameters for carbon steel in the absence and presence of various concentrations of FMSPHDHT in acidic solution.
Comp. ppm | Temp. | Ecorr (mV) | Icorr (µA/cm2) | Bc (mV/Dec) | Ba (mV/Dec) | PL (mm/y) | IE% |
blank | 293 | -239.7 | 157.54 | -141.2 | 109.3 | 1.83 | - |
303 | -245.1 | 168.95 | -160.8 | 135.0 | 1.96 | - | |
313 | -276.9 | 176.10 | -151.9 | 205.2 | 2.04 | - | |
323 | -261.9 | 186.65 | -131.2 | 219.9 | 2.17 | - | |
50 | 293 | -415.0 | 5.64 | -321.0 | 442.5 | 0.0654 | 96.5 |
303 | -448.2 | 7.16 | -397.5 | 605.3 | 0.0831 | 95.8 | |
313 | -441.4 | 8.09 | -483.5 | 511.1 | 0.0939 | 95.5 | |
323 | -434.3 | 9.48 | -423.6 | 470.2 | 0.110 | 95.0 | |
100 | 293 | -428.4 | 3.04 | -228.4 | 216.0 | 0.0353 | 98.1 |
303 | -434.3 | 3.60 | -249.6 | 242.1 | 0.0418 | 97.9 | |
313 | -440.7 | 5.49 | -393.5 | 446.7 | 0.0637 | 96.9 | |
323 | -435.8 | 6.55 | -413.1 | 422.4 | 0.0760 | 96.5 | |
150
| 293 | -440.6 | 0.98 | -146.7 | 227.5 | 0.0114 | 99.4 |
303 | -441.8 | 1.10 | -103.7 | 105.6 | 0.0128 | 99.4 | |
313 | -440.2 | 1.99 | -145.1 | 135.6 | 0.0231 | 98.9 | |
323 | -429.0 | 3.82 | -275.4 | 271.7 | 0.0443 | 98.0 |
These findings reveal that the FMSPHDHT reduces the Icorr value at all concentrations, implying that this molecule is a good corrosion inhibitor. Furthermore, FMSPHDHT results in a minor change of Ecorr to negative values and by raising inhibitor concentration, inhibition efficiency (percentage) improves. As a result, at a concentration of 150 ppm, FMSPHDHT had the highest inhibitory efficiency (99.4%) at 298 k, due to the presence of a high electronic density represented by the phenyl ring, as well as sulfur, three nitrogen atoms and Pi bonds, contribute to the formation of coordination bonds with the metal surface, resulting in the formation of a preservative layer for the metal in the acidic solution, which inhibits carbon steel [14-15]. The corrosion inhibition efficiency (IE) was estimated using the following formula from the corrosion current density.
![]()
Where Icorr (uncoated) and Icorr (coated) are the corrosion current density values without and with inhibitor, respectively. The decreasing inhibition performance as the temperature of the solution rises might be explained to an increase in the mobility of the inhibitor molecules, resulting in a reduction in the contact between the inhibitor molecules and the carbon steel surface [16-17].
3.2 Kinetic and Thermodynamic of Corrosion
The influence of temperature on the efficacy of corrosion inhibition using the inhibitor FMSPHDHT at temperatures ranging from 293-323 K with and without the inhibitor is part of the thermodynamic investigation. The values of thermodynamic functions are shown in Table 4. After adding the inhibitor to the medium, the data reveal a noticeable difference. The addition of the inhibitor caused the reaction to be directed to locations with high activation energy values, slowing the corrosion rate. Arrhenius equation was used to compute the energy of the activation process [18]:
![]()
Where A is the pre-exponential factor of Arrhenius, T is the absolute temperature, R is the gas constant and Ea the apparent activation energy of the corrosion reaction. The slope of the linear plot of log (icorr) versus 1/T as shown in Fig.5, as well as the activation energy values obtained mentioned in Table 4, were used to calculate Ea.
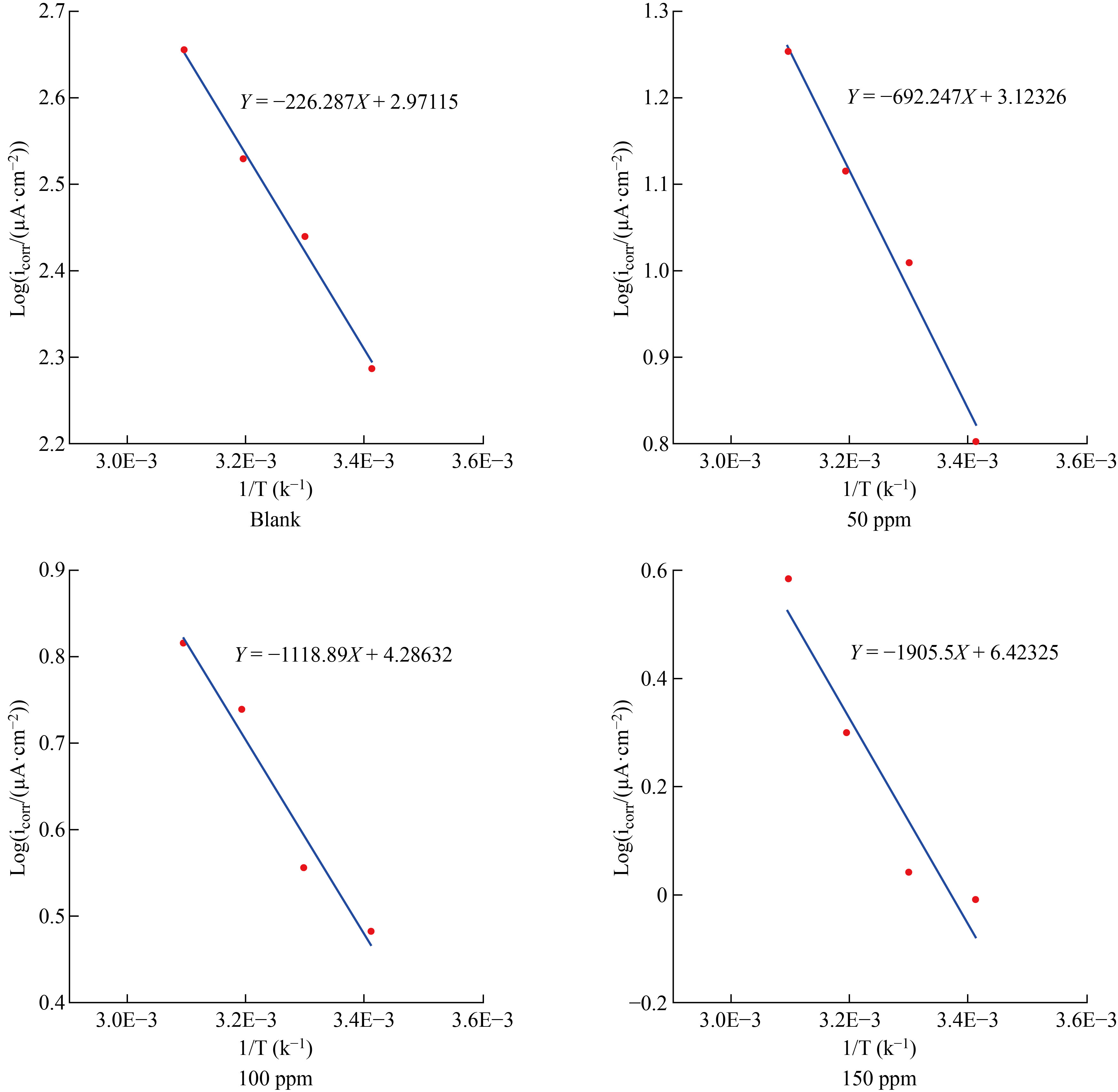
Fig.5 Arrhenius plots for CS in 1 M HCl solution without and with varied quantities of additives inhibitor FMSPHDHT at different temperatures.
The activation parameters, activation enthalpy (ΔH*), and activation entropy (ΔS*), were computed using the Eyring transition state equation [19]:
Log (CR / T) = Log (R/Nh) + ΔS# /2.303 R - ΔH#/2.303 R T
CR stands for corrosion rate, h for Planck's constant, and N for Avogadro's number. Figure 6 transition stage was a plot of log(CR/T) vs 1/T produced a straight line with an intercept of [log(R/Nh) + (ΔS*/2.303R)] and a slope of (ΔH*/2.303R). ΔH* and ΔS* were calculated from this data and are listed in Table [4]. The following thermodynamic relationship can be used to compute the change in activation free energy ΔG* for the corrosion process at each temperature.
ΔG* = ΔH* -T ΔS*
Table 4 Activation parameters of carbon steel corrosion in the absence and presence of inhibitor FMSPHDHT in 1 M HCl.
Sample | T(K) | Ea (kJ/mole) | ΔH# (kJ/mol) | ΔS# (kJ/mol.K) | ΔG# (kJ/mol) |
Blank | 293 | 4.333
| 1.776
| -0.197
| 0.060 |
303 | 0.062 | ||||
313 | 0.064 | ||||
323 | 0.066 | ||||
50 ppm | 293 | 13.255
| 10.698
| -0.194
| 0.068 |
303 | 0.07 | ||||
313 | 0.072 | ||||
323 | 0.074 | ||||
100 ppm | 293 | 21.424
| 18.867
| -0.171
| 0.069 |
303 | 0.071 | ||||
313 | 0.073 | ||||
323 | 0.075 | ||||
150 ppm | 293 | 36.485 | 33.929 | -0.131 | 0.073 |
303 | 0.074 | ||||
313 | 0.075 | ||||
323 | 0.077 |
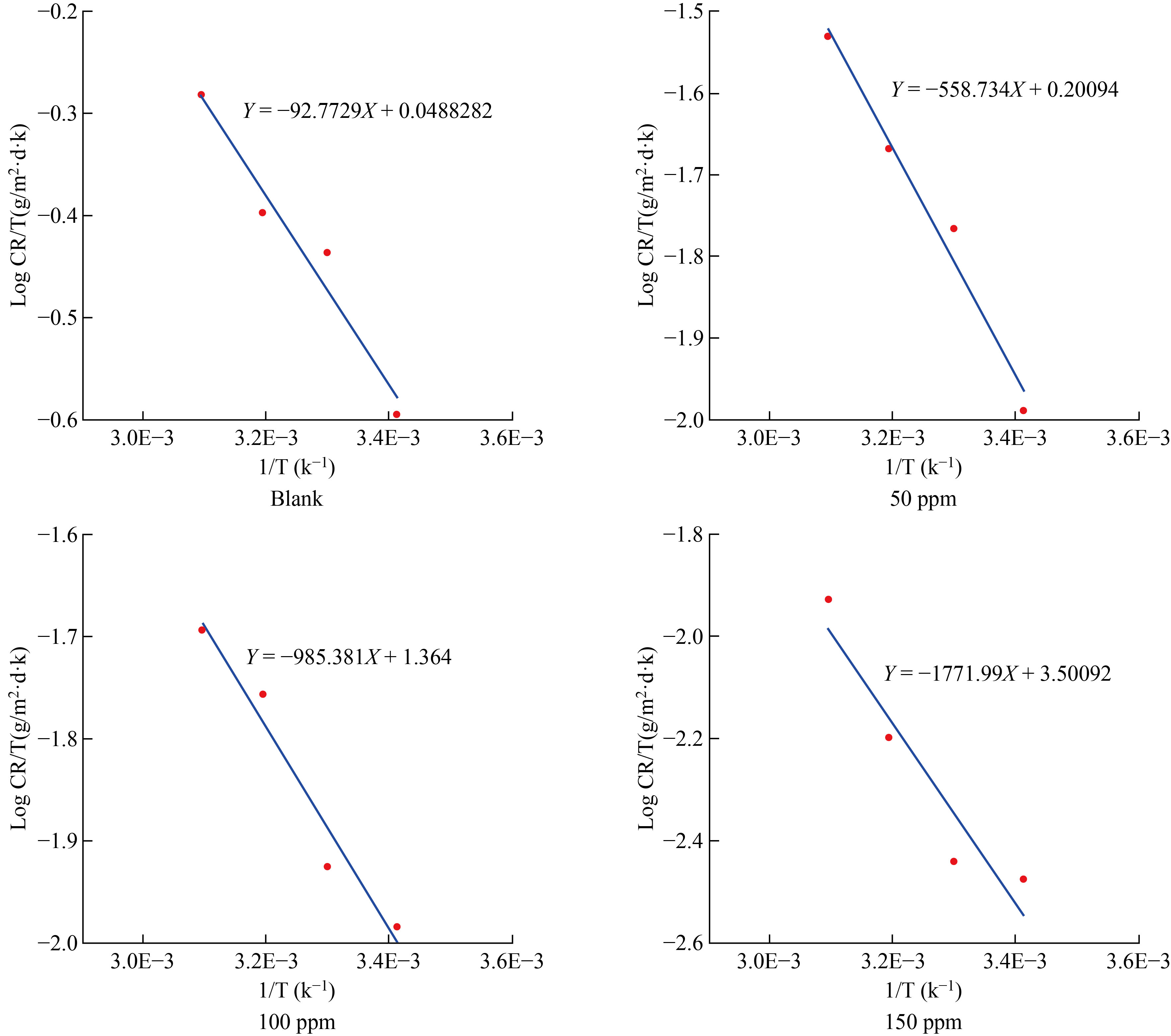
Fig.6 Transition state plots for carbon steel corrosion in the absence and presence of FMSPHDHT inhibitor.
Table 3 demonstrates that the value of H# for carbon steel corrosion in acidic solution (1.776) in the absence of the inhibitor rose in the presence of the inhibitor, with the greatest value of the enthalpy of inhibition (150 ppm) indicating improved protection efficiency and an endothermic nature of the carbon steel dissolution [20]. The negative inhibitory entropy ΔS* suggests a high degree of adsorption regularity during the activation phase, indicating the creation of the activated complex and a reduction in degrees of freedom [21-23]. The obtained values of ΔG* are listed in Table 2. ΔG* values were positive and increased slightly as temperature increased, indicating that the inhibitor lowered the thermodynamic feasibility of corrosion [24].
Surface study
Atomic force microscopy (AFM) was used to analyze the surface of a carbon steel sample in acid solution (1M HCl) in the absence and presence of the inhibitor FMSPHDHT. Figure 7 shows that in the presence of the acidic solution, the surface topography of the steel sample has been damaged, and the surface roughness (40.7 nm) is clearly visible as shown in Figure 7a, but after adding the inhibitor FMSPHDHT, the surface roughness (7.78 nm) of the carbon steel has been reduced as clearly shown in Figure 7b [25-26].
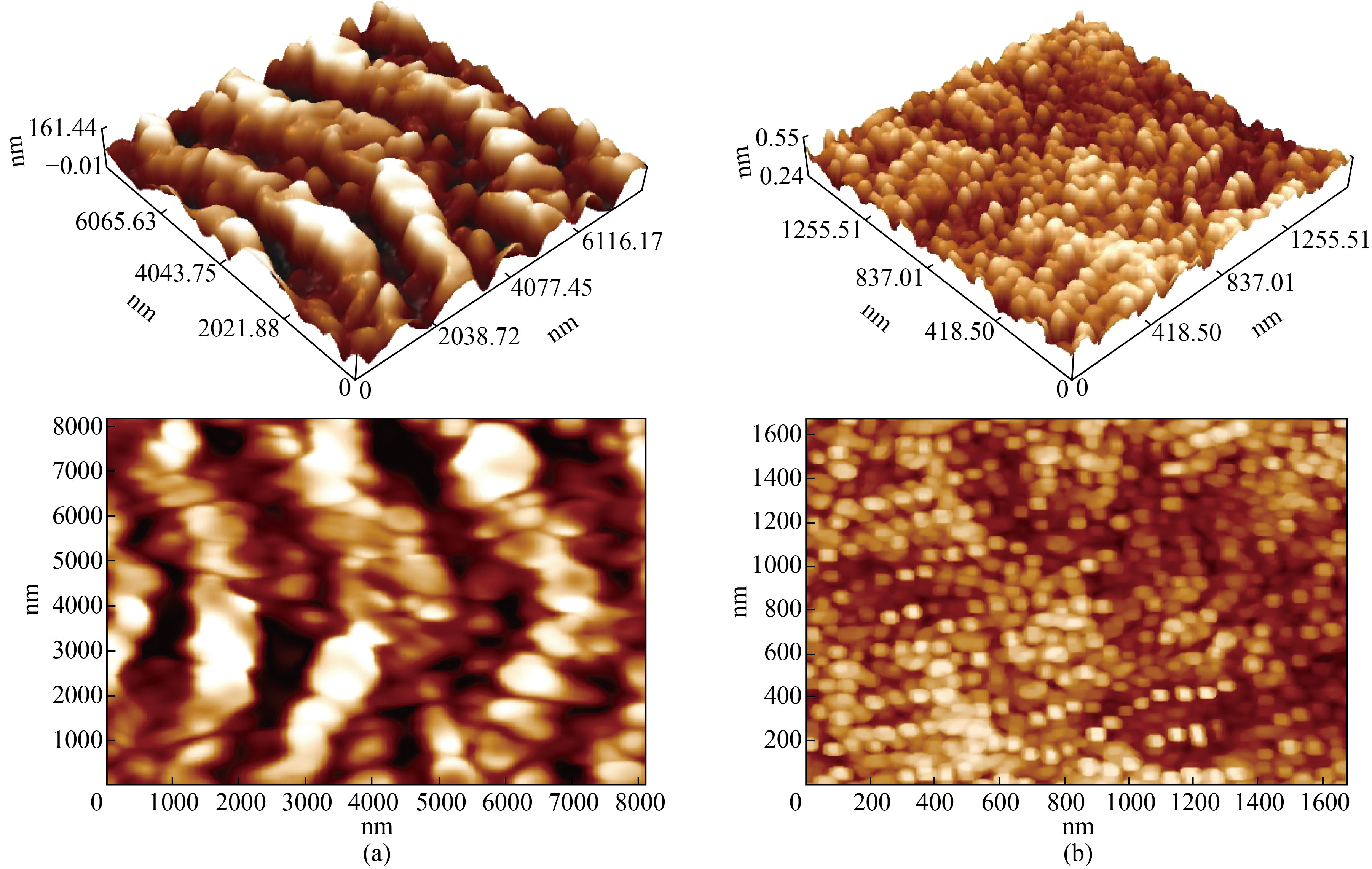
Fig.7 Micrograph (AFM) of the surface of carbon steel in acidic solution (a) in the absence of an inhibitor (b) in the presence of an inhibitor.
According to the results, the adsorption of these molecules at the metal-solution interface may explain the inhibitory effect of the novel furan derivative on carbon steel corrosion in 1 M HCl solution. The inhibitory effect of FMSPHDHT is related to the interaction of -electrons phenyl and furan ring, as well as the presence of electron donor groups (N, O, and S) via which it forms bonds with carbon steel [27]
Computational study
Theoretical details
The structural and electronic properties of the new furan derivative 5-(furan-2-ylmethylsulfonyl-4-phenyl-2,4-dihydro[1,2,4] triazole-3-thione molecule were investigated using density functional theory (DFT) and frequency calculations at the Lee, Yang, and Parr ( B3LYP/6-311++G (d, p)) basis set [28]. All calculations were done with the Gaussian 09W program [29]. The structure of the molecule FMSPhDHT (Fig.8) was drawn using the ChemDraw of Mopac tool (ver. 10), the bond lengths and dihedral angles are listed in Tables 5 and 6.
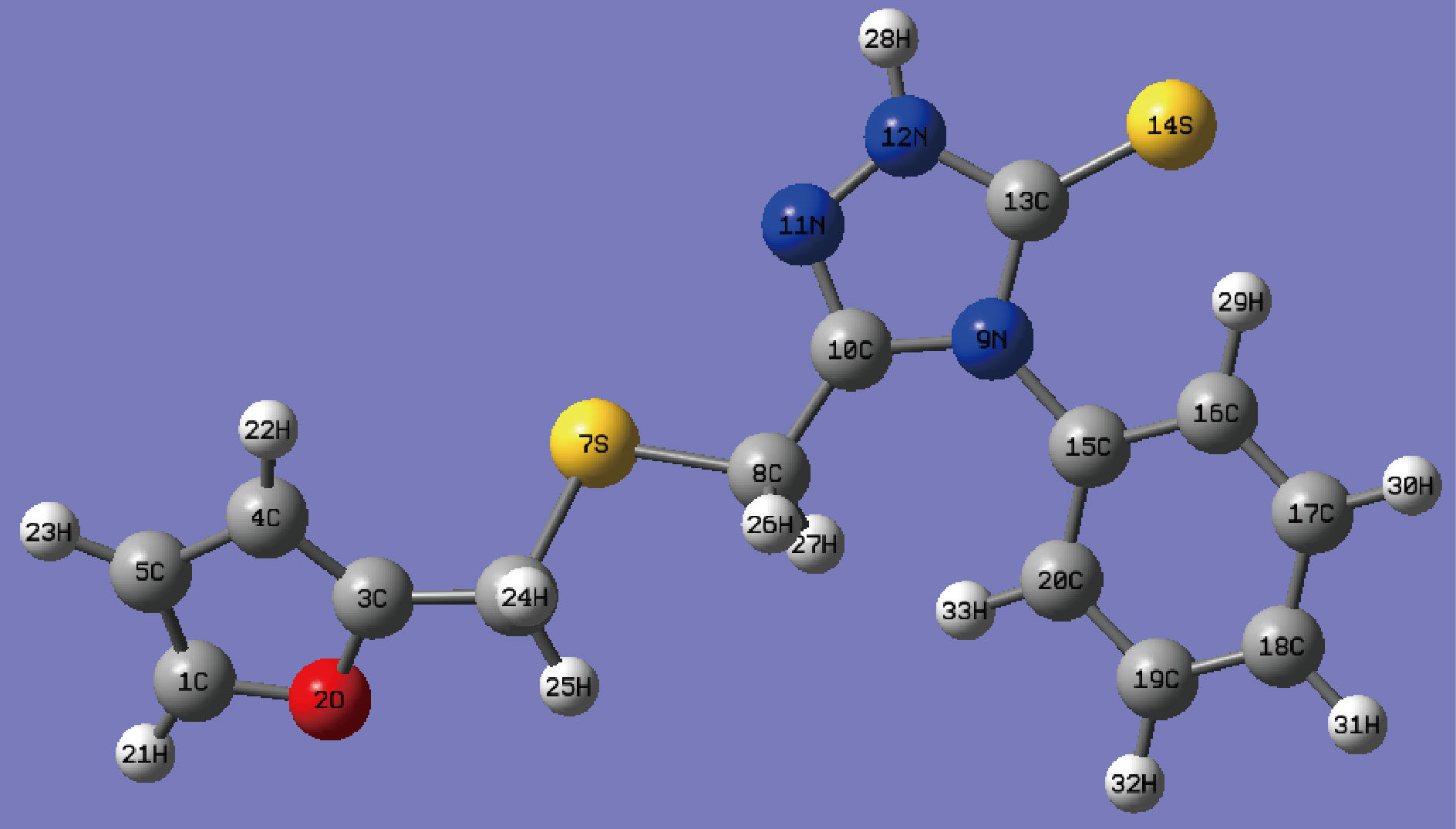
Fig.8 Optimized geometry of 5-(furan-2-ylmethylsulfonyl-4-phenyl-2,4- dihydro[1,2,4] triazole-3-thione.
Table 5 The length of the bond for FMSPhDHT compound using ChemDraw of Mopac tool.
Atoms | Bond length (Å) | Atoms | Bond length (Å) |
C20-H33 | 1.095 | C17- C18 | 1.398 |
C1-H32 | 1.1026 | C16-C17 | 1.3908 |
C18-H31 | 1.1017 | C15-C16 | 1.4201 |
C17-H30 | 1.1026 | N9-C15 | 1.4455 |
C16-H29 | 1.0981 | C13-S14 | 1.5766 |
N12-H28 | 1.0074 | C13-N9 | 1.3386 |
C8-H27 | 1.115 | N12-C13 | 1.3695 |
C8-H26 | 1.1147 | N11-N12 | 1.3483 |
C6-H25 | 1.115 | C10-N11 | 1.2804 |
C6-H24 | 1.1147 | N9-C10 | 1.4636 |
C5-H23 | 1.0961 | S7-C8 | 1.8233 |
C4-H22 | 1.096 | C6-S7 | 1.8178 |
C1-H21 | 1.0942 | C3-C6 | 1.5048 |
C8-C10 | 1.5105 | C5-C1) | 1.3605 |
C20-C15 | 1.4168 | C4-C5 | 1.4307 |
C19-C20 | 1.3919 | C3-C4 | 1.3669 |
C18-C19 | 1.3973 | O2 -C3 | 1.3685 |
|
| C1-O2 | 1.3625 |
Table 6 Angles of dihedral for FMSPhDHT compound using ChemDraw of Mopac tool.
Atoms | Angle of dihedral (deg.) | Atoms | Angle of dihedral (deg.) |
S7-C8-C10-N9 | -168.802 | H25-C6 -S7-C8 | -60.3329 |
S7-C8-C10-N11 | 14.1491 | O2-C3-C6-S7 | 166.7385 |
H26-C8-C10-N9 | 68.7814 | O2-C3-C6-H24 | -70.6522 |
H26-C8-C10-N11 | -108.268 | O2-C3-C6-H25 | 44.9162 |
H27-C8-C10-N9 | -49.8947 | C4-C3-C6-S7 | -14.2536 |
H27-C8-C10-N11 | 133.0564 | C4-C3-C6-H24 | 108.3558 |
C19-C20-C15-N9 | -179.915 | C4-C3-C6-H25 | -136.076 |
C19-C20-C15-C16 | -3.5831 | C4-C5-C1-O2 | -0.1084 |
H33-C20-C15-N9 | 0.5644 | C4-C5-C1-H21 | 179.707 |
H33-C20-C15-C16 | 176.8964 | H23-C5-C1-O2 | -179.861 |
C18-C19 - C20-C15 | 1.0929 | H23-C5-C1-H21 | -0.0453 |
C18-C19-C20-H33 | -179.36 | C3-C4-C5-C1 | 0.1886 |
H32-C19-C20-C15 | -179.382 | C3-C4-C5-H23 | 179.9414 |
H32-C19-C20-H33 | 0.1653 | H22-C4-C5-C1 | -179.466 |
C17-C18-C19-C20 | 1.3599 | H22-C4-C5-H23 | 0.2872 |
C17-C18-C19-H32 | -178.169 | O2-C3-C4-C5 | -0.2032 |
H31-C18-C19-C20 | 179.4038 | O2-C3-C4-H22 | 179.4397 |
H31-C18-C19-H32 | -0.1247 | C6-C3-C4-C5 | -179.311 |
C16-C17-C18-C19 | -1.0843 | C6-C3-C4-H22 | 0.3319 |
C16-C17-C18-H31 | -179.13 | C1-O2-C3-C4 | 0.1383 |
H30-C17-C18-C19 | 177.1191 | C1-O2-C3-C6 | 179.3113 |
H30-C17-C18-H31 | -0.9266 | C5-C1-O2-C3 | -0.0136 |
C15-C16-C17-C18 | -1.6563 | H21-C1-O2-C3 | -179.858 |
C15-C16-C17-H30 | -179.85 |
|
|
H29-C16-C17-C18 | 175.7057 |
|
|
H29-C16-C17-H30 | -2.4876 |
|
|
N9-C15-C16-C17 | -179.774 |
|
|
N9-C15-C16-H29 | 3.0241 |
|
|
H24-C6-S7-C8 | 58.2905 |
|
|
Molecular reactivity
The following relationships [30-31] are used to calculate quantum chemical parameters such as dipole moment (), electro negativity (χ), hardness (), softness (S), ionization potential (IP), electron affinity (EA), the fractions of electrons transferred (∆N), the electrophilicity index (ω), energy of highest occupied molecular orbital (EHOMO), energy of lowest unoccupied molecular orbital (ELUMO), energy gap (Egap) and Mulliken charge distribution in vacuum and DMSO solvent. Table 7 summarizes the results.
IP = -HOMO
EA = -LUMO
![]()
![]()
![]()
![]()
Table 7 Chemical parameters at the quantum level for FMSPhDHT compound calculated using DFT / B3LYP/6-311++G (d,p) in DMSO solvent and vacuum.
Parameters | DMSO | Vacuum |
Total Energy | -1577.4184 a.u. | -1577.3967 a.u. |
HOMO (eV) | -0.2295 | -0.21309 |
LUMO (eV) | -0.04044 | -0.04499 |
Egap (eV) | 0.189 | 0.168 |
( Debye) | 8.4513 | 5.7725 |
IP (eV) | 0.2295 | 0.21309 |
EA (eV) | 0.0404 | 0.0450 |
χ (eV) | 0.2699 | 0.2581 |
(eV) | 0.189 | 0.1681 |
S (eV)-1 | 5.291 | 5.948 |
(eV) | 0.192 | 0.198 |
The energy value of FMSPhDHT's for highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) in DMSO solvent and vacuum are shown in Table 7. One interesting finding is energy value of HOMO for DMSO solvent is great than value in the vacuum. The energy value of HOMO indicates a molecule's ability to donate electrons to an acceptor molecule; in general, the higher the EHOMO value, the greater the molecule's tendency to donate electrons to an acceptor molecule, and the lower the ELUMO value (the molecule's ability to accept electrons), the greater the tendency to accept electrons, As a result of the FMSPhDHT compound's ability to take electrons from metal iron, it has a greater inhibitory efficiency, which is consistent with experimental results [32].
Another important electrical characteristic that emerges from the non-uniform distribution of charges on the various atoms in a molecule is the dipole moment. Stronger intermolecular attraction is caused by higher dipole moments [33]. In the DMSO solvent, the dipole moment of FMSPhDHT is greater than in the vacuum. Adsorption between a chemical substance and a metal surface is likely to be enhanced by a strong dipole moment [34]. The inhibitor FMSPhDHT has 8.4513 Debye, this indicates a higher increase in reactivity than the medium without a solvent.
In DMSO solvent and vacuum, the inhibitor's FMSPhDHT electron density distributions, frontier molecular orbital molecular optimization, maximum occupied molecular orbital energy, and lowest unoccupied molecular orbital energy are shown in Fig.9.
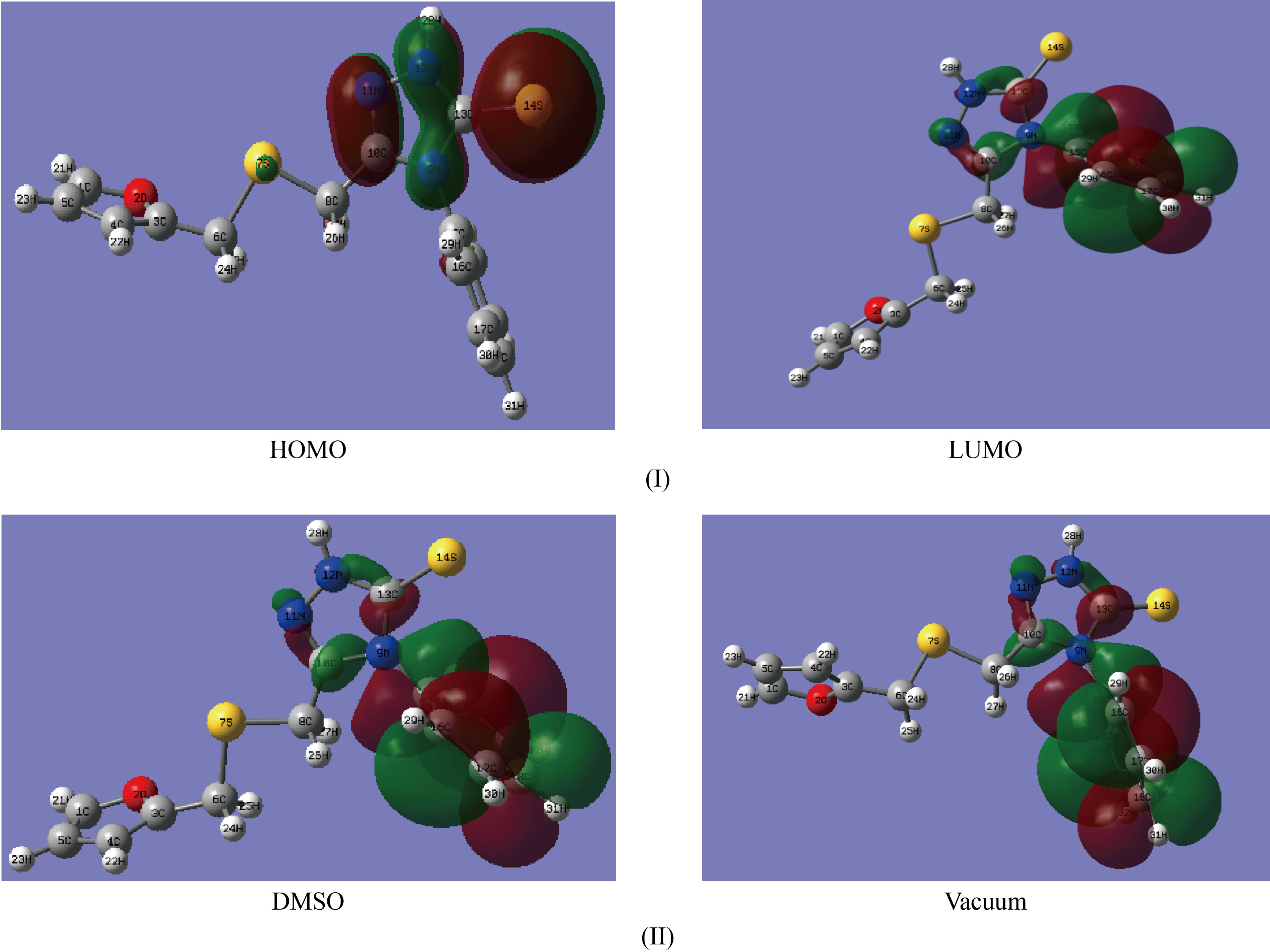
Fig.9 (I) HOMO and (II) LUMO in DMSO solvent and
vacuum of the inhibitor FMSPhDHT using DFT / B3LYP / 6-311 ++G (d, p).
The electronic density of the HOMO molecular orbitals is completely concentrated on the heteroatoms three nitrogen atoms and sulfur, but the LUMO molecular orbitals exhibit an important electronic density scattered throughout the majority of the phenyl ring and a small density over 3 nitrogen atoms (triazole ring). This finding is consistent with the Mulliken charge distribution and the direction of the dipole moment seen in Figure 10 and stated in Table 8.
Active sites of the inhibitor
Mulliken charges distribution (MCD) are an important characteristic to consider when investigating the adsorption center of inhibitor compounds. FMSPhDHT molecule has greater nucleophilic and electrophilic electrical charge values in DMSO than in vacuum [35]. Mulliken's charge population study for the FMSPhDHT molecule in two mediums (DMSO and vacuum) and the direction of dipole moment are shown in Fig.10 and Table 7.
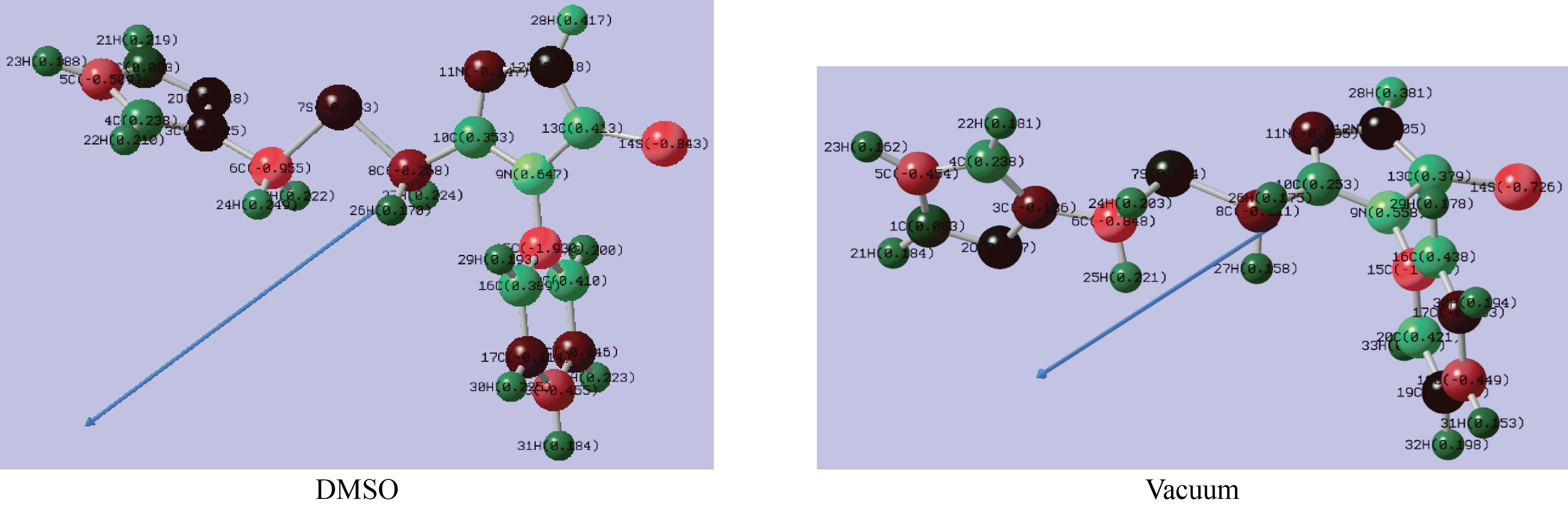
Fig.10 Atomic charge distributions for the FMSPhDHT compound.
Table 8 Mulliken charge values of the FMSPhDHT compound.
Symbol of Atom | C1 | O2 | C3 | C4 | C5 |
MCD | 0.083* 0.083** | 0.017 -0.018 | -0.136 -0.025 | 0.238 0.238 | -0.454 -0.5092 |
Symbol of Atom | C6 | S7 | C8 | N9 | C10 |
MCD | -0.848 -0.954 | 0.024 -0.063 | -0.211 -0.268 | 0.558 0.647 | 0.253 0.353 |
Symbol of Atom | N11 | N12 | C13 | S14 | C15 |
MCD | 0.095 -0.147 | -0.005 0.017 | 0.379 0.413 | -0.726 -0.843 | -1.948 -1.930 |
symbol of Atom | C16 | C17 | C18 | C19 | C20 |
MCD | 0.438 0.389 | -0.083 -0.113 | -0.449 -0.455 | -0.008 -0.146 | 0.421 0.410 |
Symbol of Atom | H21 | H22 | H23 | H24 | H25 |
MCD | 0.18 0.218 | 0.181 0.210 | 0.162 0.187 | 0.203 0.248 | 0.221 0.221 |
Symbol of Atom | H26 | H27 | H28 | H29 | H30 |
MCD | 0.175 0.169 | 0.158 0.224 | 0.381 0.417 | 0.178 0.193 | 0.194 0.224 |
Symbol of Atom | H31 | H32 | H33 |
|
|
MCD | 0.153 0.183 | 0.198 0.223 | 0.163 0.200 |
|
|
* vacuum ** DMSO
In order to investigate the effects of charge distribution on the adsorption process. Table 8 shows the order of the nucleophilic reactive sites of FMSPhDHT inhibitor is as follows: C15>C6>S14>C5>C18>C8>N11>C19, while the order of the electrophilic reactive sites is as follows N9>C13>S20>C16>C10. The most interesting aspect of this results, is that FMSPhDHT molecule is an effective inhibitor that matches experimental results [36-37].
As well as to charge distribution study, the electrostatic potential (ESP) map is a common tool for detecting where a molecule's electron density is high or low. Electrostatic potential may be used to forecast the reaction center of molecules when they come into contact with other materials. Figure 11 shows the electrostatic potential (ESP) map derived using the B3LYP/6-31++G(d,p) approach. The locations with the highest positive, negative, and zero electrostatic potential, respectively, are represented by the blue, red, and green regions in Figure 11. According to Figure 11, the largest negative electron density zone is found near S14, N9, C13, C15, S7, N11, C6 whereas the positive electron density region is mostly found in hydrogen and certain carbon atoms as well as phenyl ring.
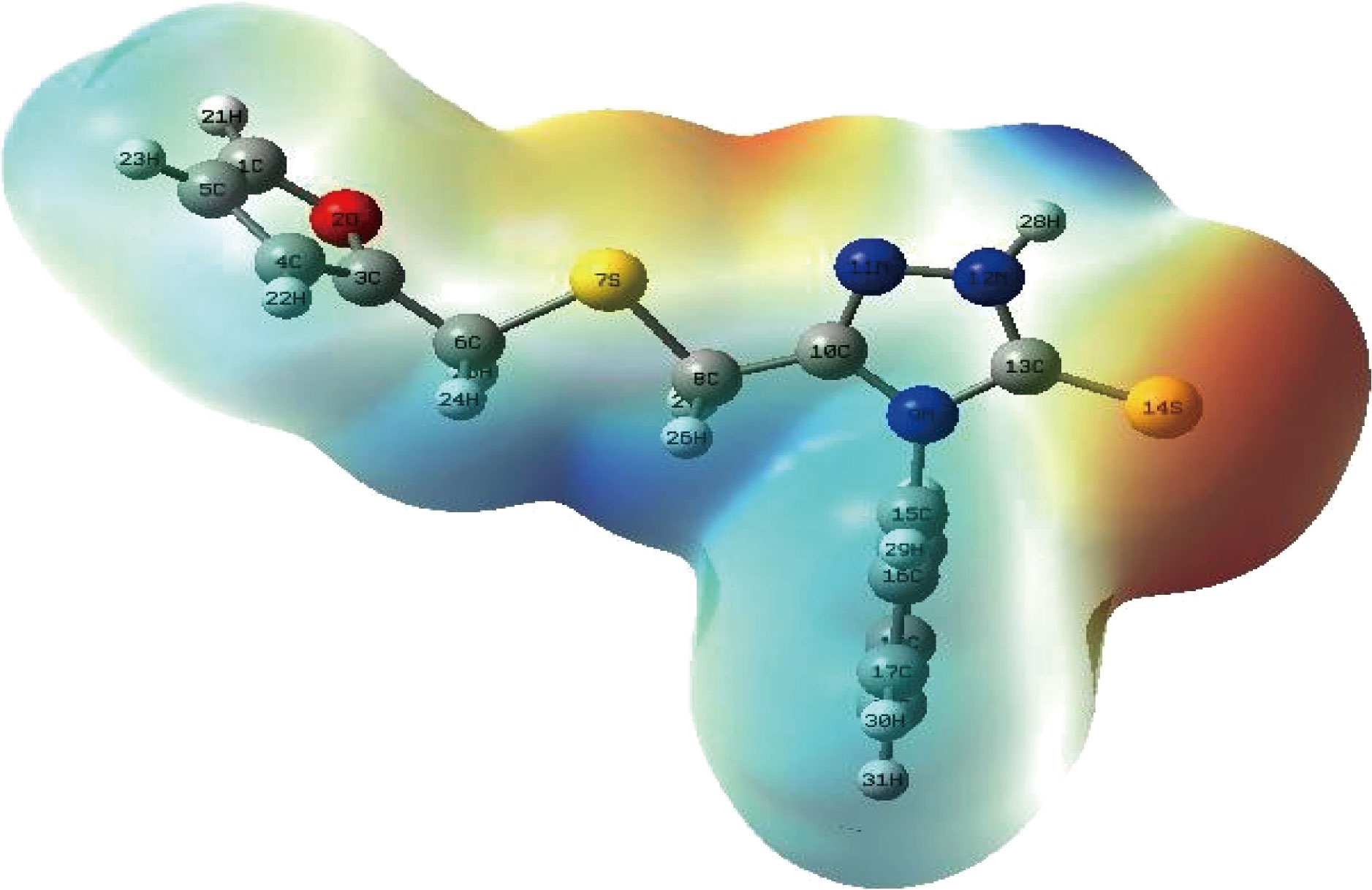
Fig.11 Electrostatic potential (ESP) maps for the studied FMSPhDHT compound.
The electrostatic potential results were in accord with the charge distribution results when it came to the influence of hetero atoms on the adsorption of the FMSPhDHT compound on the surface of carbon steel and its resistance to corrosion in an acidic medium [38].
Conclusion
The following are some of the study's findings:
- The experimental results revealed that the novel furan derivative 5-(furan-2-ylmethylsulfonyl-4-phenyl-2,4-dihydro[1,2,4]triazole-3-thione (FMSPhDHT) is an efficient corrosion inhibitor for carbon steel in acidic solutions.
- The investigated inhibitor's corrosion inhibition efficacy was 99.4 percent.
- The inhibitor reduced the thermodynamic feasibility of corrosion by increasing ΔG* values, which were positive and rose marginally as temperature climbed. While the system's entropy decreases as a result of the thermodynamic analysis.
- The creation of a protective barrier on the carbon steel surface is suggested by the potentiodynamic polarization curve and AFM investigation.
- DFT (B3LYP / 6-311 ++G (d, p) based quantum chemistry calculations of parameters related to the electronic structures of FMSPhDHT back up the experimental findings.
- The charge distribution research revealed the impacts of FMSPhDHT on the adsorption process by revealing nucleophilic and electrophilic inhibitor reactive sites.
The findings show that the FMSPhDHT compound is a potential inhibitor that may be utilized to reduce the corrosion of oil tanker pipes as well as prevent corrosion of metal mechanisms in industrial facilities.
Conflict of Interests
The authors declare that no competing interest exists.
References
- Cherrak, K., Dafali, A., Elyoussfi, A., al, 2017. Two new benzothiazine derivatives as corrosion inhibitors for mild steel in hydrochloric acid medium. J. Mater. Environ. Sci., 8 (2), 636–647.
- Ouici, H., Tourabi, M., Benali, O., Selles, C., Jama, C., Zarrouk, A., Bentiss, F., 2017. Adsorption and corrosion inhibition properties of 5-amino 1,3,4-thiadiazole-2-thiol on the mild steel in hydrochloric acid medium: thermodynamic, surface and electrochemical studies. J. Electroanal. Chem., 803, 125–134.
- Ansari, K.R., Quraishi, M.A., Singh, A., 2014. Schiffs base of pyridyl
substituted triazoles as new and effectivecorrosion inhibitors for
mild steel in hydrochloric acid solution. Corros. Sci., 79, 5–15. - Benali, O., Larabi, L., Traisnel, M., Gengembre, L., Harek, Y., 2007.
Electrochemical, theoretical and XPS studies of 2-mercapto-1-
methylimidazole adsorption on carbon steel in 1 M HClO4. Appl.
Surf. Sci. 253 (14), 6130–6139. - Cano, E., Polo, J.L., Iglesia, A.La., Bastidas, J.M., 2004. A study on the
adsorption of benzotriazole on copper in hydrochloric acid using
the inflection point of the isotherm. Adsorption 10 (3), 219–225. - Lebrini, M., Traisnel, M., Lagrene´ e, M., Mernari, B., Bentiss, F., 2008.
Inhibitive properties, adsorption and a theoretical study of 3,5-bis
(n-pyridyl)-4-amino-1,2,4-triazoles as corrosion inhibitors for mild
steel in perchloric acid. Corros. Sci. 50 (2), 473–479. - Kubba RM., Mohammed M. A. and Ahamed L.S. 2021, DFT Calculations and Experimental Study to Inhibit Carbon Steel Corrosion in Saline Solution by Quinoline-2-One Derivative: Carbon Steel Corrosion. Baghdad Science Journal. 18, 1, 0113.
- Kubba RM. and Mohammed MA. 2022, Theoretical and Experimental Study of Corrosion Behavior of Carbon Steel Surface in 3.5% NaCl and 0.5 M HCl with Different Concentrations of Quinolin-2-One Derivative. Baghdad Sci.J. 19(1), 0105.
- Loto R. T., Loto C. A., Joseph O. and Olanrewaju G., 2016, Adsorption and corrosion inhibition properties of thiocarbanilide on the electrochemical behavior of high carbon steel in dilute acid solutions. Results in Physics 6, 305–314.
- Kacimi Y. El, Azaroual M. A., Touir R., Galai M. and Alaoui K., 2017, Corrosion inhibition studies for mild steel in 5.0 M HCl by substituted phenyltetrazole, Euro-Mediterr. J Environ Integr, 2, 1-11.
- Haque J., Verma C., Srivastava V., Quraishia M.A. and Ebenso Eno E., 2018, Experimental and quantum chemical studies of functionalized
tetrahydropyridines as corrosion inhibitors for mild steel in 1 M
hydrochloric acid. Results in Physics 9, 1481–1493. - Kannan P., Shukla S. K., RaoT. S. and Rajendran N., 2016. Adsorption, thermodynamic and quantum chemical studies of 3-(4-Chlorobenzoylmethyl) benzimidazoliumbromide in inhibition effect on carbon steel, J. Mater. Environ. Sci. 7 (4), 1154-1171,
- Luma S. A., 2018. Synthesis of New Five-Membered Hetrocyclic Compounds from 2-Furfuryl Mercaptan Derivative and Evaluation of their Biological Activity, J. Glob. Pharma. Technol., 11, 298.
- AL-Sammarraie A.M., 2020. Role of carbon dioxide on the corrosion of carbon steel reinforcing bar in simulating concrete electrolyte. Baghdad Science Journal, 17(1), 93.
- Sherine B., Nasser A. J. A. and Rajendran S., 2010. Inhibitive action of hydroquinone – Zn2- system in controlling the corrosion of carbon steel in well water. Inter., J. Eng., Sci., Tech., 2(4): 341-357.
- Khudhair N. A., Synthesis, identification and experimental studies for carbon steel corrosion in hydrochloric acid solution for polyimide derivatives." AIP Conference Proceedings. Vol. 2290. No. 1. AIP Publishing LLC, 2020.
- Khudhair N. A., Khadom M. M. and Khadom A. A., 2021, Effect of Trimethoprim Drug Dose on Corrosion Behavior of Stainless Steel in Simulated Human Body Environment: Experimental and Theoretical Investigations. Journal of Bio-and Tribo-Corrosion, 7.3, 1-15.
- Benali, O., Larabi, L., Tabti, B., Harek, Y., 2005. Influence of 1-
methyl 2-mercapto imidazole on corrosion inhibition of carbon
steel in 0.5M H2SO4. Anti-Corros. Method Mater. 52, 280–285. - Fusco M. A., Yasar Ay, Casey A. H.M., Bourham M. A. and Winfrey A. L., 2016. Corrosion of single layer thin film protective coatings on steel substrates for high level waste containers, Journal Progress in Nuclear Energy, 89, 159-169.
- El Ouali I., Hammouti B., Aouniti A., Ramli Y., Azougagh M.and Essassi
EM., 2010. Thermodynamic characterisation of steel corrosion
in HCl in the presence of 2-phenylthieno (3, 2-b) quinoxaline.
Journal of Materials and Environmental Science, 1(1), 1-8. - Kubba R. M. and Al-Joborry N. M., 2020. Theoretical and Experimental Study of a New Imidazo (1,2-a) Pyridine Derivative as a Corrosion Inhibitor for the Carbon Steel Surface in the Saline Media. ANJS, 23 (1), 13 – 26.
- Oguzie EE., 2007. Corrosion inhibition of aluminium in acidic and
alkaline media by Sansevieria trifasciata extract. Corrosion Science, 49(3), 1527-1539. - Ahamad I. and Quraishi M.A., 2009. Bis (benzimidazol-2-yl) disulphide: an efficient water soluble inhibitor for corrosion of mild steel in acid media, Corros. Sci. 51, 2006-2013.
- Al-Mashhdani H. A. Y., Al-Saadie K. A., Hayfaa A. A. and Duha a., 2015. Cactus as a green inhibitor for the corrosion of carbon steel in seawater, PCAIJ, 10(4), 111-120,
- ARehan T., Lami N.A., and Khudhair N.A., 2021. Synthesis, characterization and anti-corrosion activity of new triazole, thiadiazole and thiazole derivatives containing imidazo [1, 2-a] pyrimidine moiety. Chemical Methodologies, 285-295.
- AL-Thib A.T. and Khudhair N.A., 2016. Spectroscopic study of some aromatic hydrazones derivated from aromatic substituted benzophenones and benzaldydes. PCAIJ, 11(1), 24-33.
- Zaafarany I.,2009, Phenyl phthalimide as corrosion inhibitor for corrosion of C-Steel in sulphuric acid solution. Portugaliae Electrochimica Acta, 27 (50), 631–643.
- Lee C., Yang W. and Parr G G., 1988. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density‖ .Phys. Rev. B37 (2), 785-789.
- Frisch MJ., Trucks GW., Schlegel HB., Scuseria GE., Robb MA., Cheeseman JR., Scalmani G., Barone V., Mennucci B., Petersson GA., Nakatsuji H., Caricato M., Li X., Hratchian HP., Izmaylov AF., Bloino J., Zheng G., Sonnenberg JL., Hada M., Ehara M., Toyota K., Fukuda R., Hasegawa J., Ishida M., Nakajima T., Honda Y., Kitao O., Nakai H., Vreven T., Montgomery Jr JA., Peralta JE., Ogliaro F., Bearpark M., Heyd JJ., Brothers E., Kudin KN., Staroverov VN., Kobayashi R., Normand J., Raghavachari K., Rendell A., Burant JC., Iyengar SS., Tomasi J., Cossi M., Rega N., Millam JM., Klene M., Knox JE., Cross JB., Bakken V., Adamo C., Jaramillo J., Gomperts R., Stratmann RE., Yazyev O., Austin AJ., Cammi R., Pomelli C., Ochterski JW., Martin RL., Morokuma K., Zakrzewski VG., Voth GA., Salvador P., Dannenberg JJ., Dapprich S., Daniels AD., Farkas Ö., Foresman JB., Ortiz JV., Cioslowski J. and Fox DJ., 2009, Gaussian 09; Gaussian, Inc, Journal Wallingford, CT, 32, 5648-5652.
- Rauk A., 2001. Orbital interaction Theory of Organic Chemistry. 2nd Ed, John Wiley & Sons: NewYork.
- Singh A., Ansari K.R., Kumar A., Liu W., Songsong C. and Lin Y., 2017. Electrochemical, surface and quantum chemical studies of novel imidazole derivatives as corrosion inhibitors for J55 steel in sweet corrosive environment. J. Alloys Compd., 712, 121–133.
- Jisha M., Zeinul Hukuman N. H., Leena P. and Abdussalam A. K., 2019. Electrochemical, computational and adsorption studies of leaf and floral extracts of Pogostemon quadrifolius (Benth.) as corrosion inhibitor for mild steel in hydrochloric acid, J. Mater. Environ. Sci., 10(9), 840-853.
- Dwivedi A. and Misra N., 2010. Quantum chemical study of Etodolac (Lodine), Der Pharma Chemica. 2(2), 58-65.
- Li X., Deng S., Fu H. and Li T., 2009. Adsorption and inhibition effect of 6- benzylaminopurine on cold rolled steel in 1.0 M HCl, Electrochim. Acta
54, 4089–4098. - Ramya K. and Joseph A., 2015. Synergistic effects and hydrogen bonded interaction of alkyl benzimadazoles and thiourea pair on mild steel in hydrochloric acid, J. Taiwan Inst. Chem. Eng., 52,127 -139.
- Fergachia O., Benhibae F., Rbaab M., Touira,d R. and Ouakkic M., 2018. Experimental and Theoretical Study of Corrosion Inhibition of Mild Steel in 1.0 M HCl Medium by 2(-4( hloro phenyl-1H- benzo[d]imidazol)-1-yl)phenyl) methanone, Mater. Res., 21, 1-11.
- Tian U. and Yuan K., 2021, Performance and Mechanism of Alkylimidazolium Ionic Liquids as Corrosion Inhibitors for Copper in Sulfuric Acid Solution. Molecules, 26 (16), 4910.
- Tian U. and Yuan K. , 2021, Performance and Mechanism of Alkylimidazolium Ionic Liquids as Corrosion Inhibitors for Copper in Sulfuric Acid Solution. Molecules, 26 (16), 4910.
Copyright© Israa M. H. Al-Mousawi, Noor Ali Khudhair, and Lama S. Ahmed. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.