Dual Degradation of Hexavalent Chromium (VI) and Cotton Blue Dye by Reduced and PVP-capped Silver Nanoparticles Using Fruit Extract of Ficus carica
Rama Jyothi Narjala1ǂ, Susmila Aparna Gaddam 2ǂ*, Sangeeta C. Casimeer3, Siva Gayathri Velakanti 4, Ramamurthy Nadipi5, Vishali Batyala 4,
Lakshmi Narayana Suvarapu 6, Venkata Subbaiah Kotakadi 7*
1.Department of Chemistry, Sri Padmavathi Mahila Visvavidyalayam (Women’s University), Tirupati, Andhra Pradesh, India
2. Department of Virology, Sri Venkateswara University, Tirupati, Andhra Pradesh, India
3.Department of Physics, Sri Padmavathi Mahila Visvavidyalayam (Women’s University), Tirupati, Andhra Pradesh, India
4. Department of Basic Science and Humanities, School of Engineering and Technology, Sri Padmavathi Mahila Visvavidyalayam (Women’s University), Tirupati, Andhra Pradesh, India
5. Department of Botany, PVKN Govt. College(A), Chittor, Andhra Pradesh, India
6. Department of Chemistry, Sri Venkateswara University, Tirupati, Andhra Pradesh, India
7. DST PURSE centre, Sri Venkateswara University, Tirupati, Andhra Pradesh, India
*Corresponding authors. E-mail: kotakadi72@gmail.com; susmilaaparna@gmail.com
ǂ: contributed equally to the work
Received: Jul. 22, 2022; Accepted: Oct. 10, 2022; Published: Oct. 20, 2022
Citation: Rama Jyothi Narjala, Susmila Aparna Gaddam, Sangeeta C. Casimeer, Siva Gayathri Velakanti, Ramamurthy Nadipi, Vishali Batyala, Lakshmi Narayana Suvarapu and Venkata Subbaiah Kotakadi, Dual Degradation of Hexavalent Chromium (VI) and Cotton Blue Dye by Reduced and PVP-capped Silver Nanoparticles Using Fruit Extract of Ficus carica. Nano Biomed. Eng., 2022, 14(2): 123-135.
DOI: 10.5101/nbe.v14i2.p123-135
Abstract
Recent investigations on green synthesis of silver nanoparticles (AgNPs) have been widely used in various therapeutic and industrial applications. So in the present study, AgNPs and PVP coated AgNPs were biosynthesized using fig fruit (Ficus carica) named as FF-AgNPs PVP-FF-AgNPs. The FF-AgNPs and PVP-FF-AgNPs revealed the surface Plasmon resonance band at 446 nm and 460 nm respectively. The FT-IR analysis of both nanoparticles reveals that different bioactive components of the fruit extract were actively involved in reduction of AgNPs. The SEM revealed that the particles are roughly spherical and irregular in shape and size, EDX analysis confirms the formation of complete reduction of silver to elemental silver. DLS studies also revealed similar results with both the nanoparticles are within the range of 10 ± 5 nm to 35 ± 5 nm. The zeta potential studies reveal negative potential values were as follows FF-AgNPs has -13.8 mV and PVP-FF-AgNPs has -17.1 mV. They also exhibit good antimicrobial activity. Another important application of these nanoparticles is dual detection of toxic chromium (VI) and photocatalytic dye degradation of cotton blue by H2O2 quenching and without quenching. It is concluded that, biosynthesized FF-AgNPs and PVP-FF-AgNPs have multiple applications of economic importance and environmental pollution.
Keywords: Ficus carica (Fig Fruit); AgNPs; PVP coated; Spectral characterization; antimicrobial activity; dye degradation; H2O2 quenching; chromium reduction
Introduction
Nanotechnology have been broadly spread in various multidisciplinary areas such as DNA and RNA detection, disease diagnose, optical, biosensor, agriculture, electrochemical and food industries [1-3]. Several studies shows that silver acts as good antimicrobial and antibacterial agent and silver nanoparticles due to its controlled size and morphology it plays a prominent role in nanotechnology [4]. Composite and polymer materials are designed by using ultrafine silver nanoparticles [5]. Silver nanoparticles have been used in various biomedical and biotechnology applications extensively due to its bacterial resistance and stability, so they are useful in treatment of several infection [6]. Various bio-mediated methods were used to biosynthesize silver nanoparticles and other metal nanoparticles from plant parts and several fruit extracts to reduce the cost and hazardous effects of toxic chemicals [7]. Presently green synthesis of silver nanoparticles used vastly due to its large surface area, reactivity, high catalytic activity and they also acts as very good biocidal agents [3, 8-9]. Several scientists have reported green synthesis of silver nanoparticles using various plant parts like leaves, flowers, fruits and roots etc., [10-12]. Silver nanoparticles has a good binding affinity with polymers like PVP and they form as coated to enhance its stability and reduces in forming lumps or aggregations and they were also useful in several biological and industrial applications [13].
Ficus carica is commonly known as fig which is edible and belongs to family Moraceae. Since ancient times it has been cultivated widely throughout the world (Mediterranean and western Asia) and it is used both as fruit and ornamental plant. Fig peel as it gets matured, it changes from green to black-violet and it indicates Anthocyanins are the pigments present in higher level in the peel. The Food industry from few decades have been producing artificial coloring agents which is causing several health problems on its consumption. These fig peel are seems to be contemporary replaceable materials to artificial coloring agents [14]. Fig fruit consists of various phytochemicals which are used very much in traditional medicines. Ficin (Cysteine protease) and Collagenase (Serine Protease) are involved in digestion of collagen, fig fruit extract has a very good industrial application in partial digestion of collagen [15]. The latex released from Ficus carcia is widely used for the treatment of warts, skin cancer and also inhibits the growth of mice tumors caused by cancer [16].
Chromium [Cr (VI)] is highly toxic, carcinogenic and mutagenic which is confirmed by the International Agency for Research on Cancer [17]. Cr (VI) contamination is highly present in industrial areas such as tanning, pigments, paints, pulp and paper production industries. Cr (VI) contaminates water in other industrial areas like electroplating, steel manufacturing and in wood preservation [18]. According the World Health Organization (WHO) the maximum concentration of Cr (VI) should not exceed 0.05 mg/L [19]. So the researchers have been working on reduction of Cr (VI) contamination since a decade to find out best alternative methods to reduce Cr (VI) to Cr (III), because Cr (III) supposed to be less toxic in nature , have poor fluidity and easily get precipitated by various natural adsorbents.
Organic dyes are confirmed to be highly toxic in nature, industrial contaminated water and soils contains toxic elements due to the effluents emitted by dye industries. The contaminants are the major root cause for several diseases and disorders. These organic dyes are also harmful to the aquatic ecosystem by reducing and blocking the sunlight and in agriculture the crops grown in contaminated soils contains toxic elements. The consumption of food grains, edible greens grown in these soils causes diseases like anemia, skin disorders and gastrointestinal problems [20-22]. Presently the removal of toxic organic dyes is a major problem in several industrial areas, is a major challenge for the scientists to overcome this problem. So they are looking for alternative solutions to overcome the leading problem. The scientists have identified several nano metal oxides for this purpose, which acts as photoactive catalyst for dye degradation and removal of toxic elements from water and soil.
Presently scientists have been working on various metallic nanoparticles for improvement of environmental protection and bioremediation. There are several studies on nano-materials and their impact on environmental hazardous effects. So in the present study we have biosynthesized silver nanoparticles (AgNPs) and PVP AgNPs using Fig fruit extract, these metal nanoparticles have been used to study antibacterial activity, reduction of toxic Cr (VI) and Cotton blue dye degradation.
2. Materials and methods
2.1. Green synthesis of silver nanoparticles FF-AgNPs
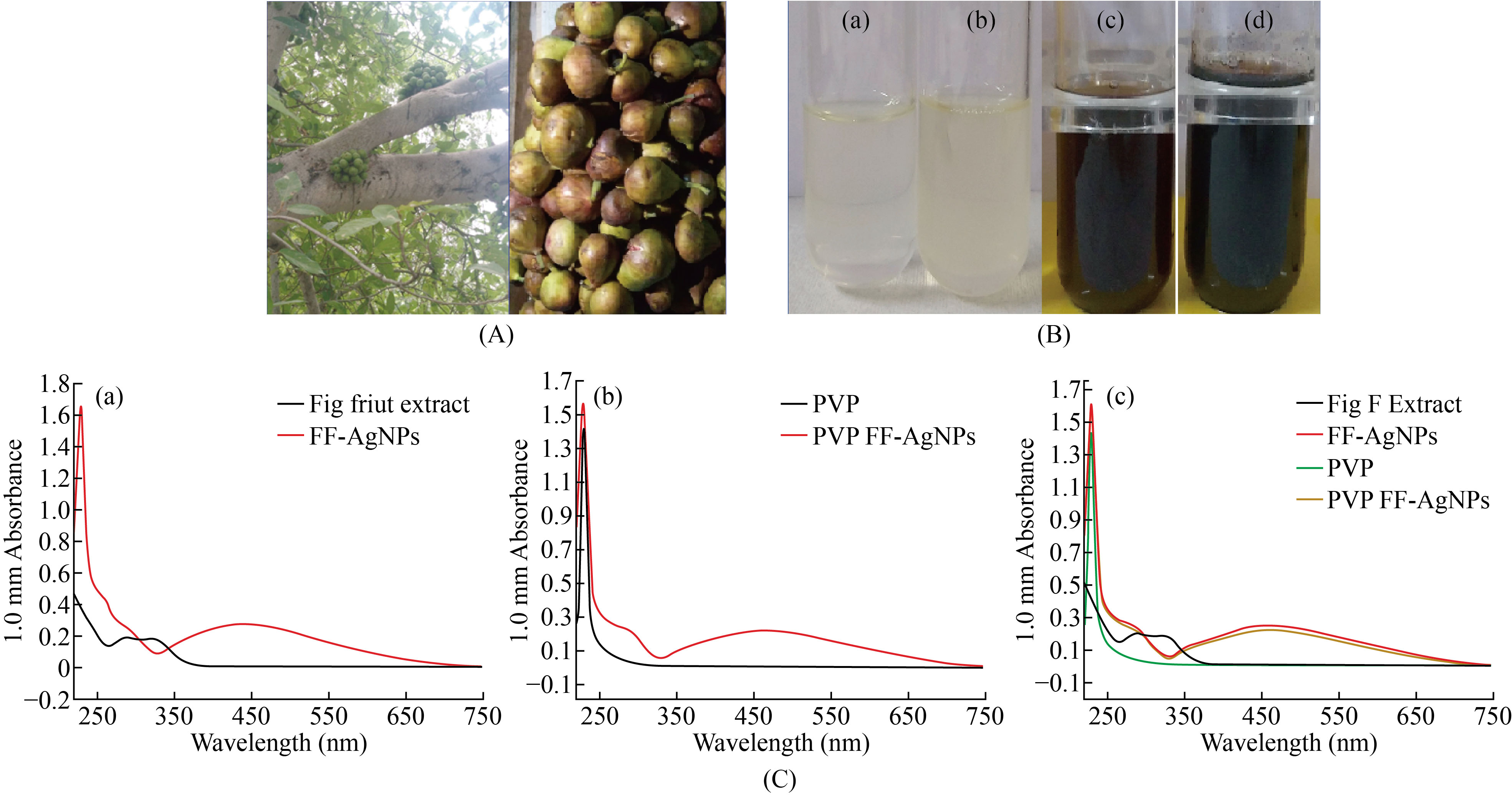
The Fig fruits (Ficus carica) [Fig.1A]. were collected from the trees growing in Sri Padmavathi Mahila Visvavidyalayam (Women’s University) of Tirupati and Chittoor district of Andhra Pradesh, India in the month of September & October. The fresh fruits were collected and made into fine paste with the help of electric blender. The fruit extract was prepared, by adding 10g of freshly prepared paste into 100 ml of Milli Q ultra pure distilled water in a sterile conical flask. The sample was stirred at room temperature for 30 minutes and filtered through sterile muslin cloth followed by whatmann No.1 filter paper. This filtrate solution was used as source of extract for preparation of silver nanoparticles and was utilized in subsequent procedures.
To the 5ml diluted fig fruit filtrate, 10ml of 0.02M AgNO3 was added and the sample was left at room temperature, until the color of solution changed from pale brown color to dark brown and subsequently blackish dark brown. The solution containing AgNPs was confirmed by the dark brown color and the blank was prepared with 5 ml of distilled water and 10 ml of 0.02M AgNO3 [Fig.1B]. So, in this study the green synthesis of silver nanoparticles (AgNPs) with Fig fruit (Ficus carica) extract was prepared without any toxic chemical which is popularly known as “Green synthesis”. The AgNPs green synthesized with Fig fruit were named as FF-AgNPs.
Fig. 1 (A) Fig plant and Ripen Fig Fruits; (B) (a) Blank (b) Fruit extract + 0.02 M AgNo3, (c) FF-AgNPs, (d) PVP coated FF-AgNPs; (C) (a) UV-Visible Spectra of FF-Fruit Extract and FF-AgNPs, (b) UV-Visible Spectra of PVP and PVP coated FF-AgNPs, (c) UV-Visible Spectra of FF extract, FF-AgNPs, PVP and PVP coated FF-AgNPs
2.2. Synthesis of PVP coated FF-AgNPs
The green synthesized FF-AgNPs were coated with 0 .2% poly vinyl pyrolidine (PVP) to obtain PVP coated FF-AgNPs. To 20 ml of FF-AgNPs 20 mg of PVP was added and stirred on magnetic stirrer for one hour to obtain PVP-FF-AgNPs.
2.3. Spectral Characterization of FF AgNPs and PVP-FF-AgNPs
The green synthesized silver nanoparticles with the Fig fruit extract were characterized by different spectral methods. The biosynthesized FF-AgNPs and PVP coated FF-AgNPs were monitored periodically by sampling of the 1ul on UV-Vis spectrometer and the optical absorbance of the silver nanoparticles was recorded on Nanodrop 8000 UV-Vis spectrometer in the wavelength range of 220–750nm. The FT-IR analysis of both FF-AgNPs and PVP-FF-AgNPS were carried out by using Brucker alpha Germany. Particle size and zeta potential measurement experiments of both samples were carried out by using a Nanopartica (Horiba Nanopartica SZ-100 instrument) SEM and EDAX was performed by Oxford Inca Penta FeTX3 EDS instrument attached to Carl Zeiss EVO MA 15 Scanning Electron Microscope (200 kV) machine with a line resolution 2.32 (in Å). These images were taken by coating FF-AgNPs and PVP-FF-AgNPs on a glass pieces separately. Energy Dispersive Absorption Spectroscopy photograph of AgNPs were carried out by the Scanning Electron Microscopy (SEM) equipment, as mentioned above. X-Ray Diffractometry (XRD) study was conceded to prove the crystalline nature of the FF-AgNPs and PVP-FF-AgNPs using Malvern Aeris Panalytical X-ray powder diffractometer (UK) by means of Cu Kα radiation source (Sri Padmavathi Mahila university, Tirupati).
2.4. Antimicrobial activity of FF-AgNPs and PVP-FF-AgNPs
The antibacterial efficiency of bio-synthesized FF-AgNPs and PVP-FF-AgNPs were determined against four bacterial strains of both gram-negative bacteria such as Escherichia coli, Pseudomonas aeruginosa and gram positive such as Staphylococcus aureus and Bacillus megatorium, by using Agar disc-diffusion method as described by Kotakadi et al [23-25]. Different concentration of biosynthesized FF-AgNPs and PVP-FF-AgNPs were loaded on 5 mm of sterile Whatman No 1 filter paper discs as follows 25 micrograms (25 mcg), 50 mcg, 75 mcg and 100 mcg. Overnight cultures of the above bacterial strains were spread on freshly prepared agar plates and the above different concentrated Whatman No 1 filter paper discs along with FF-AgNPs and PVP-FF-AgNPs were placed on agar medium and incubated for overnight at 37±1oC. Amoxyclav 30 mcg was used as a standard drug for comparison zone of inhibition (ZOI).
2.5.Catalytic activity of FF-AgNPs and PVP-FF-AgNPs
2.5.1. Colorimetric detection of Cr (VI) by FF-AgNPs and PVP-FF-AgNPs
Reduction of chromium (Cr-VI) by biosynthesized FF-AgNPs and PVP-FF-AgNPs was chosen as a replica method to quantify the catalytic activity. In the present study colorimetric detection of Cr(VI) was carried out by adding 350 μl of Cr(VI) to 650 μl of freshly prepared FF-AgNPs and PVP-FF-AgNPs and checked for instant coloration. Absorption spectra of the final mixture were recorded using Nanopdrop-8000 spectrophotometer within the range of 220 nm to 750 nm with 1 cm path length.
2.5.2. Catalytic reduction of Cotton blue Dye Degradation by FF-AgNPs and PVP-FF-
AgNPs
10 mg of Cotton blue dye was added to 1000 mL of double distilled water used as stock solution. About 250 µl of biosynthesized both silver nanoparticles were added to 2.5 mL of Cotton blue dye solution. A blank was maintained without addition of silver nanoparticles FF-AgNPs and PVP-FF-AgNPs. The reaction suspension was mixed well to the equilibrium of the working solution. The suspension was used to evaluate the photocatalytic degradation of dye. The absorbance spectrum of the suspension was subsequently measured at different time interval using Nanodrop-8000, UV-Vis spectrophotometer at the different wavelength 220 nm to 750 nm. Concentration of dye during degradation was calculated by the absorbance values and percentage of dye degradation was estimated. At the same time the reaction was also repeated by adding hydrogen peroxide (H2O2), to see the quenching and catalytic efficacy of both FF-AgNPs and PVP-FF-AgNPs in dye degradation.
3. RESULTS AND DISCUSSION
3.1. UV–Visible spectral analysis of FF- AgNPs and PVP-FF-AgNPs
UV-visible spectroscopy is prominent technique for detecting silver and other metal nanoparticles in aqueous solution. The biosynthesized FF-AgNPs and PVP-FF-AgNPs has free electrons, which gives rise to surface plasma resonance (SPR) absorption band. The Fig.1.C.(a)-(c) reveals a surface plasma resonance spectrum of FF-AgNPs and PVP-FF-AgNPs which were obtained at 446 nm and 460 nm respectively. The aqueous reaction of FF-AgNPs indicates color changes from colorless to light dark brown color whereas the PVP-FF-AgNPs indicates dark brown color respectively (Fig.1.B). The metal nanoparticles can be synthesized by reducing metal ions using some organic chemicals which are highly toxic in nature. Presently, green synthesis of metal nanoparticles using various parts of plants, their extracts act as reducing agent for generation of metal nanoparticles. The previous reports on biosynthesis of AgNPs and PVP coated AgNPS by various plant extracts like seed extract of Annona squamosa L, Ficus fruit extract, Terminalia belarica and Capsicum annuum L. revealed similar type of results regarding SPR of both coated and uncoated AgNPs[24, 26-28].
3.2. FT-IR analysis of FF- AgNPs and PVP-FF-AgNPs
FTIR analysis of biosynthesized FF-AgNPs and FF-PVP-AgNPs shown in (Fig. 2). The IR peaks for FF-AgNPs found at 3563, 3491, 3449, 3424, 1637, 820, 620 cm-1. The peak at 3563 corresponds to O-H stretch of free hydroxyl alcohol and phenols, The IR band at 1637 cm-1 are due to N-H bond of primary amines. The IR band at 820 cm-1 corresponds to C-N stretch of aromatic amines, aliphatic amine groups. The bio synthesised FF-PVP-AgNPs showed some prominent peaks in FTIR analysis such as 3479, 3448, 3422 1627 & 617 cm-1. The peaks at 3479 cm-1 was due to O-H bonds of alcohol and phenols the intensity of the peak is reduced when compared with the extract, peak at 1627 cm-1 corresponds to stretch of N-H bending of primary amines. The results revealed that the different fruit phyto-constituents like flavonoids, polyphenols, sugars, tannins, of the fruit extract have been actively participated in reduction of silver nitrate to FF-AgNPs and in capping and stabilization of the nanoparticles The results were similar to earlier reports on silver nanoparticle and PVP capped silver nanoparticles [29].
Fig. 2 (a) FTIR analysis of FF-AgNPs; (b) FTIR analysis of PVP coated FF-AgNPs.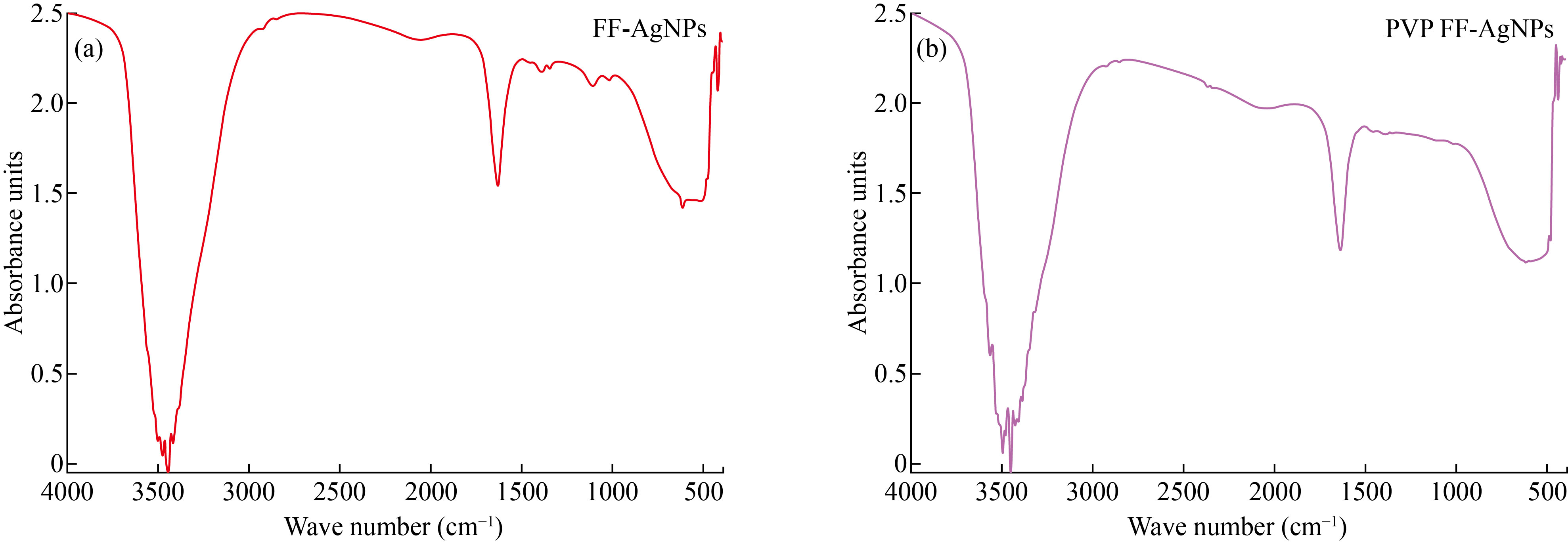
3.3. Particle Size analysis of FF- AgNPs and PVP-FF-AgNPs
Dynamic Light Scattering (DLS) is simple and efficient method to detect the particle size of biosynthesized FF AgNPs and PVP-FF-AgNPs in the colloidal solution. The results reveals that the size of biosynthesized FF AgNPs and PVP-FF-AgNPs are polydispered in nature and the distribution of nanoparticle is as follows 10% -10.6 nm; 50% - 11.9 nm 90%-12.9 nm with an the average particle size of FF-AgNPs is 11.8 nm. Similarly the PVP coated silver nanoparticles - PVP-FF-AgNPs size distribution is as follows 10% -35.3 nm 50% -37.2 nm 90% -39.1 nm the average particle size of PVP-FF-AgNPs is 37.1 nm [Fig.3(a)] indicating that both the FF AgNPs and PVP-FF-AgNPs are poly dispersed in nature. As per a recent study, the effect of coating on AgNPs with various biological agents like PVP, BSA, Chitosan revealed the SPR shift and also they change in the size of the nanoparticles depending on the nature of capping agent [30].
Fig. 3 (a) Particle size analysis of FF-AgNPs, PVP -FF-AgNPs; (b) Zeta Potential analysis of FF-AgNPs, PVP -FF-AgNPs.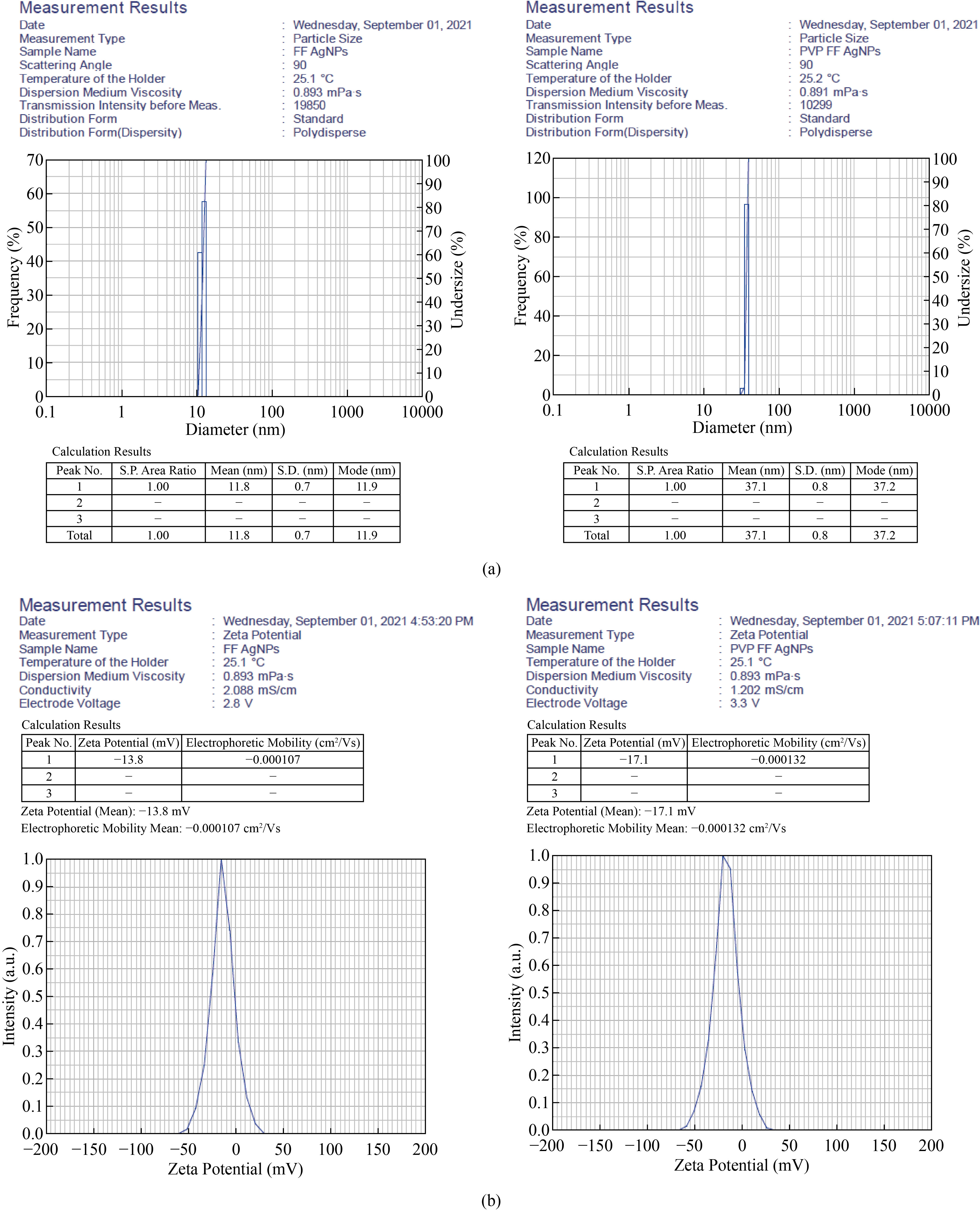
3.4. Zeta potential analysis of FF-AgNPs and PVP-FF-AgNPs
The stability of any metal nanoparticles depends on the surface charge which plays major role in preventing from agglomeration in the medium leading to the long term stability. Thus the electrostatic repulsive force between the nanoparticles depends on the charge. In the current study for both FF-AgNPs and PVP-FF-AgNPs have negative zeta potential values which confirm the repulsion among the particles and increase the stability of both colloidal solutions. The Zeta potential of nanoparticles having +25 mV or -25 mV and above are said to be highly stable. In the present study FF-AgNPs have negative potential around -13.8 mV and PVP-FF-AgNPs have -17.1 mV [Fig.3b], indicating that both the biosynthesized metal nanoparticles were moderately stable. Similar results were observed in previous reports the coated AgNPs have slightly higher potential when compared to non-coated AgNPs [30].
3.5. SEM and EDX analysis of FF-AgNPs and PVP-FF-AgNPs
The approximate size and morphology of biosynthesized FF-AgNPs and PVP-FF-AgNPs in the colloidal solution were determined by SEM images [Fig.4]. It reveals that FF-AgNPs (Fig. 4. a-b) and PVP-FF-AgNPs (Fig. 5. a-b) nanoparticles are roughly spherical and irregular in shape. The EDX results reveals very strong signal to silver, which confirms the complete reduction of silver ions into elemental silver possibly originated from the molecules attached to the surface of FF-AgNPs (Fig.4.c-d) and PVP-FF-AgNPs (Fig.5.c-d). The previous reports were also showed similar results in EDX analysis. Both the FF-AgNPs and PVP-FF-AgNPs showed strong emission energy at 3 KeV for silver and some of the weak signals from C,O, Na, Ca, Mg were observed [10-11, 27-29].
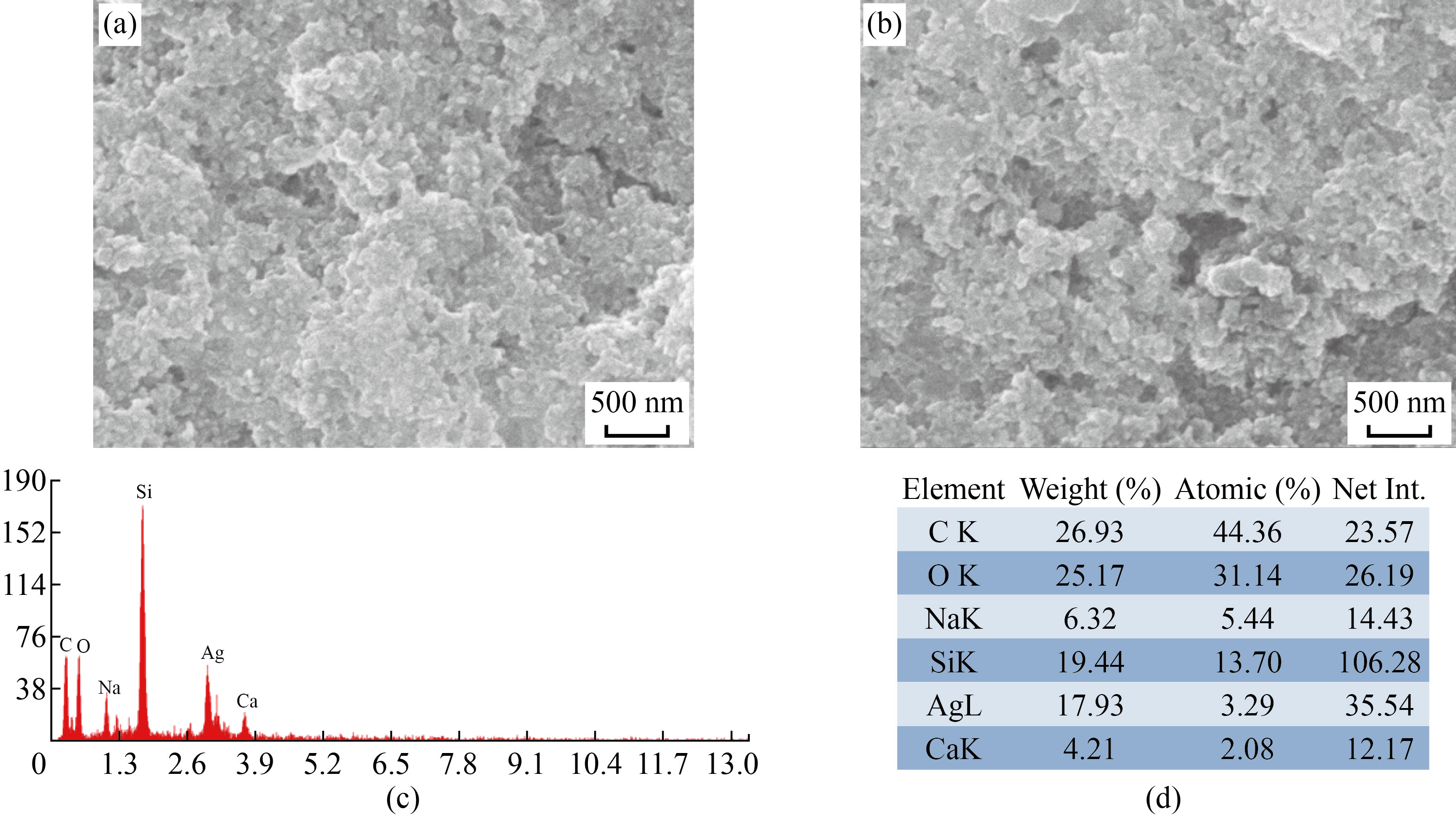
Fig. 4 (a-b) SEM analysis of FF-AgNPs; (c-d) EDAX analysis of FF-AgNPs.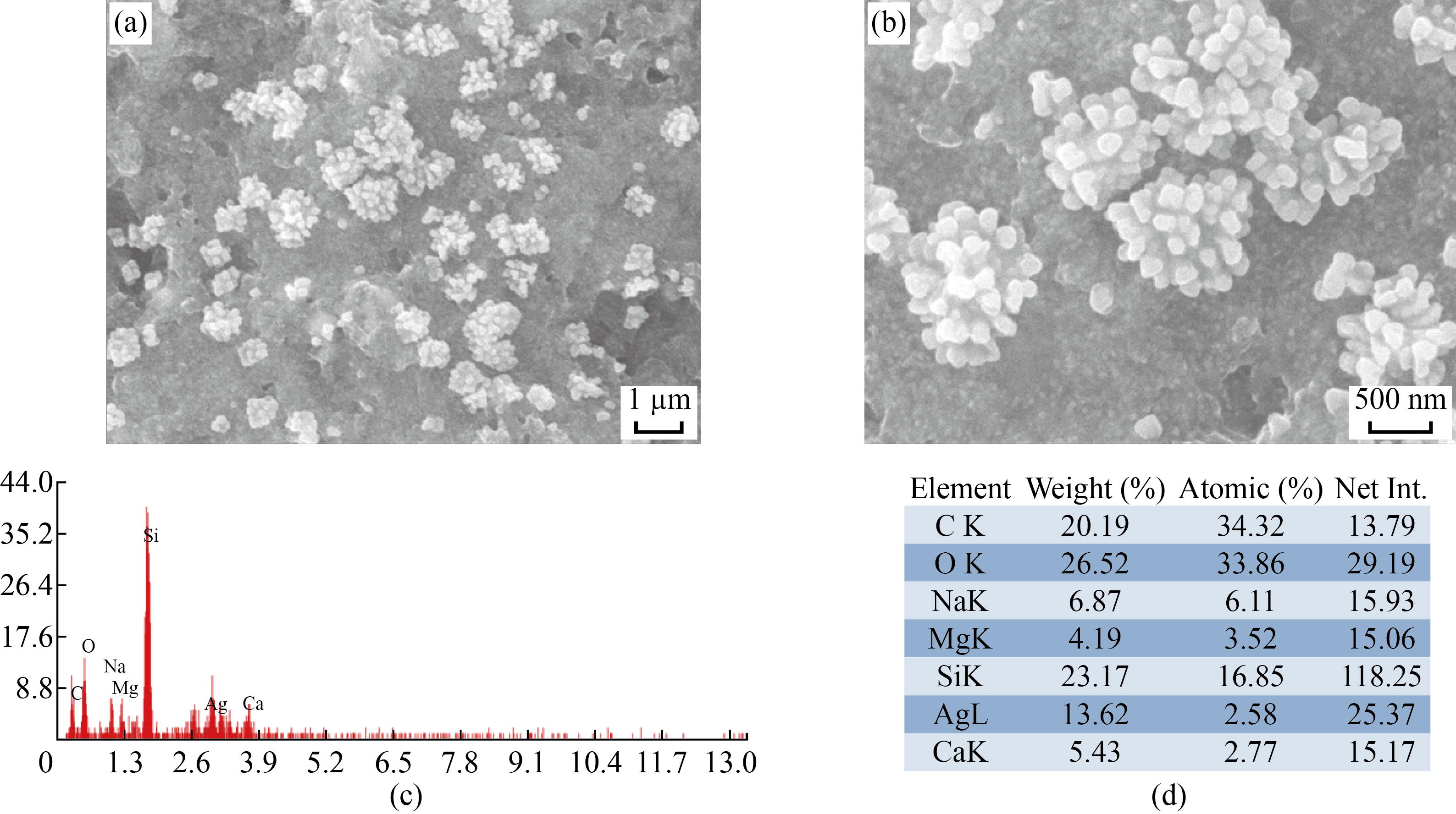
Fig. 5 (a-b) SEM analysis of PVP- FF-AgNPs; (c-d) EDAX analysis of PVP-FF-AgNPs.
3.6. XRD analysis of FF-AgNPs and PVP-FF-AgNPs
The crystalline nature and size of biosynthesized FF-AgNPs and PVP-FF-AgNPs were evaluated by using XRD. The results of XRD analysis are shown in (Fig.6), FF-AgNPs reveals distinctive diffraction peaks at 31.6683, 45.7144, 64.0875 and 76.9554 corresponding to planes of (111), (200), (220) and (311) respectively. The planes are indexed according to the facets of the face-centered cubic crystal structure of Silver oxide (JCPDS card no. 04-0783). The PVP-FF-AgNPs also revealed distinctive diffraction peaks at 37.6052, 45.7144 and 64.0875. The present results are consistent with the previous reports on silver nanoparticles [9, 11, 31].
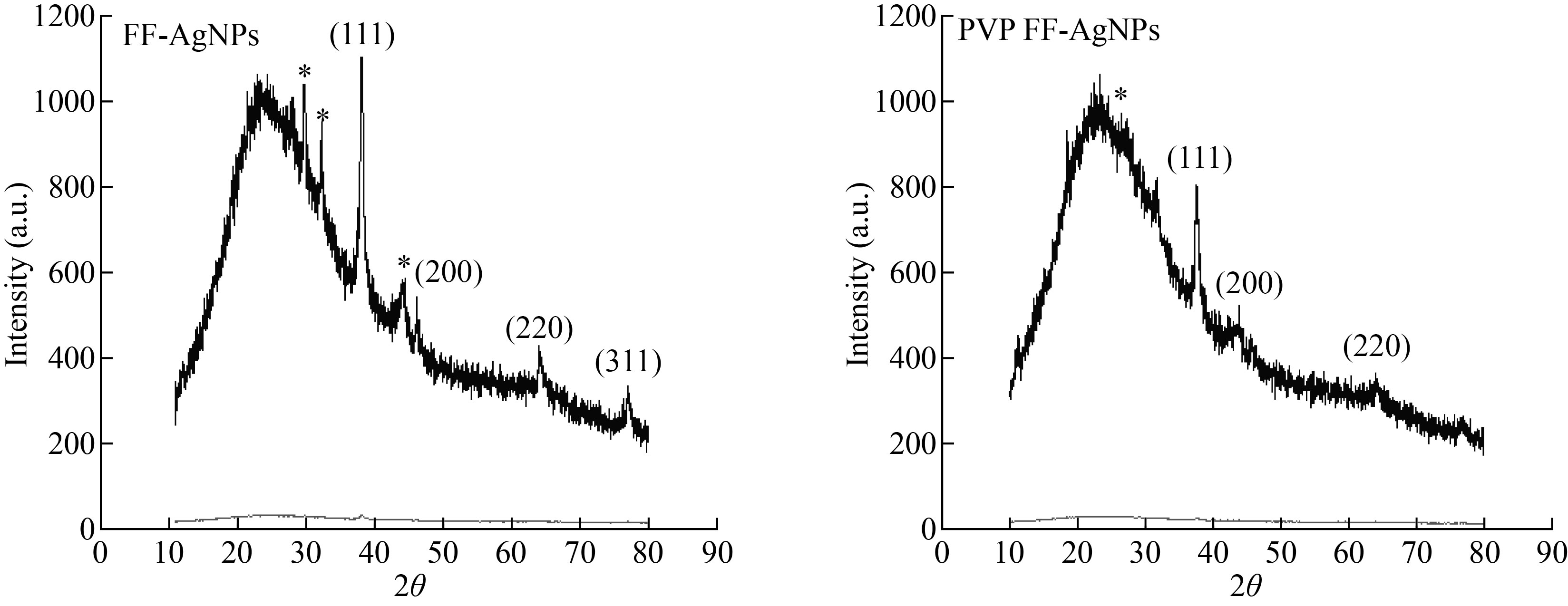
Fig. 6 XRD analysis of FF-AgNPs and PVP-FF-AgNPs.
3.7. Antimicrobial activity of FF-AgNPs and PVP-FF-AgNPs
The antibacterial activity of Fig fruit extract mediated biosynthesised FF-AgNPs and PVP-FF-AgNPs revealed good antibacterial activity against four bacterial isolates Gram +ve Staphylococcus aureus, Bacillus megatrium, and Gram –ve Escherichia coli, and Pseudomonas aeruginosa . The zone of inhibition (ZOI) of FF-AgNPs and PVP-FF-AgNPs at different concentration of 25 mcg, 50 mcg, 75 mcg and 100 mcg in different bacterial cultures was shown in (Fig.7.a-c) and ZOI values in mm were measured and tabulated in (Table 1. Supplemental Data). It is concluded that the FF-AgNPs and PVP-FF-AgNPs at lower and higher concentration revealed good zone of inhibition. The MIC of FF-AgNPs and PVP-FF-AgNPs was detected to be less 10 mcg. The results reveal that the biosynthesized FF-AgNPs and PVP-FF-AgNPs can used as effective antibacterial agents. The shape of silver nanoparticles also plays prominent role in antimicrobial property. Similarly various biosynthesised AgNPs using by various plant extracts exhibited very good antibacterial activities on both gram negative and gram positive bacteria [11, 23-24 ,27]. Similarly the PVP coated biosynthesised AgNPs also exhibit very good antibacterial activity on both gram negative and gram positive bacteria [32- 34].
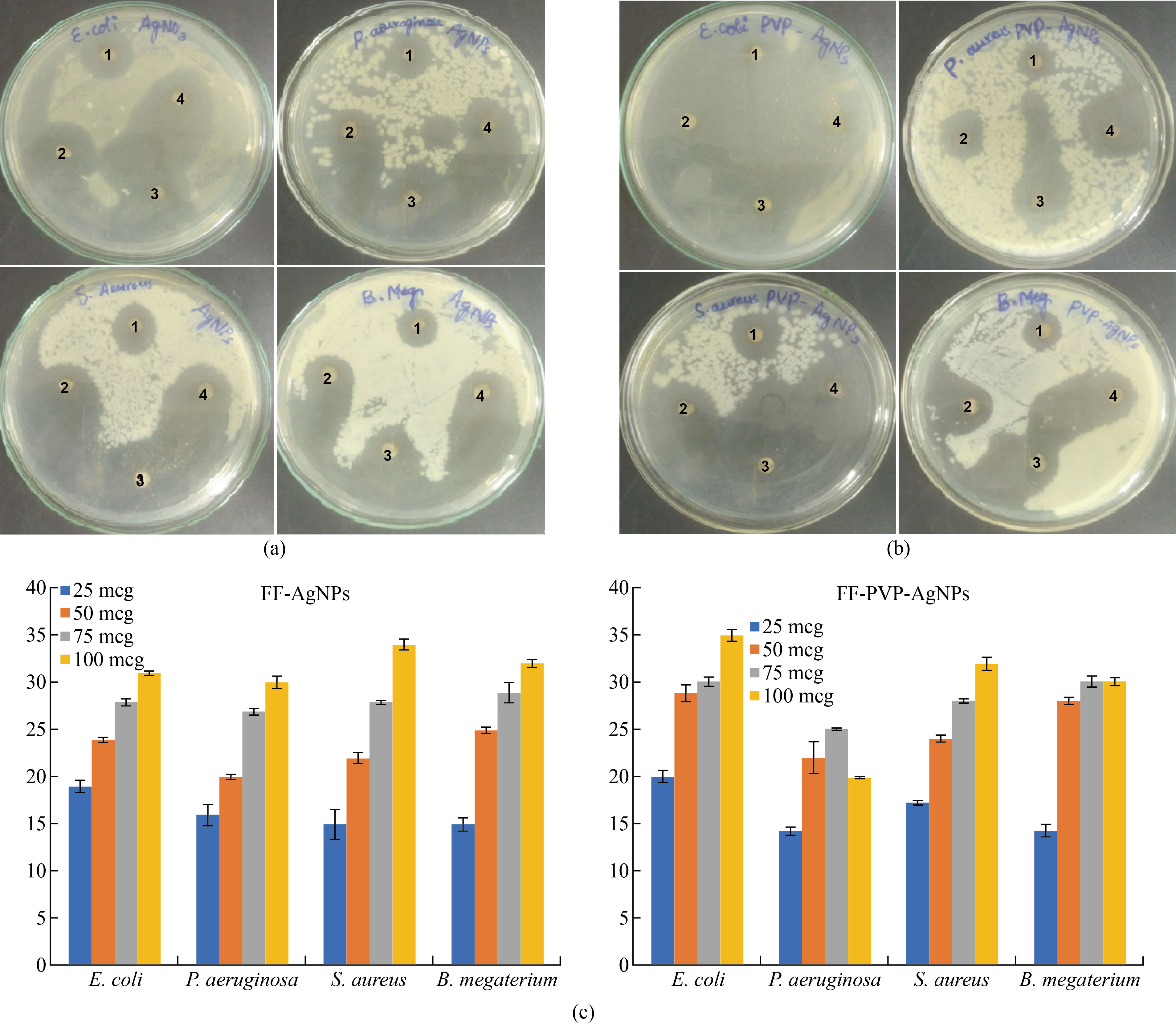
Fig. 7 (a) Antimicrobial activity of FF-AgNPs (1. 25 mcg, 2. 50 mcg, 3. 75 mcg, 4. 100 mcg); (b) Antimicrobial activity of PVP-FF-AgNPs (1. 25 mcg, 2. 50 mcg, 3. 75 mcg, 4. 100 mcg); (c) Graphical representation of Antimicrobial activity of FF-AgNPs and PVP-FF-AgNPs.
Table 2 (a) Dye degradation of Cotton blue by FF-AgNPs (b) Dye degradation of Congo red by PVP-FF-AgNP absorbance.
S. No. | Sample | Time Interval | 590 nm |
1 | Cotton blue (Control) | 0 min | 1.531 |
2 | CB+ FF-AgNPs | 0 min | 1.453 |
3 |
| 1 min | 1.363 |
4 |
| 5 min | 1.359 |
S. No. | Sample | Time Interval | 590 nm |
1 | Cotton blue (Control) | 0 min | 1.620 |
2 | CB+ FF-AgNPs+ H2o2 | 0 min | 1.248 |
3 |
| 1 min | 1.195 |
4 |
| 5 min | 1.108 |
5 |
| 10 min | 1.055 |
6 |
| 15 min | 0.977 |
7 |
| 20 min | 0.925 |
S. No. | Sample | Time Interval | 590 nm |
1 | Cotton blue (Control) | 0 min | 1.620 |
2 | CB+ PVP -FF-AgNPs | 0 min | 1.478 |
3 | CB+ PVP -FF-AgNPs | 1 min | 1.336 |
4 | CB+ PVP -FF-AgNPs | 5 min | 1.308 |
S. No. | Sample | Time Interval | 590 nm |
1 | Cotton blue (Control) | 0 min | 1.620 |
2 | CB+PVP- FF-AgNPs+ H2o2 | 0 min | 1.412 |
3 | CB+PVP- FF-AgNPs+ H2o2 | 1 min | 1.220 |
4 | CB+PVP- FF-AgNPs+ H2o2 | 5 min | 1.193 |
5 | CB+PVP- FF-AgNPs+ H2o2 | 10 min | 1.150 |
6 | CB+PVP- FF-AgNPs+ H2o2 | 15 min | 0.935 |
7 | CB+PVP- FF-AgNPs+ H2o2 | 20 min | 0.860 |
Table 2 Reduction of Hexavalent chromium
| Sample | Absorbance (360 nm) |
1 | Chromium (VI) | 7.648 |
2 | FF-AgNPs +Cr(VI) | 2.910 |
3 | PVP-FF-AgNPs + Cr(VI) | 2.514 |
3.8. Photocatalytic reduction of Cotton blue Dye Degradation by FF-AgNPs and PVP-FF-AgNPs
The photo-catalytic reduction activity of the FF-AgNPs and PVP-FF-AgNPs was deter-mined by using the detoxification of cotton blue (CB) dye for specific time interval. First, the catalytic degradation of the dyes CB in the presence of the bio-synthesized FF-AgNPs and PVP-FF-AgNPs was observed by their color change. The color intensity of CB dye change from dark blue to light blue, thereby confirming the effective catalytic dye degradation activity of the biosynthesized FF-AgNPs and PVP-FF-AgNPs. Whereas in our experiment we have observed that the efficacy of nanoparticles was only up to five minutes, later on there was not much change in the color and the OD values shown in (Fig. 8a). (Table.2a Supplemental Data). so we have carried out the same experiment with addition of hydrogen perioxide (H2O2) as a quenching agent to enhance the phytocatalytic activity of both biosynthesized nanoparticles. Moreover, the absorbance OD values were recorded and the results were shown in (Fig.8.b) and (Table. 2b Supplemental Data). The results reveal that the addition of quenching agent enhances fast dye degradation. The OD values of CB dye at 0 min is 1.531 and 5 min is 1.359 for FF-AgNPs and for PVP-FF-AgNPs, the OD values at 0 min is 1.620 and 5 min is 1.308 both the values were recorded without quenching. Whereas the color change and OD values were rapidly changed by the addition of hydrogen peroxide to the reaction mixture as follows 0 min- 1.620, 5 min- 1.108, 10 min – 1.055, 15 min- 0.977 and 20 min - 0.925 for FF-AgNPs and the OD values for PVP-FF-AgNPs is as follows 0 min- 1.620, 5 min- 1.193, 10 min – 1.150, 15 min- 0.935 and 20 min - 0.860. After quenching with hydrogen peroxide, it was clearly revealed that the catalytic activity was enhanced in both samples of nanoparticles. Finally it is concluded that the FF-AgNPs and PVP-FF-AgNPs both acts as excellent phytocatalytic dye degradation agents. The PVP coated FF-AgNP has enhanced dye degradation effect than FF-AgNPs at the end of reaction. These may be due to the coating of FF-AgNPs with PVP, as per earlier reports PVP along with silver nanoparticles enhances phytocatalytic dye degradation.
The effluents released from textile industries contaminates the ground water and also drinking water resources which leads to several kinds of toxic effects on gastrointestinal tract problems, skin and eye irritation problems [35-37] . The decrease in the peak intensity could be due to the SPR property of the biosynthesized FF-AgNPs and PVP-FF-AgNPs. The CB solution was exposed for a time interval of 0–20 min. which showed a significant decrease in the peak intensity [Fig. 8 (a-b)]. Therefore, confirming the CB dye degradation that could have occurred due to the SPR property of the biosynthesized FF-AgNP and coating of PVP on the surface of FF-AgNPs., similar results were also observed in the case of the Crystal violate dye degradation study by using Cd-AgNPs by another researcher [38] and by silver nanoparticles [39].
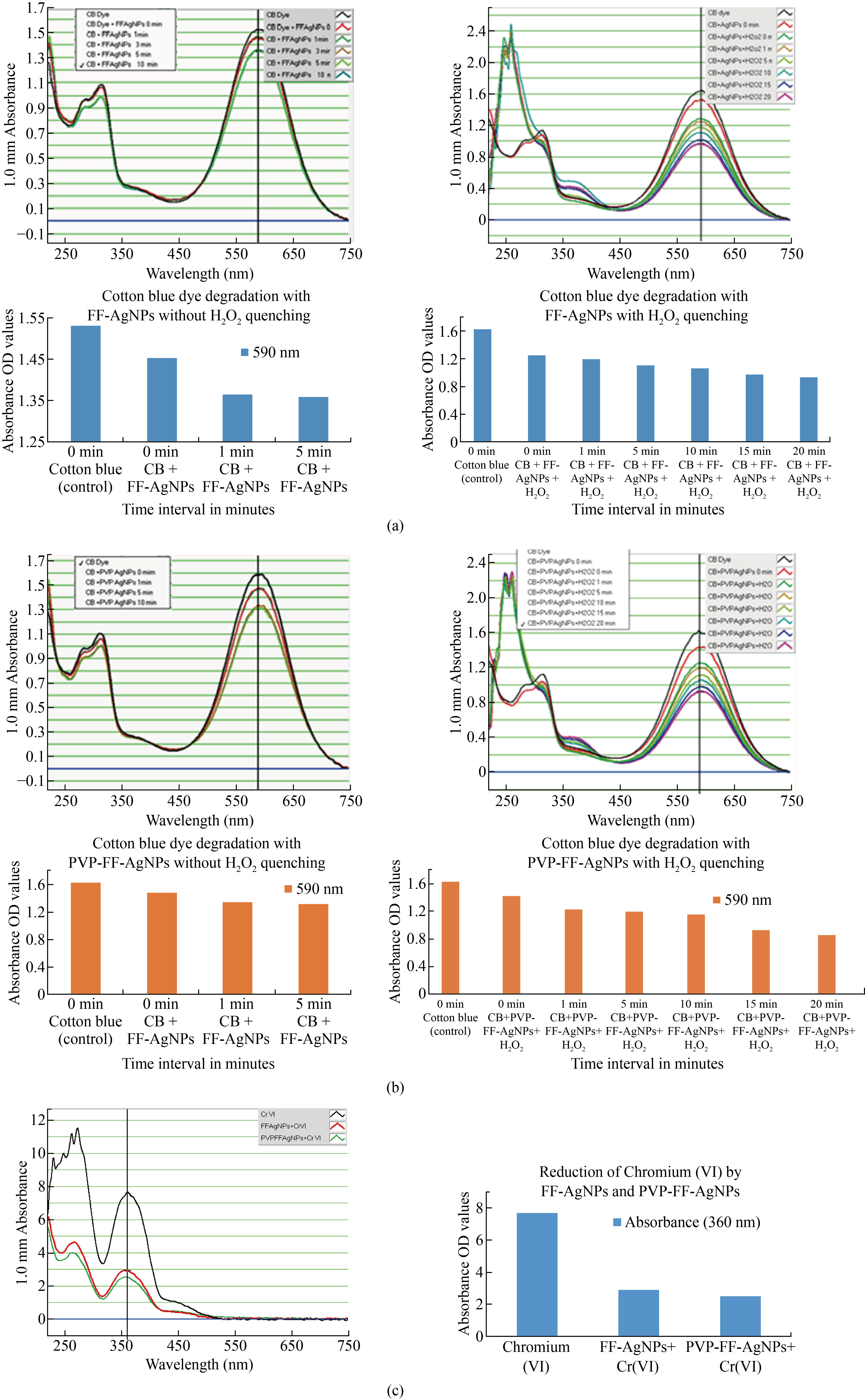
Fig. 8 (a) Cotton blue dye degradation by FF-AgNPs without and with H2O2 quenching; (b) Cotton blue dye degradation by PVP-FF-AgNPs without and with H2O2 quenching; (c) Colorimetric detection and reduction of Cr(VI) by FF-AgNPs and PVP-FF-AgNPs.
3.9. Colorimetric detection of Cr (VI) by FF-AgNPs and PVP-FF-AgNPs
The results revealed that the both prepared colloidal FF-AgNPs and PVP-FF-AgNPs have strong surface Plasmon resonance bands. The 1 mM Cr (VI) stock solution was prepared and its absorbance peak was recorded at 360 nm with maximum optical density (O.D.) at 7.648 mm, whereas after the addition of FF-AgNPs and PVP-FF-AgNPs to 1mM Cr(VI) solution the maximum absorbance was decreased to 2.910 (FF-AgNPs) and 2.515 (PVP-FF-AgNPs) indicates the interaction of AgNPs with chromium solution , the color of Cr(VI) was changed from orange color to pale to dark purple color. The results were tabulated and shown in (Fig.8c ). Similar type of results were observed by using hexavalent chromium catalytic activity by biogenic Pd(0) nanoparticles [40] and rapid detection of Cr(VI) by AgNPs biosynthesized by leaf extract of Anacardium occidentale [41], Inorganic nanoparticles for reduction of hexavalent chromium and Farooqi et al used inorganic nanoparticles for reduction chromium (VI) [42].
Summary and Conclusion
In our study we report a simple and efficient method which is environment friendly method for biosynthesis of silver nanoparticles. In present studies results revealed that the biosynthesized FF-AgNPs and PVP-FF-AgNPs using Fig fruit extract have important therapeutic and industrial applications. The biosynthesized FF-AgNPs and PVP-FF-AgNPs were analyzed by various spectral methods and studied their antimicrobial and toxicological studies. The SPR spectra of FF-AgNPs and PVP-FF-AgNPs were obtained at 446 nm and 460 nm. SEM analysis results reveal that FF-AgNPs and PVP-FF-AgNPs nanoparticles are spherical in shape and irregular in nature. EDX analysis revealed that both FF-AgNPs and PVP-FF-AgNPs complete reduction of silver ions to elemental silver elemental. The DLS analysis of FF-AgNPs and PVP-FF-AgNPs have revealed that the biosynthesized nanoparticles were polydispered in nature, the average particle size of FF-AgNPs is 11.8 ± 2 nm with an polydispered index is 0.254 and the average particle size of PVP-FF-AgNPs is 37.1 ± 2 nm with an polydispered index is 0.365 respectively. Zeta-potential of both nanoparticles was determined to be -13.8 mV for FF-AgNPs and -17.1 mV for PVP-FF-AgNPs. The FF-AgNPs and PVP-FF-AgNPs have proved to be good antimicrobial agents against both gram +ve and gram –ve bacteria. At the same time both the FF-AgNPs and PVP-FF-AgNPs were proved to be effective agents for the dual detection of Cr(VI) and photocatalytic dye degradation .
Acknowledgement
The author RJ and VSK is grateful to DST-PURSE Centre, S.V.University, Tirupati for providing facility to carry out the research.
Disclosure statement
The authors declare that there are no conflicts of interest and financial support for the present research article.
Data availability statement
All the data related to our research article is present in manuscript submitted
ORCID
N. Rama Jyothi https://orcid.org/0000-0002-8746-8954; Susmila Aparna Gaddam https://orcid.org/0000-0001-7671-3136; S. Lakshmi Narayana https://orcid.org/0000-0002-5483-2633;Venkata Subbaiah Kotakadi https://orcid.org/0000-0001-7315-3575
REFERENCES
[1] Yazdi, M.E.T., M. Modarres, M.S. Amiri, and M. Darroudi. 2018. Phyto-synthesis of silver nanoparticles using aerial extract of Salvia leriifolia Benth and evaluation of their antibacterial and photo-catalytic properties. Res. Chem. Intermediat. 45: 1105-1116.
[2] Menon, S., K.S. Shrudhi Devi, R. Santhiya, S. Rajesh Kumar, and S. Venkat Kumar. 2018. Selenium nanoparticles: A potent chemotherapeutic agent and an elucidation of its mechanism. Colloids and Surfaces B: Biointerfaces 170(1):280-92.
[3] Kotakadi VS, Kolapalli B , Gaddam SA, D.V.R Sai Gopal, Dual Synthesis of Silver and Iron
Oxide Nanoparticles from Edible Green Amaranthus Viridis and their In vitro Antioxidant
Activity and Antimicrobial Studies, Current Biotechnology 2021; 10(3) .
https://dx.doi.org/10.2174/2211550110666211206101654
[4] Dell'Erba, I.E, F.D. MartõÂnez, C.E. Hoppe, G.E. ElicËabe, M. CeolõÂn, I.A. Zucchi, and W.F. Schroeder. 2017. Mechanism of Particle Formation in Silver/Epoxy Nanocomposites Obtained through a Visible-Light-Assisted in Situ Synthesis. Langmuir 33(39): 10248-10258. https: //doi.org/10.1021/acs.langmuir.7b01936 . PMID: 28874051.
[5] Hai-Lei, C., H.B. Huang, Z. Chen, B. Karadeniz, J. Lü, and R. Cao. 2017. Ultrafine Silver Nanoparticles Supported on a Conjugated Microporous Polymer as High-Performance Nanocatalysts for Nitrophenol Reduction. ![]()
![]()
![]()
![]()
![]() ACS Appl. Mater. Interfaces 9: 5231−5236.
ACS Appl. Mater. Interfaces 9: 5231−5236.
[6] Panáček, A., M. Slekalova, M. Killanova, R. Prucek, K. Bogdanova, R. Vecerova, M. Kolar, M. Hardova, G.A. Plaza, J. Chojnaik, R. Zboril, and L. Kvitek. 2015. Strong and nonspecific synergistic antibacterial efficiency of antibiotics combined with silver nanoparticles at very low concentrations showing no cytotoxic effect. Molecules 21(1): 26.
[7] Jacob, S.J.P., V.L. Siva Prasad, S. Sivasankar, and P. Muralidharan. 2017. Biosynthesis of silver nanoparticles using dried fruit extract of Ficus carica - Screening for its anticancer activity and toxicity in animal models. Food and Chemical Toxicology 109(2):951-956.
[8] Palithya S, Gaddam SA, Kotakadi VS, et al. Biosynthesis of silver nanoparticles using leaf extract of Decaschistia crotonifolia and its antibacterial, antioxidant, and catalytic applications, Green Chemistry Letters and Reviews. 2021(a); 14(1): 136-151.
[9] Subramanyam G.K, SA Gaddam, VS Kotakadi, J Penchalaneni, S Palithya, V N
Challagundla (2021). Argyreia nervosa (Samudra pala) leaf extract mediated silver
nanoparticles and evaluation of their antioxidant, antibacterial activity, in vitro anticancer
and apoptotic studies in KB oral cancer cell lines. Artificial Cells, Nanomedicine, and
Biotechnology. Volume 49, Issue 1 pages 635-650.
https://doi.org/10.1080/21691401.2021.1996384
[10] Gaddam SA, VS Kotakadi, G K Subramanyam, J Penchalaneni, V N Challagundla, VR
Pasupuleti.(2021) Multifaceted phytogenic silver nanoparticles by an insectivorous plant Drosera spatulata Labill var. bakoensis and its potential therapeutic applications. Scientific Reports 11, 21969 (2021). https://doi.org/10.1038/s41598-021-01281-8
[11] Palithya S, Gaddam SA, Kotakadi VS, et al. Green synthesis of silver nanoparticles using
flower extracts of Aerva lanata and their biomedical applications. Particulate Science and Technology. 2021(b); DOI: 10.1080/02726351.2021.1919259
[12] Fahmy, H.M., A.M. Mosleh, A.A. Elghany, E. Shams-Eldin, E.S.A. Serea, S.A. Ali, and A.E. Shalan 2019. Coated silver nanoparticles: synthesis, cytotoxicity, and optical properties, RSC Adv. 9: 20118-20136.
[13] Wang, Z., Y. Cui, A. Vainstein, S. Chen, and H. Ma. 2017. Regulation of Fig (Ficus carica L.) Fruit Color: Metabolomic and Transcriptomic Analyses of the Flavonoid Biosynthetic Pathway Frontiers in Plant Science, 8: 1–15.
[14] Arvaniti, O. S., Y. Samaras, G. Gatidou, N.S. Thomaidis, and A.S. Stasinakis. 2019. Review on fresh and dried figs: Chemical analysis and occurrence of phytochemical compounds, antioxidant capacity and health effects. Food Research International, 119: 244–267.
[15] Salman, N.A, G. Davies, F. Majidy, F. Shakir, H. Akinrinade, D. Perumal and G.H. Ashrafi. 2017. Association of High Risk Human Papillomavirus and Breast cancer: A UK based Study. Sci. Rep. 7: 43591.
[16] Yao, Y.J., J. Zhang, H. Chen, M. J. Yu, M. X. Gao, Y. Hu, S.B. Wang. 2018. Ni- encapsulated in N-doped carbon nanotubes for catalytic reduction of highly toxic hexavalent chromium. Appl. Surf. Sci. 440: 421–431.
[17] Jobby, R., P. Jha, A.K. Yadav, N. Desai. 2018. Biosorption and biotransformation of hexavalent chromium Cr(VI): A comprehensive review. Chemosphere 207: 255–266.
[18] Aslani, H., T.E. Kosari, S. Naseri, R. Nabizadeh, M. Khazaei. 2018. Hexavalent chromium removal from aqueous solution using functionalized chitosan as a novel nano-adsorbent: modeling and optimization, kinetic, isotherm, and thermodynamic studies, and toxicity testing. Environ. Sci. Pollut. Res. 25: 20154–20168.
[19] Brown, M.A., S.C. de Vito. 1993. Critical reviews in environmental science and technology, Critical Rev. Environ. Sci. Technol. 23: 249–324.
[20] Rani, B., S. Punniyakoti, N. K. Sahu. 2018. Polyol asserted hydrothermal synthesis of SnO2 nanoparticles for the fast adsorption and photocatalytic degradation of methylene blue cationic dye. New J. Chem. 42: 943–954.
[21] Bhattacharjee, A., M. Ahmaruzzaman, T.B. Devi, J. Nath. 2016. Photodegradation of methyl violet 6B and methylene blue using tin-oxide nanoparticles (synthesized via a green route), J. Photochem. Photobiol. A Chem. 325:116–124.
[22] Kotakadi VS, S A Gaddam, S K Venkata and D.V.R. Sai Gopal.(2015a). “New generation
of bactericidal silver nanoparticles against different antibiotic resistant Escherichia coli
strains”. Appl Nanosci., 5, pages 847–855 (2015).
https://doi.org/10.1007/s13204-014-0381-7.
[23] Kotakadi VS, SA Gaddam, S K Venkata and D.V.R. Sai Gopal.(2015). “Ficus fruit-
mediated biosynthesis of silver nanoparticles and their antibacterial activity against
antibiotic resistant E.coli strains. Current NanoScience 2015b. 11(4): 527 – 538.
DOI: 10.2174/1573413711666150126225951
[24] Kanagala P, Gaddam SA, Gunji P, et al. Synthesis of Bio-Inspired Silver Nanoparticles by
Ripe and Unripe Fruit Extract of Tinospora cordifolia and Its Antioxidant, Antibacterial and
Catalytic Studies. Nano Biomed. Eng. 2020; 12(3): 214-226.
[25] Jose, V., L. Raphel, K.S. Aiswariya, K.S. et al. 2021. Green synthesis of silver
nanoparticles using Annona squamosa L. seed extract: characterization, photocatalytic and
biological activity assay. Bioprocess Biosyst Eng 44: 1819–1829.
https://doi.org/10.1007/s00449-021-02562-2
[26] Kumaram VS, Gaddam, SA, Kotakadi VS, et al. Multifunctional Silver Nanoparticles by
Fruit Extract of Terminalia belarica and their Therapeutic Applications: A 3-in-1 System. Nano Biomed. Eng. 2018; 10: 279-274.
[27] Saravanakumar, K., X. Hu, R. Chelliah, D. H. Oh, K. Kathiresan, and M. H. Wang. 2020. Biogenic silver nanoparticles-polyvinylpyrrolidone based glycerosomes coating to expand the shelf life of fresh-cut bell pepper (Capsicum annuum L. var. grossum (L.) Sendt). Postharvest biology and technology, 160: 111039. doi: 10.1016/j.postharvbio.2019.111039.
[28] Mirzaei, A., K. Janghorban, B. Hashemi, B. et al. 2017. Characterization and optical studies of PVP-capped silver nanoparticles. J Nanostruct Chem 7: 37–46. https://doi.org/10.1007/s40 097-016-0212-3.
[29] Makama, S., S. K. Kloet, J. Piella, H. V. D. Berg, N. C. A. de Ruijter, V. F. Puntes, I. M.
C. M. Rietjens, N. W. V. D. Brink. 2018. Effects of Systematic Variation in Size and
Surface Coating of Silver Nanoparticles on Their In Vitro Toxicity to Macrophage RAW
264.7 Cells, Toxicological Sciences, 162(1): 79–88. https://doi.org/10.1093/toxsci/kfx228
[30] Netala VR , VS Kotakadi , S B Ghosh, P Bobbu , V Nagam , Sharma K.K , VTartte (2014)
“Biofabrication of silver nanoparticles using aqueous laef extract of Melia dubia , characterization and antifungal activity”. Int J Pharm Pharm Sci, Vol 6, Issue 10, 298-300
[31] Bhatia, D., A. Mittal, D. K. Malik. 2016. Antimicrobial activity of PVP coated silver nano particles synthesized by Lysinibacillus varians. Biotech. 6(2):196. doi:10.1007/s 13205-016-0514-7.
[32] Dey, A., A. Dasgupta, V. Kumar, et al. 2015. Evaluation of the of antibacterial efficacy of polyvinylpyrrolidone (PVP) and tri-sodium citrate (TSC) silver nanoparticles. Int Nano Lett 5: 223–230. https://doi.org/10.1007/s40089-015-0159-2.
[33] Palle SR, Penchalaneni J, Lavudi K, et al. Green Synthesis of Silver Nanoparticles by Leaf
Extracts of Boerhavia erecta and Spectral Characterization and Their Antimicrobial,
Antioxidant ad Cytotoxic Studies on Ovarian Cancer Cell Lines. Letters in Applied
NanoBioScience 2020; 9: 1165–1176.
[34] Ashokkumar, S., Ravi, S., Velmurugan, S. 2013. Green synthesis of silver nanoparticles
from Gloriosa superba L. Leaf extract and their catalytic activity. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 115: 388-392
[35] Chimentao, R.J., I. Kirm, F. Medina, X. Rodriguez, Y. Cesteros, and P. Salagre, P. 2013. Different morphologies of silver nanoparticles as catalysts for the selective oxidation of styrene in the gas phase. Chem. Commun. 846-847.
[36] Oliveira, L.S., A.S. Franca, T.M. Alves, S.D. Rocha, 2008. Evaluation of untreated coffee husks as potential biosorbents for treatment of dye contaminated waters. J. Hazardous Mater. 155: 507-512.
[37] Bharathi, D., M. Diviya Josebin, S. Vasantharaj, 2018. Biosynthesis of silver nanoparticles using stem bark extracts of Diospyros montana and their antioxidant and antibacterial activities J. Nanostruct. Chem. 8: 83-92.
[38] Adoni M, Yadam M, Gaddam SA, et al. Antimicrobial, Anti Oxidant and Dye Degradation
Properties of Biosynthesized Silver Nanoparticles From Artemisia Annua L. Letters in Applied BioNanoScience. 2021; 10 (1):1981-1992.
[39] Tripathi, R.M., S.J. Chung, 2020. Reclamation of hexavalent chromium using catalytic activity of highly recyclable biogenic Pd(0) nanoparticles. Sci Rep 10: 640. https://doi.org/10.1038 /s41598-020-57548-z.
[40] Balavigneswaran, C.K., T. Sujin Jeba Kumar, R. Moses Packiaraj, et al. 2014. Rapid detection of Cr(VI) by AgNPs probe produced by Anacardium occidentale fresh leaf extracts. Appl Nanosci 4: 367–378. https://doi.org/10.1007/s13204-013-0203-3.
[41] Farooqi, Z. H., M. W. Akram, R. Begum, W. Wu, A. Irfan. 2021. Inorganic nanoparticles for reduction of hexavalent chromium: Physicochemical aspects, J Hazard. Mater. 402: 123535. https://doi.org/10.1016/j.jhazmat.2020.123535.
Copyright© Rama Jyothi Narjala, Susmila Aparna Gaddam, Sangeeta C. Casimeer, Siva Gayathri Velakanti, Ramamurthy Nadipi, Vishali Batyala, Lakshmi Narayana Suvarapu and Venkata Subbaiah Kotakadi. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.