Green Synthesis of α- Fe2O3 Nanoparticles Mediated Musa Acuminata: A study of Their Applications as Photocatalytic Degradation and Antibacterial Agent
T. Indumathi1, N. Krishnamoorthy2, R. Valarmathy3, K. Saraswathi4, S. Dilwyn5, S. Prabhu6*
1Department of Chemistry, CHRIST (Deemed to be University), Bangalore, Karnataka, India
2Department of Physics, Sri Eshwar College of Engineering, Coimbatore, Tamil Nādu, India
3Department of Chemistry, Hindusthan College of Engineering and Technology, Coimbatore, Tamil Nādu, India
4Department of Civil Engineering, Hindusthan College of Engineering and Technology, Coimbatore, Tamil Nādu, India
5Department of Food Technology, Hindusthan College of Engineering and Technology, Coimbatore, Tamil Nādu, India
6Department of Physics, Hindusthan College of Engineering and Technology, Coimbatore, Tamil Nādu, India
*Corresponding author. E-mail: prabhucsa@gmail.com
Received: Jun. 18, 2022; Revised: Oct. 02, 2022; Accepted: Nov. 18, 2022; Published: Nov. 30, 2022
Citation: T. Indumathi, N. Krishnamoorthy, R. Valarmathy, et al. Green synthesis of α-Fe2O3 nanoparticles mediated musa acuminata: a study of their applications as photocatalytic degradation and antibacterial agent. Nano Biomedicine and Engineering, 2022, 14(3): 254–262.
DOI: 10.5101/nbe.v14i3.p254-262
Abstract
The present study was aimed to green synthesize of α-Fe2O3 nanoparticles (NPs) using flower extract of Musa acuminata and examination of their antibacterial and photocatalytic activities. The
synthesized NPs were investigated using UV-visible spectroscopy, which exhibited a colour change pattern, and the maximum absorption peak at 265 nm confirmed the formation of α-Fe2O3 NPs. The FTIR analysis showed the presence of various functional groups coated over the synthesized α-Fe2O3 NPs. The XRD pattern showed that the formation of rhombohedral structure with an average crystallite size was 21.86 nm. FESEM micrographs revealed that α-Fe2O3 NPs were roughly spherical in shape. EDX spectrum confirmed the presence of Fe and O elements. By TEM analysis, the average particle size was calculated to be 32 nm. Using the well diffusion method, the antibacterial activity of α-Fe2O3 NPs was tested against both gram positive and negative bacterial strains of Staphylococcus aureus (S. aureus) and Escherichia coli (E. coli). The NPs exhibited good antibacterial activity against the tested bacteria. Finally, the synthesized α-Fe2O3 NPs demonstrated the photocatalytic degradation of Crystal Violet (CV) dye under sunlight. The efficiency of degradation within 150 min was determined to be 90.27% for CV. This effective removal method under sunlight may support a cost-effective method for degradation of CV dyes from wastewater.
Keywords: Green synthesis; α-Fe2O3 NPs; Musa acuminata; Photoluminescence and dye
degradation; Antibacterial activity
Introduction
Iron oxide nanoparticles (IO NPs) attract tremendous attention in various fields owing to their unique optical, electrical, chemical, magnetic and biomedical properties [1–4]. These versatile properties of IO NPs widen its area of applications in fluorescence cell imaging [5, 6], drug delivery [7], gas sensors [8], memory devices [9] and super-capacitors [10]. Iron oxide (IO) exists in three different crystalline forms: FeO, Fe2O3 and Fe3O4. Among these nanocrystalline phases, Fe2O3 exists in 4 different polymorphic forms, namely, alpha, beta, gamma and epsilon Fe2O3. Especially, α-Fe2O3 (hematite) phase is the most thermodynamically stable form of Fe2O3. Additionally, its biocompatibility, magnetic, fluorescence and promising catalytic properties allow hematite phase for much more investigation as an ideal material for multifunctional applications [11–13]. Recently, much attention has been diverted to explore the properties of α-Fe2O3, as a catalyst for the light assisted degradation of complex organic dye [14] studied the role of reducing agent (NH4OH) in the synthesis of α-Fe2O3 NPs and its effect in the photocatalytic degradation of organic dye. Further, Silva et al. studied the photocatalytic activity for TiO2 and Ag hybridized α-Fe2O3 nanocatalyst [15].
Various techniques such as spray pyrolysis [13], hydrothermal method [8], solvothermal method, microwave-assisted method [2], co-precipitation method [16], and sol-gel method [4, 17] have been employed to synthesise α-Fe2O3 (hematite) nanocrystals. However, most of the preparation techniques were complex that require usage of templates or substrates and high temperature processing. Additionally, by the usage of hazardous organic solvents as reducing agents and further washing the NPs using highly reactive solvents to remove the impurities, makes the α-Fe2O3 NPs toxic [18]. Hence huge number of researchers are tending towards the synthesis of toxic free IO NPs using plant extract and another biological system as reducing and capping agent [19, 20]. In addition to that, green synthesis method has many advantages, including simple process, natural organic compounds as stabilizing agents and good crystallization of the nanoparticles. Recently, it was reported that the plant extract (leaf, root, flower and fruit) possesses natural antioxidant, carotenoids, flavonoids, poly-phenols and bioactive compounds. These natural chemical complexes from the plant extract reduce the metal ions in a single step process and prevent the aggregation of nanoparticles [20, 21].
Musa acuminata-Red Dacca, commonly known as red banana, belongs to musa family which is cultivated in most of the tropical countries. The flower extract of Musa acuminata possesses unique importance in traditional ayurvedic medicine to treat stomach cancer, blood related diseases and arthritis. The flower extract of Musa acuminata has strong antibacterial properties and contains various phytochemicals, including phenols, carbohydrate, tannins, saponins, flavonoids, alkaloids, and anthraquinones [20, 22]. These phytochemical compounds serve as strong reducing and capping agents during the synthesis of α-Fe2O3 NPs. The aqueous flower extract is capable of reducing ferric nitrate to α-Fe2O3 NPs, and the NPs thus formed have significant antimicrobial properties. Hence, we examined the possibility of preparing green synthesized α-Fe2O3 NPs using Musa acuminata suitable for antibacterial applications.
There is a significant problem with water pollution caused by hazardous chemical discharges from
industries such as textiles, leather dyeing, paper, cosmetics, and pharmaceuticals because these dyes are highly toxic and highly resistant to degradation, which makes them more likely to pollute water sources. Crystal Violet (CV) is a cationic dye used in the textile and paper industries. CV dyes are commonly discharged into the environment during dyeing. Numerous health problems may result from their consumption, including eye irritation, breathing difficulties, allergic dermatitis, and nervous system dysfunction. It has been found that CV dyes are highly toxic and are soluble in water. Hence, it is essential to remove such dyes from contaminated wastewater. The photocatalytic degradation of the dye derivatives CV using α-Fe2O3 NPs has not been reported. Therefore, we have undertaken a study on the photo degradation of CV using sun light irradiation. In order to remove the pollutants, various methods have been used to treat wastewater, including precipitation, ion exchange, carbon adsorption, reverse osmosis, membrane filtration, and microfiltration [23, 24].
The present study focuses on the synthesis of α-Fe2O3 NPs using Musa acuminata flower extract.
The NPs photocatalytic degradation efficiency under sunlight and antibacterial activities have been investigated. The novelty of the present study is the use of Musa acuminata flower extract as a good reducing and capping agent for the synthesis of α-Fe2O3 NPs. The photocatalytic activity of the synthesized NPs is evaluated based on the degradation of CV dye (Fig. 1) using sunlight irradiation, which has not been reported to date. Therefore, in this study, the degradation of CV dyes was carried out. Moreover, the antibacterial activity of the α-Fe2O3 NPs was assessed against S. aureus and E. coli pathogenic bacterial strains, which showed effective antibacterial activity against both gram-positive and gram-negative pathogenic bacteria, thus establishing their potential for bacteria-based biomedical applications. The highlight of our research implies that the synthesized α-Fe2O3 NPs could be employed as an important antibacterial and cost effective catalytic agent.
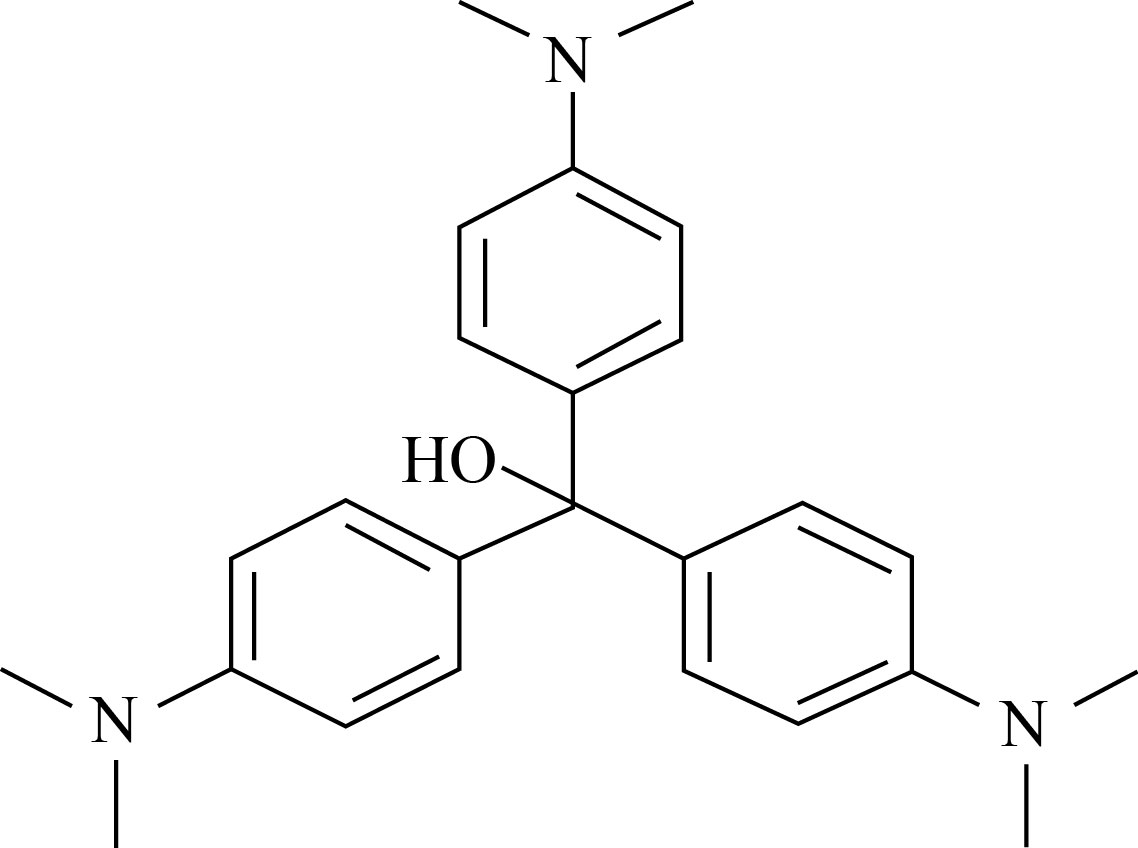
Fig. 1 Structure of crystal violet dye
Materials and Methods
Ferric nitrate (AR grade) was purchased from Hi Media Labs Pvt limited. Deionised water has been used as the solvent throughout the green synthesis process. Flower from Musa acuminata “Red Decca” variety plant was collected from the banana cultivation farm, Tamilnadu Agricultural University, Tamil Nadu, India, and was used for the present green synthesis method.
Preparation of flower extract
Fresh and healthy Musa acuminata “Red Decca” flowers were taken from the plant. The fresh flowers were washed two to three times using distilled water to remove dust particles and were cut into small pieces. In 100 mL of distilled water, 5 g of flowers were added and heated to 100℃ for 20 min. The light purple coloured extract was filtered out using whatman No. 1 grade filter paper and cooled to room temperature. The as prepared flower extract solutions were used in further experiments.
Synthesis of α-Fe2O3 NPs
The green synthesis of α-Fe2O3 NPs was carried out with minor modifications to the previous reports [18]. A 10 mL of 0.1 mol/L precursor solution of Fe (NO3)3 was prepared and vigorously stirred for 20 min. The as prepared flower extract solution of 30 mL was added into the precursor solution and made up to 150 mL. Under constant stirring, the solution turned to dark brown colour at room temperature, which confirmed the formation of α-Fe2O3 from ferric nitride through reduction process. The solution was centrifuged at 5000 r/min for 10 min and the dark brown precipitate was collected. Then the as collected precipitate was washed repeatedly using deionised water and dried under ambient condition. The dried precipitate was further annealed at 100℃ for 4 h, resulting in the formation of dark brown IO NPs. The schematic representation of the green synthesized α-Fe2O3 NPs using Musa acuminata is shown in Fig. 2.
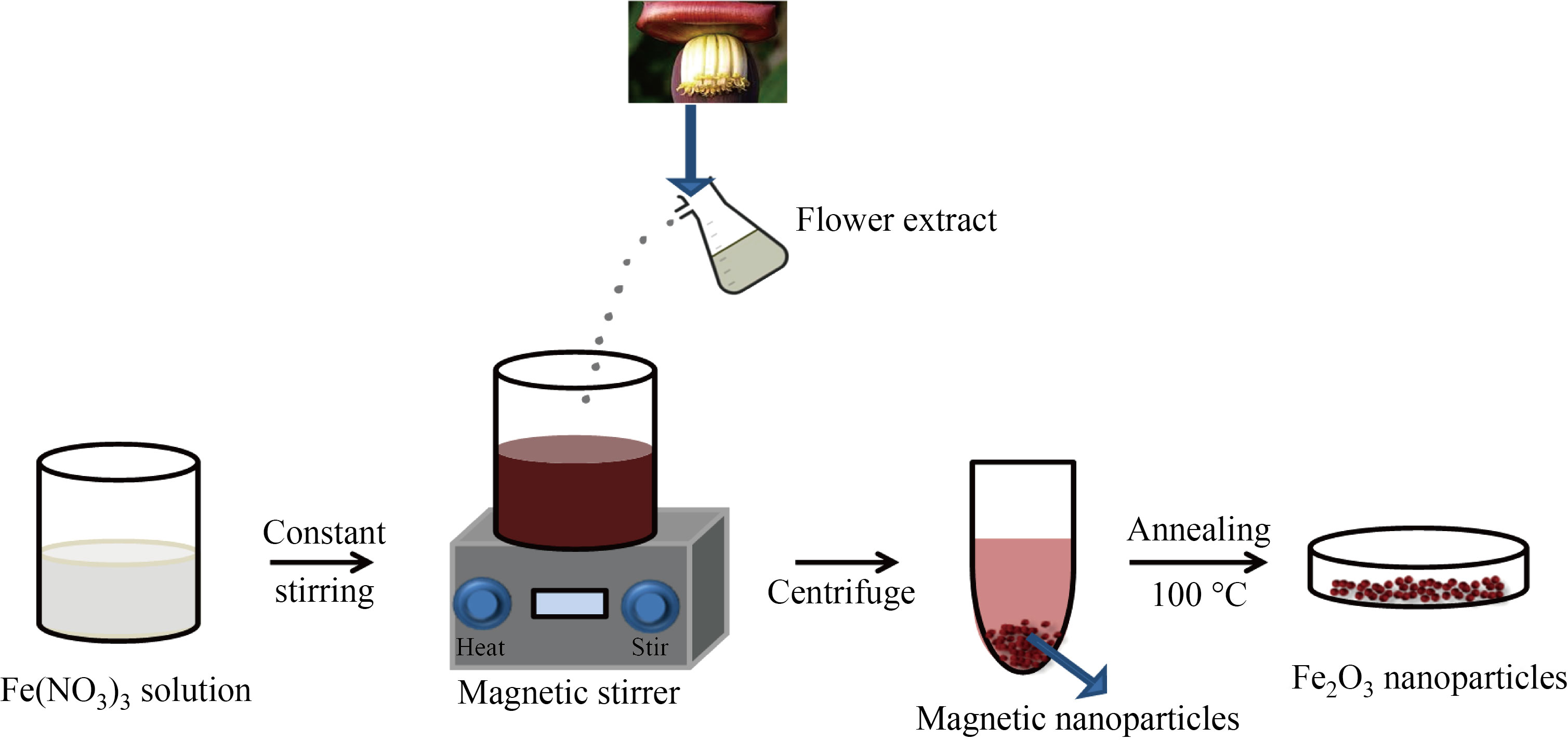
Fig. 2 Green synthesis of α-Fe2O3 NPs using Musa acuminata flower extract
Dye degradation study
The photocatalytic degradation activity of α-Fe2O3 NPs was investigated under direct sunlight, as per the procedure stated in previous works but with slight modifications [25]. To evaluate the photocatalytic activity of α-Fe2O3 NPs under sunlight, CV dye was employed as the sample pollutant. To 30 mL of the aqueous dye solution (25 µg/mL), concentration of CV solution under stirring at pH = 7 conditions. 30 mg of IO NPs were added. In order to achieve maximum adsorption, the dye solution containing the catalyst was adjusted for pH in the range of 7–9 and was exposed to direct sunlight for 150 min. In order to achieve maximum adsorption, the dye solution containing the catalyst was adjusted for pH in the range of 7–9 by adding 0.1 mol/L NaOH. At intervals of 30 min, 3 mL of the dye solution was removed and centrifuged at 5000 r/min for 5 min. The degradation efficiency of the prepared solution was examined by using UV-visible absorption spectroscopy. The maximum absorbance (λmax) of the CV solution was measured at 586 nm, and the C/C0 ratio was calculated based on the dye degradation. The photocatalytic experiments were carried out under a clear sky between 10:30 a.m. and 12:00 p.m. The average sun light intensity measured was around 9×104 lx.
Antibacterial activity
The antibacterial activity of the green synthesized α-Fe2O3 NPs was evaluated against two bacterial strains E. coli and S. aureus by well diffusion method [26]. Bacterial colonies were swabbed in sterile cotton swabs into Muller Hinton agar plates, which were then allowed to dry for 15 min before placing samples on the plates. Using sterile micropipette tips, 6 mm wells were punched in agar plates, and three different concentrations (50, 100, and 200 µg/mL) of synthesized IO NPs were taken. The plates were then incubated for 24 h at 37 ℃. After incubation, the diameter of the areas exhibiting bacterial growth inhibition was measured (in mm).
Characterization techniques
The UV-visible absorption spectrum of α-Fe2O3 NPs was analysed by using (UV-Vis DRS) Perkin Elmer Lambda 25 spectrometer (JASCO V650). Schimadzu IR Affinity 1S series spectrophotometer was used to identify the possible biomolecules responsible for the reduction and capping agent of the NPs. XRD patterns were obtained using Rigaku X-ray diffractometer with Cu-Kα radiation (λ = 1.54 Å) and scanning angle 2θ over the range of 20°–70° at room temperature. The surface morphological analysis of the α-Fe2O3 NPs was studied using scanning electron microscopy (SEM) JEOL JEM 6390, Japan using SEM equipped with an EDAX model (INCA PENTA FET X3) attachment. Photoluminescence (PL) experiments were also performed for the optical characterization of the nanoparticles using Horiba Jobin Yvon luminescence spectrometer with xenon lamp (150W) as the excitation source. Transmission Electron Microscopy (TEM) measurements were performed using JEOL 2100, Japan.
Results and Discussion
UV spectroscopy
The prepared aqueous flower extract of Musa acuminata was added drop-wise into the ferric nitrate solution and stirred vigorously. After 45 min of continuous stirring, the solution turned to dark brown colour. The observed colour change confirmed the formation of α-Fe2O3 NPs, which is shown in inset of Fig. 3. The UV-visible absorption spectrum of α-Fe2O3 NPs is shown in Fig.3. The maximum absorption spectra peak (λ) was observed at 265 nm. The peak in this spectrum matches with that observed in the previous studies [18]. Further, the optical band gap (Eg) for α-Fe2O3 NPs was computed using Tauc’s plot method and was found to be 2.2 eV (Fig. 4), which well matches with that in the previous reports [27, 28].
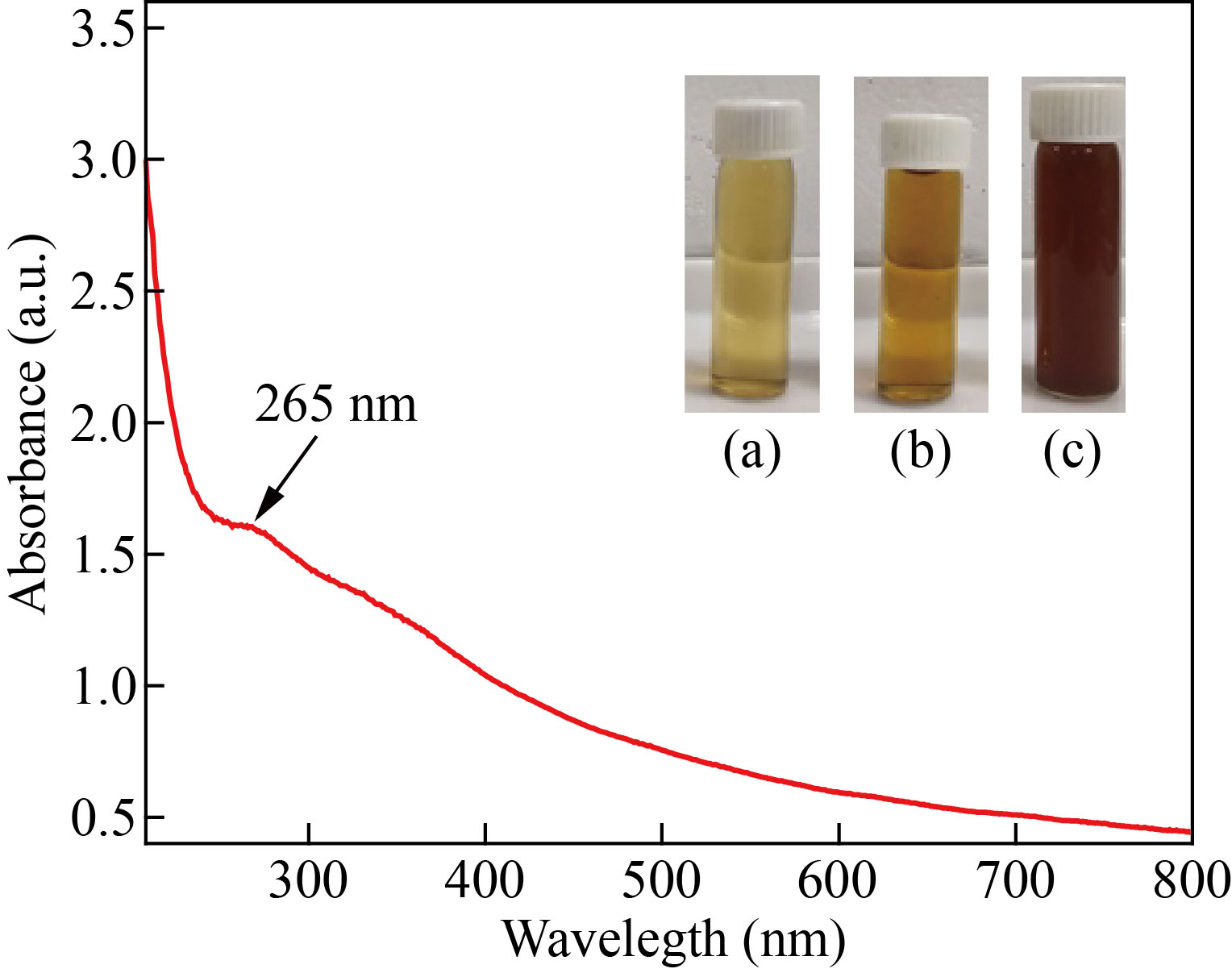
Fig. 3 UV-visible spectrum of α-Fe2O3 –NPs: (inset) (a) Ferric nitrate solution, (b) Flower extract, and (c) α-Fe2O3 NPs
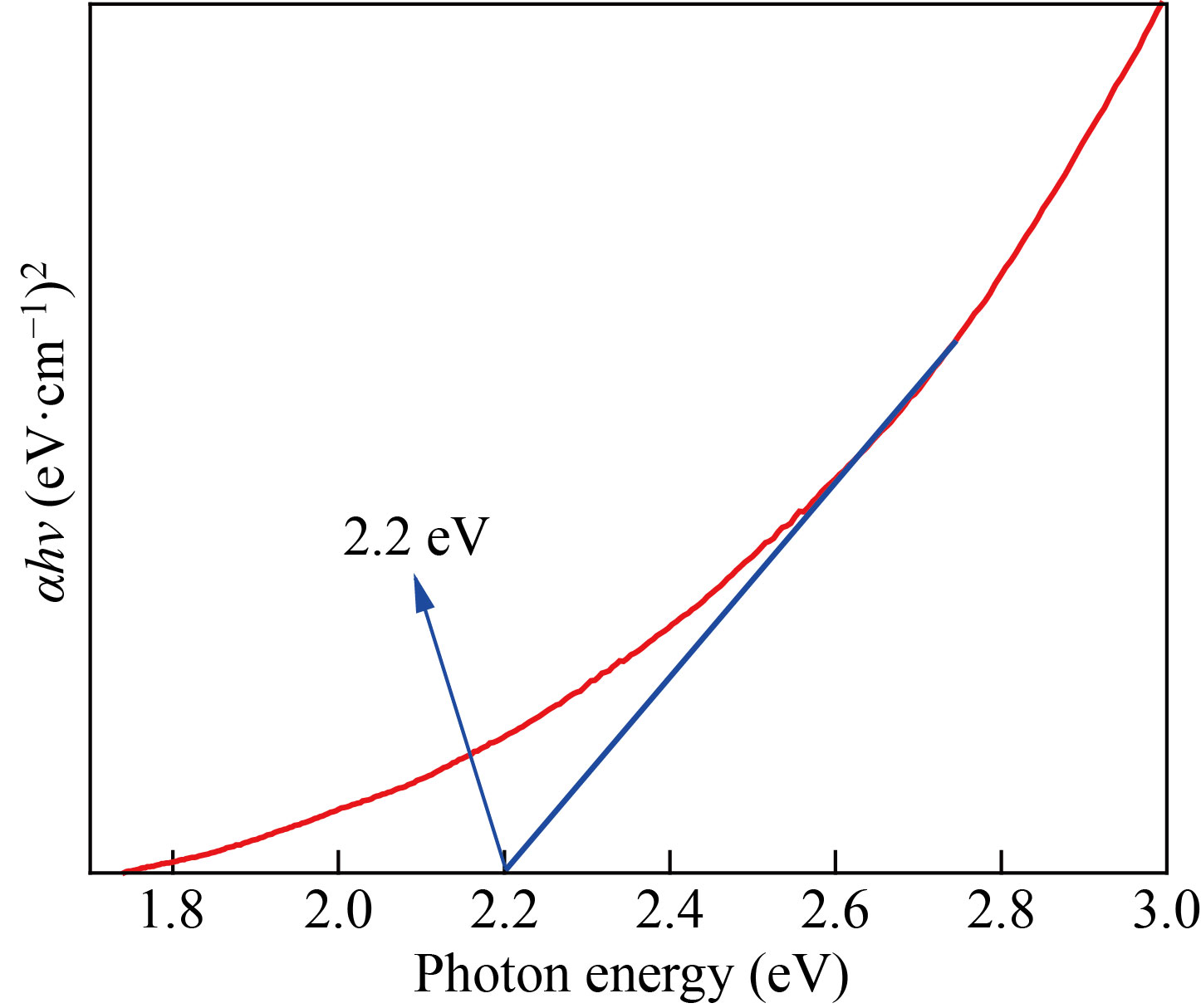
Fig. 4 Optical bandgap of α-Fe2O3 NPs
FTIR analysis
The FTIR spectrum of green synthesized α-Fe2O3 NPs gives details about the various functional groups responsible for reducing the iron ions. FTIR spectra of α-Fe2O3 NPs shows strong bands at 3311, 2887, 1643, 1340, 1047, 646, 594 and 557 cm-1 within the region of 500–4300 cm-1 (Fig. 5). The strong peaks at 557 cm-1 and 594 cm-1 can be attributed to Fe–O vibrational modes and confirms the formations of α-Fe2O3 NPs [29]. The absorption peak at 646 cm-1 was assigned to the alkyl halide of functional group. The bands at 1050 cm-1, and 1644 cm-1 are corresponding to the C–O, N–H and O–H stretching vibrations of primary amine, phenolic functional groups of biomolecules [29, 30]. The wider bands at 1343 cm-1 and 1396 cm-1 attributed to C–O and C–H stretching vibration of bioactive phytocompounds of Musa acuminata flower [27]. These results show that the bioactive compounds of flower extract involved in the formation and stabilization of Fe ions to form α-Fe2O3 NPs.
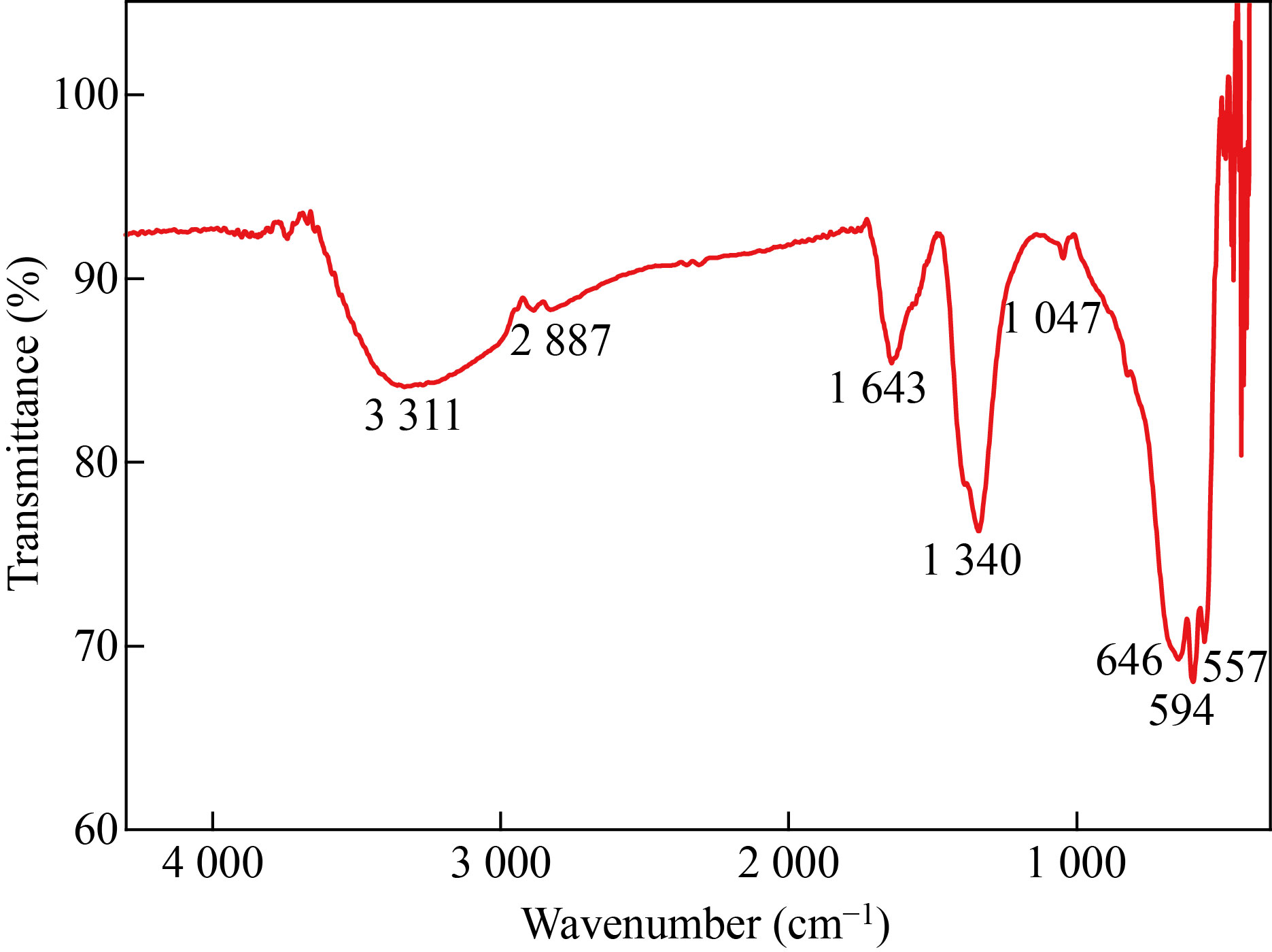
Fig. 5 FTIR spectrum of α-Fe2O3 NPs
XRD analysis
The XRD spectrum of the green synthesized α-Fe2O3 NPs is shown in Fig. 6. The characteristic peaks have been observed at 2θ values of 24.72°, 33.06°, 36.42°, 39.28°, 40.41°, 43.40°, 56.08°, 58.96°, 62.6°, and 66.56° which correspond to the planes with miller indices (012), (104), (110), (006), (113), (202), (211), (018), (214), and (125), respectively and represent the rhombohedral crystal structures of α-Fe2O3 (JCPDS card No 33-0664), which is in good agreement with previous reports [27]. The particle size was calculated using Scherrer equation:
![]() (1)
(1)
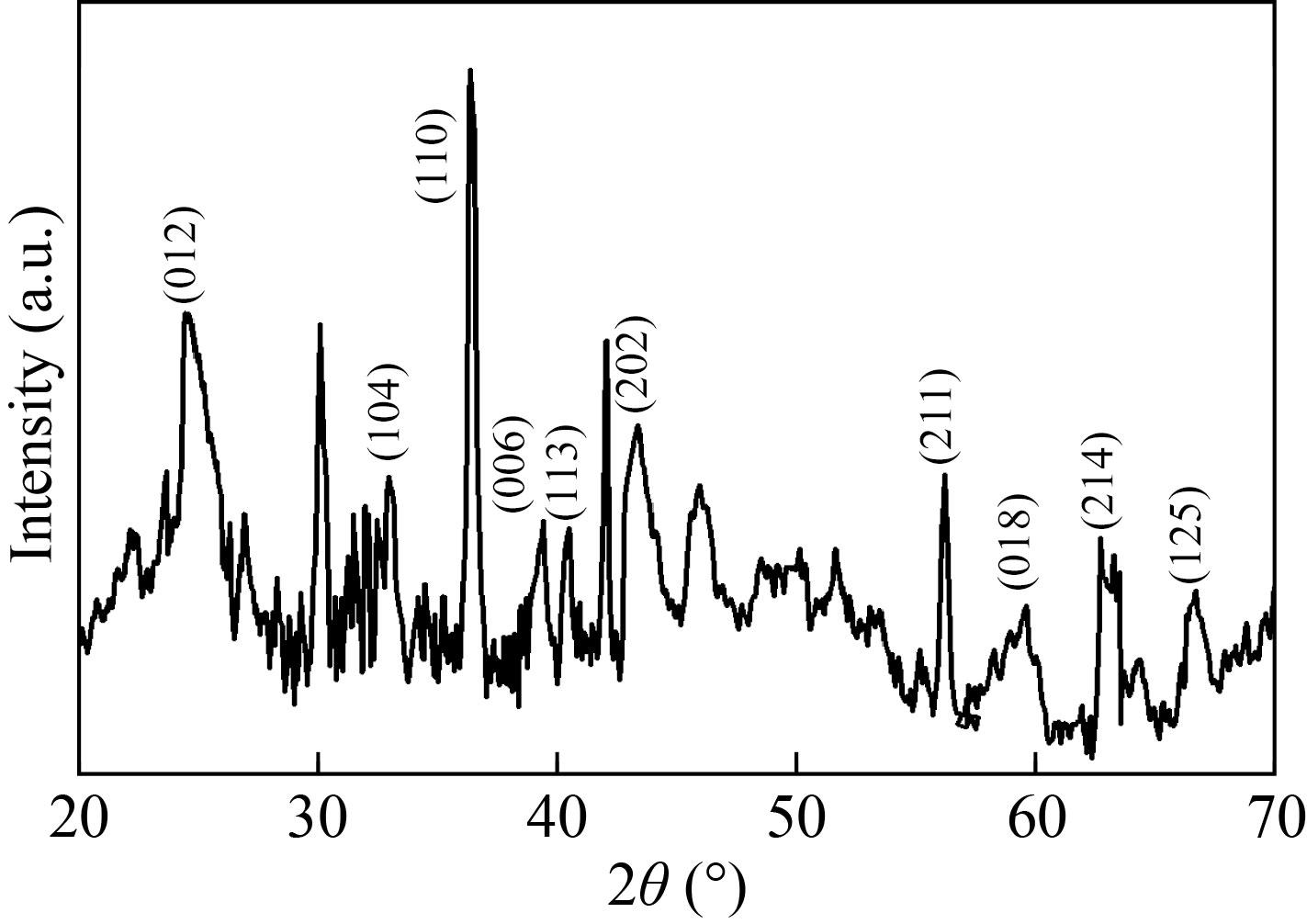
Fig. 6 XRD Pattern of α-Fe2O3 NPs
where k is the Scherrer constant, λ indicates the wavelength of the X-ray radiation, and β represents the full width at half maximum of the diffraction peak [25]. The average crystallite size was found to be 21.86 nm for the as synthesised α-Fe2O3 NPs. The obtained crystallite size of α-Fe2O3 NPs matches that in the previous reports [16].
Photoluminescence analysis
Luminescent properties of the transition metal compounds are gaining much attention in fluorescence cell imaging and photo-physical sensing applications. In the present work, luminescence properties of the as synthesized α-Fe2O3 NPs from flower extract were studied in detail. Figure 7 shows the room temperature photoluminescence spectra of the as synthesised α-Fe2O3 NPs. From the UV spectra analysis, it was found that the absorption band exists in the UV and visible region. Hence, the luminescence behaviour of α-Fe2O3 was investigated at different excitation wavelengths from 275 to 375 nm within the UV region. By increasing the excitation wavelength, the emission edges get shifted towards visible region from UV region. Thus, the excitation wavelength was fixed at 275 nm for the present analysis. The α-Fe2O3 NPs excited at 275 nm shows a sharp emission edge at 335.2 nm and broad visible emission around 380 to 480 nm. Lassoued et al. reported that when the IO NPs were excited at 350 nm, it shows a strong emission peak at 450 nm which was due to the electron transition within the α-Fe2O3 NPs [16]. Similarly in the present study, the PL analysis spectra show a sharp emission at 469 nm, and confirms the emission due to electron/hole recombination of α-Fe2O3 which agrees well with the previous reports [31].
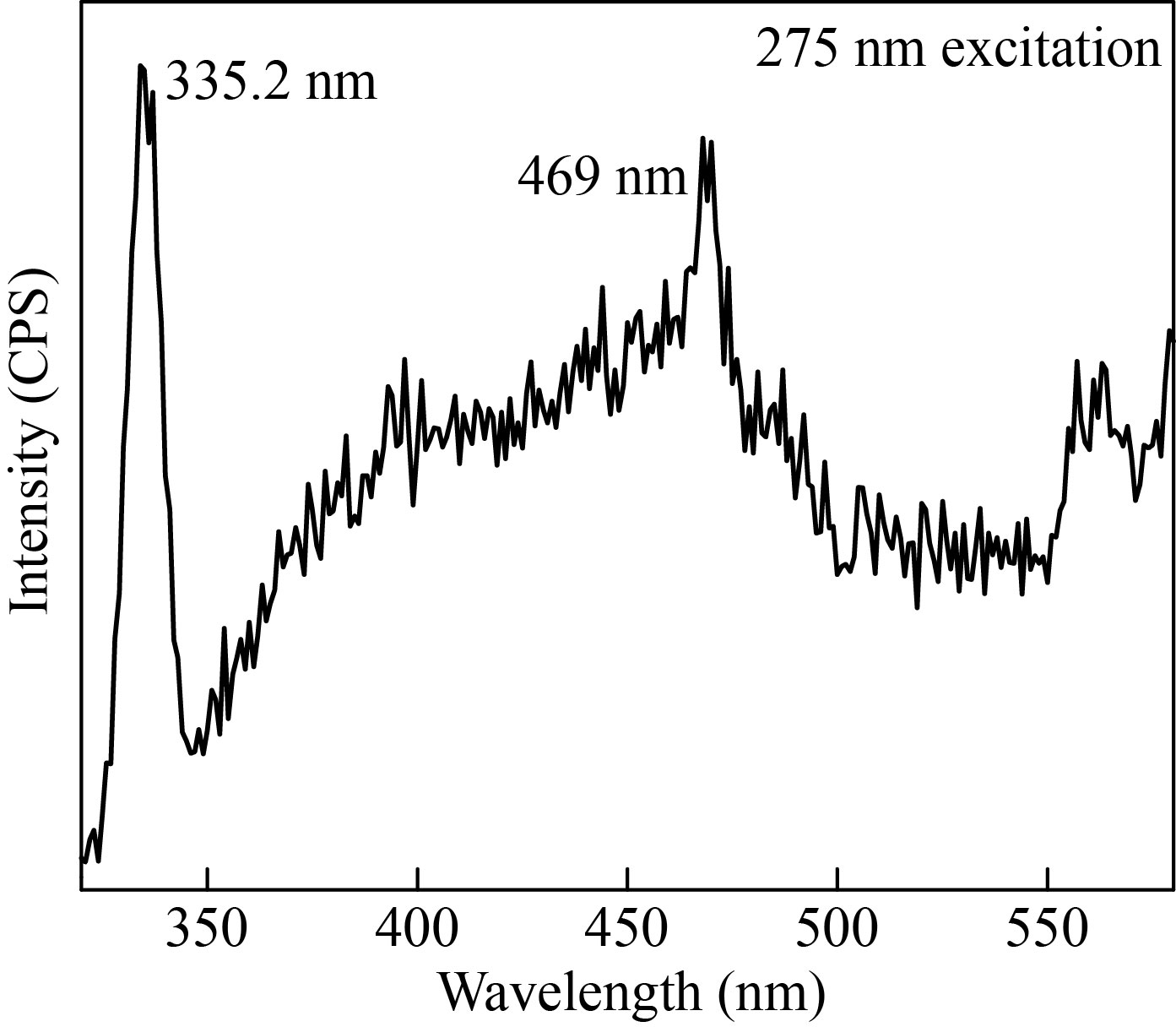
Fig. 7 Photoluminescence spectra of α-Fe2O3 NPs
The emission on the high energy side (335.2 nm) of the spectra arises from the band-to-band recombination of the photo-induced electron in the iron oxide. However, this emission is mostly by the surface defect states resulted from variable sized nanoparticles and by other imperfections at the boundaries which usually resulted from the growth conditions. Overall, these results confirm that the as synthesized iron oxide nanoparticles using Musa acuminata flower extract show excellent luminescence properties.
FESEM and EDX analyses
The particle size and surface morphology of the green synthesized α-Fe2O3 NPs were determined from the Field Emission Scanning Electron Microscope (FESEM) analysis. From the FESEM image, it is confirmed that the as synthesized α-Fe2O3 NPs are roughly spherical in shape, and agglomerations are observed among the particles, as shown in Fig. 8. Agglomeration is determined by sample preparation, electrostatic field interaction and hydrophobic magnetism which is further exhibited by HRTEM studies [26]. The energy dispersive X-ray (EDAX) spectrum analysis clearly confirms the presence of strong peaks of Fe and O elements (Fig. 9). In addition, sodium peaks were observed, which may be caused by impurities in the precursor [18].
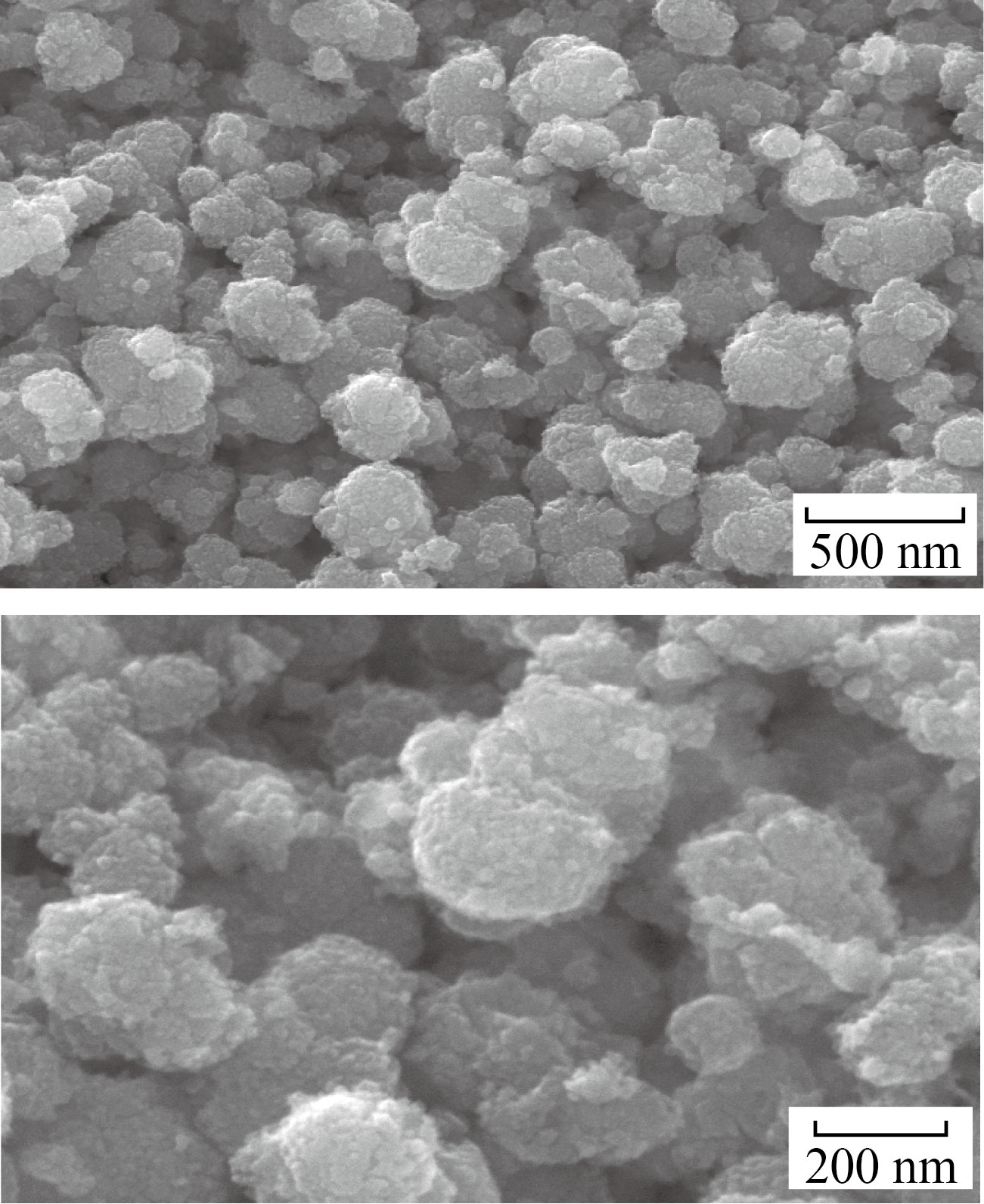
Fig.8 FESEM images of α-Fe2O3 NPs
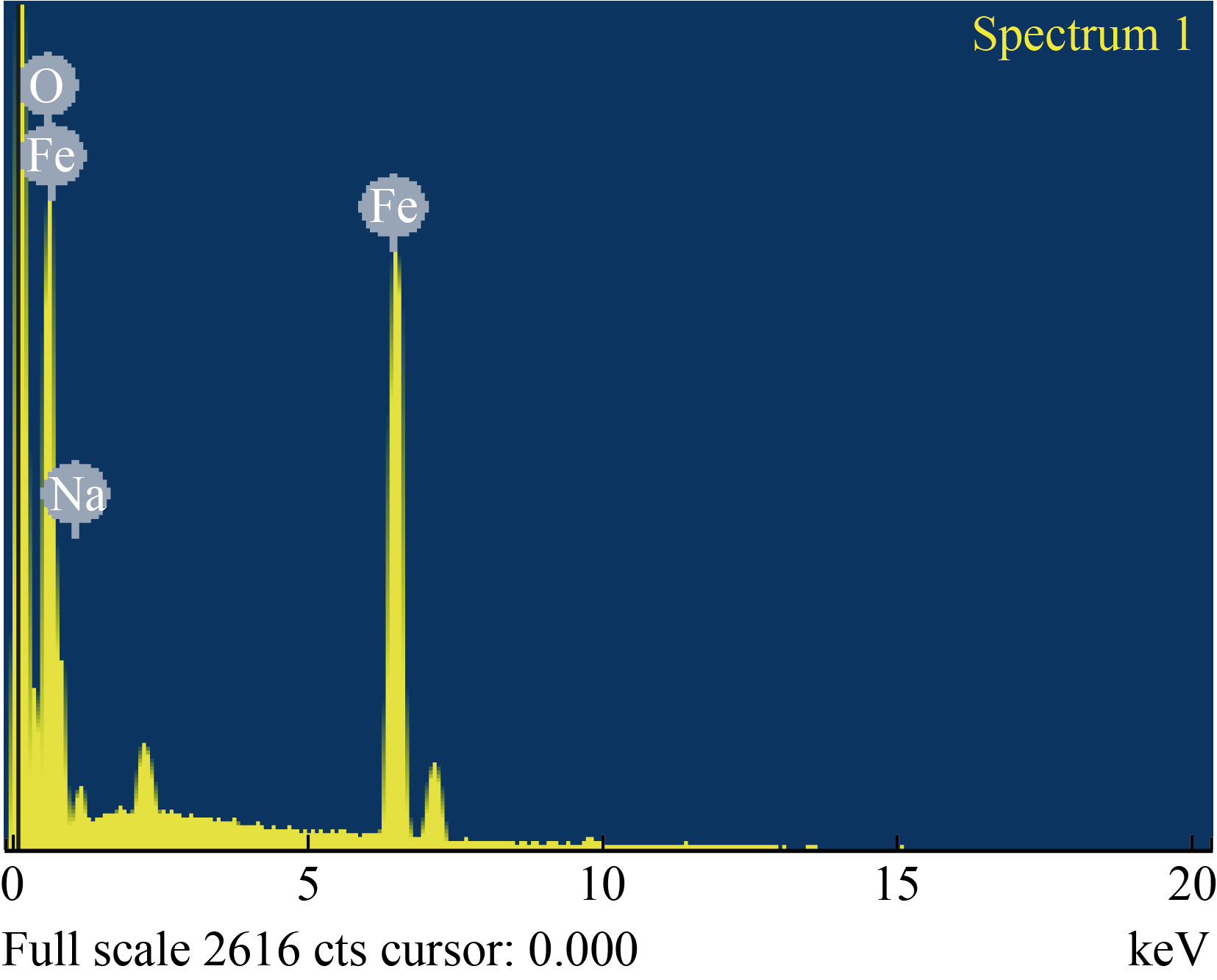
Fig. 9 EDAX spectrum of α-Fe2O3 NPs
HRTEM analysis
The HRTEM images of the α-Fe2O3 NPs at 100 nm are shown in Fig. 10(a). At this magnification, it was observed that the α-Fe2O3 NPs have a roughly spherical in shape with agglomeration between particles. The average particle size calculated from the size distribution histogram (Fig. 10(b)) was found to be 32 nm, which corroborates with the XRD results. As the particle size decreases, the surface area increases, which enhances the photocatalytic applications [32].

Fig. 10 HRTEM images of α-Fe2O3 NPs: (a) 100 nm, (b) size distribution histogram
Antibacterial activity
The green synthesis of α-Fe2O3 NPs was tested against E. coli and S. aureus bacteria by well diffusion method. α-Fe2O3 concentrations were varied as 50, 100, and 200 µg/mL, and the corresponding zones are shown in Fig. 11. We observed an increase in antibacterial activity with increasing α-Fe2O3 NP concentrations (Fig. 11(c), Table 1). For S. aureus, the highest zone of inhibition was found to be 20 mm while for E. coli was 18 nm at 200 µg/mL. In general, antibacterial activity of α-Fe2O3 NPs is influenced by their size, shape, surface area, wide band gap, reactive oxygen species (ROS), and biomolecules interaction. α-Fe2O3 NPs trigger bacterial oxidative metabolism in addition to creating ROS and destroying bacterial walls. Interactions between biomolecules and the bacterial membrane result in the rupture and collapse of the membrane, thus destroying the bacteria’s cytoplasm [26]. α-Fe2O3 NPs inhibit both tested bacteria, but better efficiency is observed against gram-positive bacteria. α-Fe2O3 NPs exhibit significant antibacterial activities due to their crystallite size of 18 nm and their particle size of 41 nm, which allows them to interact with bacterial surfaces.
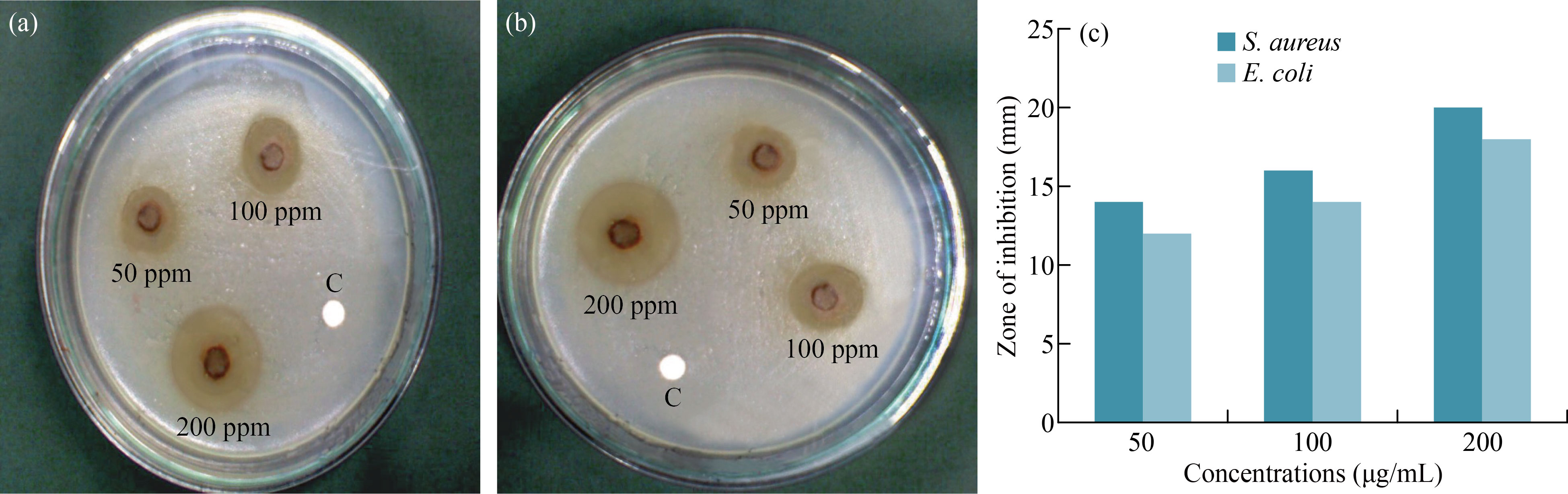
Fig. 11 Antibacterial activity of α-Fe2O3 NPs against (a) S. aureus, (b) E. coli, (c) Bacterial zone of inhibitions
Table 1 Antibacterial activity of α-Fe2O3 – NPs against S. aureus and E. coli
Microorganism | Zone of inhibition (mm) | ||
50μg/mL | 100 μg/mL | 200 μg/mL | |
S. aureus | 14 | 16 | 20 |
E. coli | 12 | 14 | 18 |
Photocatalytic studies
The photocatalytic efficiency of the green synthesized α- Fe2O3 NPs towards CV dye was examined under direct sunlight irradiation. The dye is significantly reduced when sunlight irradiates the CV dye solution containing the catalyst. The UV-visible spectrum was used to examine the photocatalytic degradation. The maximum absorbance (λmax) of the CV dyes was found to be 507 nm, which decreases gradually, demonstrating a strong reduction of the CV dye as shown in Fig 12. The violet colour of the dye ends gradually concurring that the chemical bonding of the dye molecules has disintegrated and ruptured. The efficiency of dye degradation is evaluated by the BeerLamberts equation:
![]() (2)
(2)
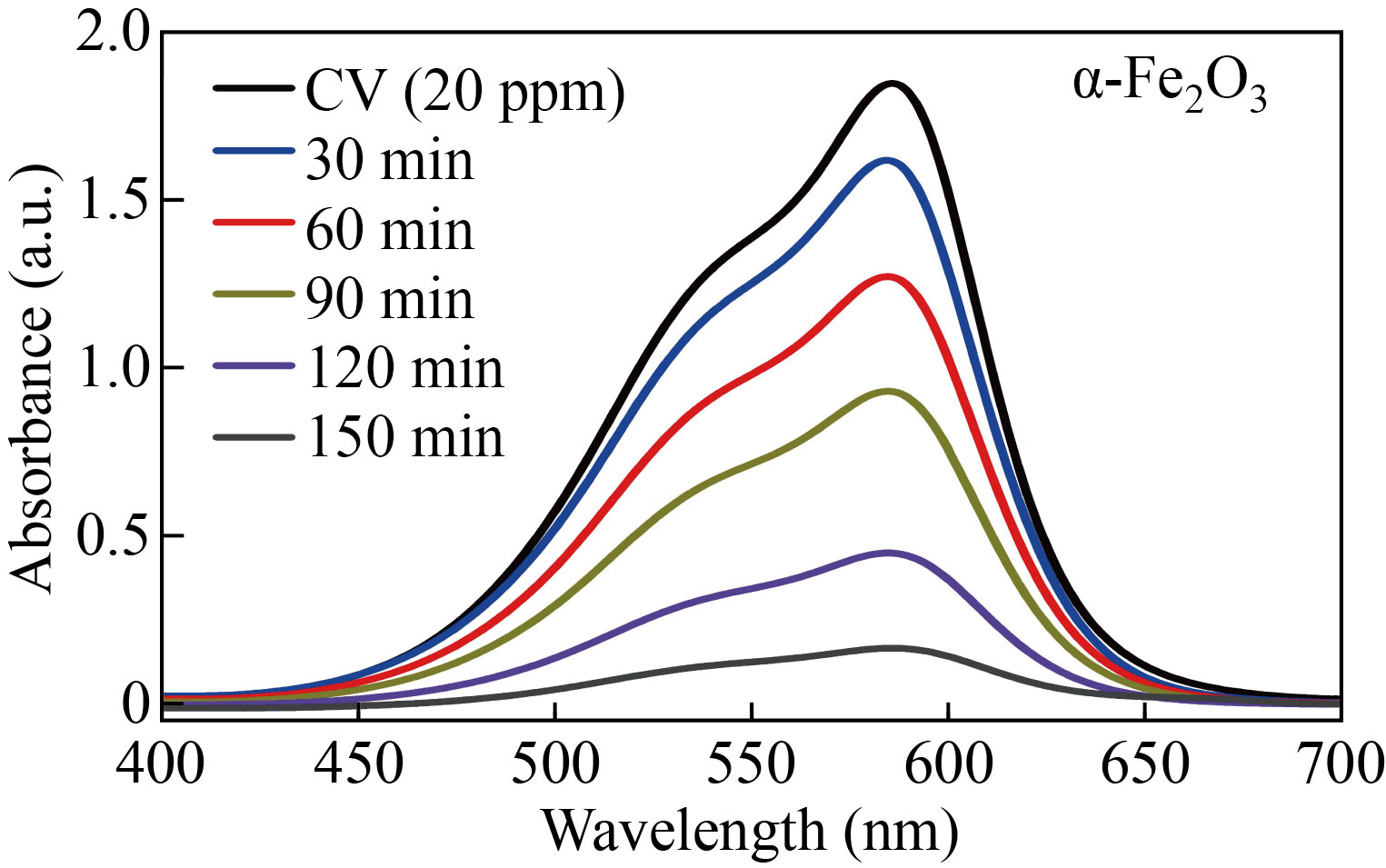
Fig. 12 Photodegradation of CV dye by α-Fe2O3 NPs
where C and C0 represent the final and initial concentrations, respectively of the CV dye. The degradation percentage of CV within 150 min was evaluated to be 90.27%. The degradation efficiency of the CV dye increases as the time of sun light radiation increases. The dye degradation of CV dye at pH = 9 significantly enhances the degradation rate under sunlight irradiations and achieves a stable photodegradation. As the pH increases, more negatively charged surfaces are available resulting in decrease in repulsion between the positively charged dye molecule and the catalyst [33]. This confirms that the CV is stable enough to be used in investigating the photocatalytic characteristics of the as-prepared photocatalyst. The catalytic activity can be influenced by various factors such as size and morphology of the catalyst [32]. Under the influence of sunlight, dye degradation occurs due to selective photooxidation resulting from electron transfer. After solar irradiation, the dye molecules adsorbed on the surface of the α-Fe2O3 NPs form a pair of electron-hole in the nanostructures. The produced electron-hole pair engages with the hydroxyl and oxygen groups, forming hydroxyl and anion radicals that interact directly with the CV dye molecules, causing dye degradation. The dye degradation process can be demonstrated as follows:
FeO + hv → e – + h+ (3)
OH– + O2 → OH (4)
OH– + O2 → OH (5)
Dye + O2– + OH → Decay products (6)
Figure 13(a) shows the plot of ln (C/C0) vs. irradiation time (t) for blank and CV dye. The graph demonstrates a linear fit (R2 = 0.98 and 0.92). The kinetic curve illustrated in Fig. 13(b) for α-Fe2O3 NPs indicates a pseudo-1st order degradation which follows the Langmuir-Hinshelwood model:
ln(C/C0) = –kt (7)
where t denotes the reaction time and k represents the degradation rate constant for the dye [25]. The degradation rates of α-Fe2O3 NPs against blank and CV dye solutions were 0.0062 and 0.032 min-1, respectively. The k value fits to the slope of the line and is related to the rate of reaction as well as the concentration of the reaction components. The dye degradation mechanism is influenced by the dye concentration, catalyst amount, irradiation time, and incident light intensity. As the time of irradiation increases, the rate of reaction becomes slow, as the size and morphology of the catalyst and the intermediate products play a key part in the degradation [33].
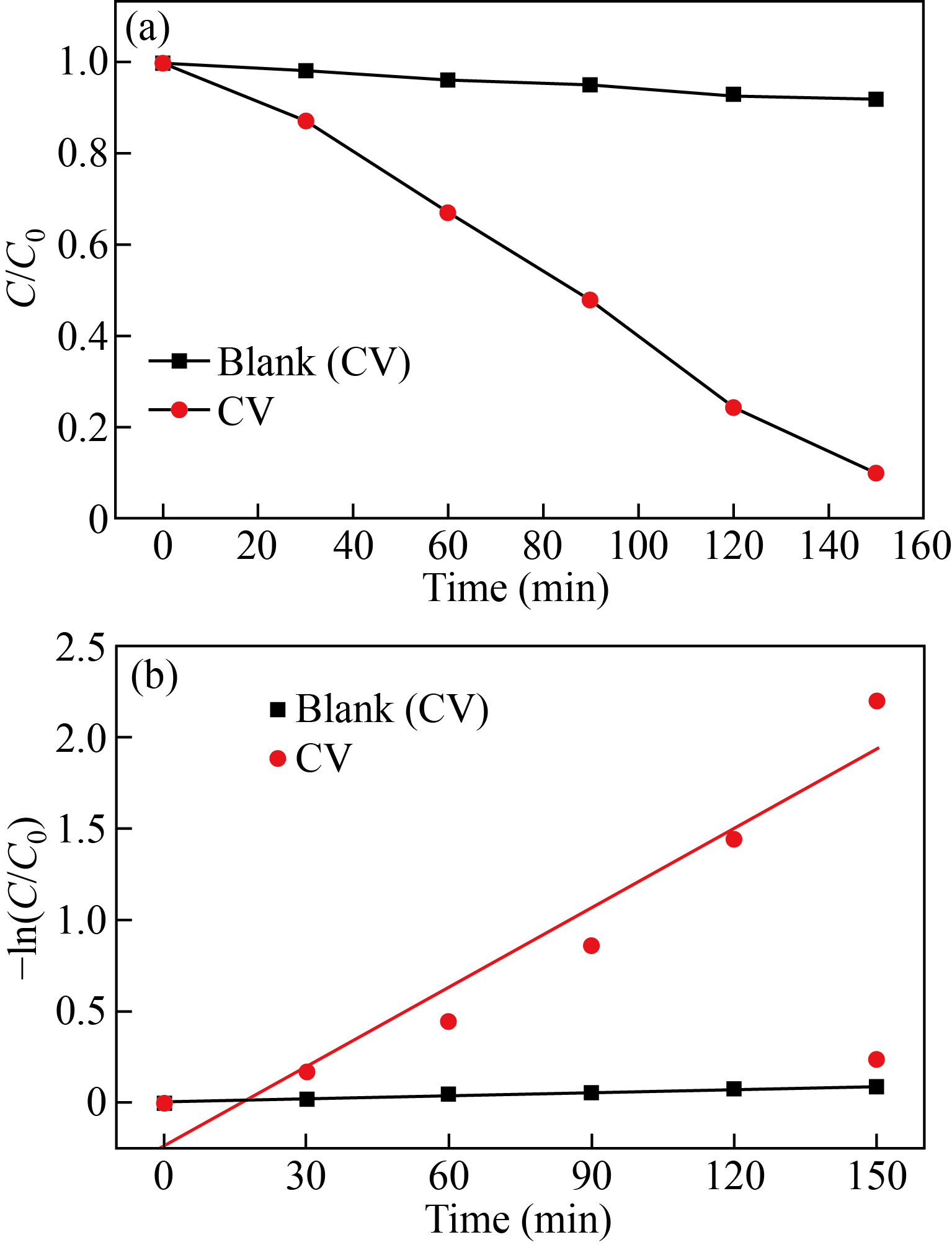
Fig. 13 (a) C/C0 vs. time interval graph of degradation, (b) –ln (C/C0) time interval graph of degradation of CV dye
Conclusion
This study provides a distinctive approach for the green synthesis of α-Fe2O3 NPs using the flower extract of Musa acuminata for the first time. The green synthesis method that takes advantage of using flower extract from a medicinal plant is costly effective and eliminates the need for toxic chemical reducing agents. FTIR and XRD results confirmed the formation of α-Fe2O3 NPs. The HRTEM micrographs showed spherical particles with an average particle size of 32 nm, which showed enhanced performances in both antibacterial and photocatalytic activities. The green synthesized α-Fe2O3 NPs demonstrated significant inhibition activity against E. coli and S.aureus bacterial strains, but superior efficiency was noticed against gram +ve S. aureus. Furthermore, α-Fe2O3 NPs demonstrated significant photocatalytic properties in the degradation of CV dye under sunlight with the degradation phenomenon obeying pseudo first-order kinetics. The results prove that the synthesized α-Fe2O3 NPs using Musa acuminata flower extract were found to be significant antibacterial and catalytic agents. By largescale green synthesis, α-Fe2O3 NPs could be used to treat wastewater, environmental remediation, and used for various bacteria-based biological applications.
Reference
[1] N.V. Srikanth Vallabani, S. Singh. Recent advances and future prospects of iron oxide nanoparticles in biomedicine and diagnostics. 3 Biotech, 2018, 8(6): 279. http://dx.doi.org/10.1007/s13205-018-1286-z
[2] W. Wu, Z.H. Wu, T. Yu, et al. Recent progress on magnetic iron oxide nanoparticles: Synthesis, surface functional strategies and biomedical applications. Science and Technology of Advanced Materials, 2015, 16(2): 023501. http://dx.doi.org/10.1088/1468-6996/16/2/023501
[3] P. Sangaiya, R. Jayaprakash. A review on iron oxide nanoparticles and their biomedical applications. Journal of Superconductivity and Novel Magnetism, 2018, 31(11): 3397–3413. http://dx.doi.org/10.1007/s10948-018-4841-2
[4] L.S. Arias, J.P. Pessan, A.P.M. Vieira, et al. Iron oxide nanoparticles for biomedical applications: A perspective on synthesis, drugs, antimicrobial activity, and toxicity. Antibiotics, 2018, 7(2): 46. https://doi.org/10.3390/antibiotics7020046
[5] Q.Y. Feng, Y.P. Liu, J. Huang, et al. Uptake, distribution, clearance, and toxicity of iron oxide nanoparticles with different sizes and coatings. Scientific Reports, 2018, 8(1): 2082. https://doi.org/10.1038/s41598-018-19628-z
[6] M. Kluenker, M.N. Tahir, R. Dören, et al. Iron oxide superparticles with enhanced MRI performance by solution phase epitaxial growth. Chemistry of Materials, 2018, 30(13): 4277–4288. https://doi.org/10.1021/acs.chemmater.8b01128
[7] A.K. Gupta, M. Gupta. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials, 2005, 26(18): 3995–4021. http://dx.doi.org/10.1016/j.biomaterials.2004.10.012
[8] M. Hjiri, M. Aida, G. Neri. NO2 selective sensor based on α-Fe2O3 nanoparticles synthesized via hydrothermal technique. Sensors, 2019, 19(1): 167. https://doi.org/10.3390/s19010167
[9] H.H. Nguyen, H.K.T. Ta, S. Park, et al. Resistive switching effect and magnetic properties of iron oxide nanoparticles embedded-polyvinyl alcohol film. RSC Advances, 2020, 10(22): 12900–12907. http://dx.doi.org/10.1039/C9RA10101B
[10] S.J. Yu, V.M. Hong Ng, F.J. Wang, et al. Synthesis and application of iron-based nanomaterials as anodes of lithium-ion batteries and supercapacitors. Journal of Materials Chemistry A, 2018, 6(20): 9332–9367. http://dx.doi.org/10.1039/C8TA01683F
[11] X.L. Mou, X.J. Wei, Y. Li, et al. Tuning crystal-phase and shape of Fe2O3 nanoparticles for catalytic applications. CrystEngComm, 2012, 14(16): 5107–5120. http://dx.doi.org/10.1039/C2CE25109D
[12] Komal, H. Kaur, M. Kainth, et al. Sustainable preparation of sunlight active α-Fe2O3 nanoparticles using iron containing ionic liquids for photocatalytic applications. RSC Advances, 2019, 9(71): 41803–41810. http://dx.doi.org/10.1039/C9RA09678G
[13] R. Kant, D. Kumar, V. Dutta. High coercivity α-Fe2O3 nanoparticles prepared by continuous spray pyrolysis. RSC Advances, 2015, 5(65): 52945-52951. https://doi.org/10.1039/c5ra06261f
[14] M. Rincón Joya, J. Barba Ortega, J.O.D. Malafatti, et al. Evaluation of photocatalytic activity in water pollutants and cytotoxic response of α-Fe2O3 nanoparticles. ACS Omega, 2019, 4(17): 17477–17486. https://doi.org/10.1021/acsomega.9b02251
[15] P.C.L. Muraro, S.R. Mortari, B.S. Vizzotto, et al. Iron oxide nanocatalyst with titanium and silver nanoparticles: Synthesis, characterization and photocatalytic activity on the degradation of Rhodamine B dye. Scientific Reports, 2020, 10(1): 3055. https://doi.org/10.1038/s41598-020- 59987-0
[16] A. Lassoued, M.S. Lassoued, B. Dkhil, et al. Synthesis, photoluminescence and Magnetic properties of iron oxide (α-Fe2O3) nanoparticles through precipitation or hydrothermal methods. Physica E: Low-dimensional Systems and Nanostructures, 2018, 101: 212–219. http://dx.doi.org/10.1016/j.physe.2018.04.009
[17] K. Raja, M. Mary Jaculine, M. Jose, et al. Sol-gel synthesis and characterization of α-Fe2O3 nanoparticles. Superlattices and Microstructures, 2015, 86: 306–312. http://dx.doi.org/10.1016/j.spmi.2015.07.044
[18] B. Ahmmad, K. Leonard, M. Shariful Islam, et al. Green synthesis of mesoporous hematite (α-Fe2O3) nanoparticles and their photocatalytic activity. Advanced Powder Technology, 2013, 24(1): 160–167. https://doi.org/10.1016/j.apt.2012.04.005
[19] S. Saif, A. Tahir, Y.S. Chen. Green synthesis of iron nanoparticles and their environmental applications and implications. Nanomaterials, 2016, 6(11): 209. https://doi.org/10.3390/nano6110209
[20] M. Kaur, D.S. Chopra. Green synthesis of iron nanoparticles for biomedical applications. Global Journal of Nanomedicine, 2018, 4(4): 68–77. https://juniperpublishers.com/gjn/pdf/GJN.MS.ID.555643.pdf
[21] P. Singh, Y.J. Kim, D.B. Zhang, et al. Biological synthesis of nanoparticles from plants and microorganisms. Trends in Biotechnology, 2016, 34(7): 588–599. http://dx.doi.org/10.1016/j.tibtech.2016.02.006
[22] M.A. Babu, M. Suriyakala, K. Gothandam. Varietal impact on phytochemical contents and antioxidant properties of Musa acuminata (banana). Journal of Pharmaceutical Sciences & Research, 2012, 4(10): 1950–1955. http://www.pharmainfo.in/jpsr/Documents/Volumes/vol4issue10/jpsr%2004121005.pdf
[23] F. Mashkoor, A. Nasar, Inamuddin, et al. Exploring the reusability of synthetically contaminated wastewater containing crystal violet dye using Tectona grandis sawdust as a very low-cost adsorbent. Scientific Reports, 2018, 8: 8314. https://doi.org/10.1038/s41598-018-26655-3
[24] S. Ledakowicz, K. Paździor. Recent achievements in dyes removal focused on advanced oxidation processes integrated with biological methods. Molecules, 2021, 26:870. https://doi.org/10.3390/molecules26040870
[25] S. Prabhu, T. Daniel Thangadurai, P. Vijai Bharathy, Pon. Kalugasalam, Investigation on the Photocatalytic and Antibacterial Activities of Green synthesized Cupric Oxide Nanoparticles using Clitoria ternatea. Iranian Journal of Catalysis, 2022, 12(1): 1–11. http://ijc.iaush.ac.ir/article_689547_0313d1a492d8eaaf1cb2115343c5a242.pdf
[26] S. Prabhu, D.T. Thangaian, P.V. Bharathy. Green-based biosynthesis of zinc oxide nanoparticles using Clitoria ternatea flower extract and its antibacterial activity. Nano Biomedicine and Engineering, 2021, 13(4): 394-400. https://doi.org/10.5101/nbe.v13i4.p394–400
[27] A. Rufus, S. N, D. Philip. Synthesis of biogenic hematite (α-Fe2O3) nanoparticles for antibacterial and nanofluid applications. RSC Advances, 2016, 6(96): 94206–94217. http://dx.doi.org/10.1039/C6RA20240C
[28] A. Lassoued, B. Dkhil, A. Gadri, et al. Control of the shape and size of iron oxide (α-Fe2O3) nanoparticles synthesized through the chemical precipitation method. Results in Physics, 2017, 7: 3007–3015. http://dx.doi.org/10.1016/j.rinp.2017.07.066
[29] C.P. Devatha, A.K. Thalla, S.Y. Katte. Green synthesis of iron nanoparticles using different leaf extracts for treatment of domestic waste water. Journal of Cleaner Production, 2016, 139: 1425–1435. http://dx.doi.org/10.1016/j.jclepro.2016.09.019
[30] S. Vasantharaj, S. Sathiyavimal, P. Senthilkumar, et al. Biosynthesis of iron oxide nanoparticles using leaf extract of Ruellia tuberosa: Antimicrobial properties and their applications in photocatalytic degradation. Journal of Photochemistry and Photobiology B: Biology, 2019, 192: 74–82. http://dx.doi.org/10.1016/j.jphotobiol.2018.12.025
[31] M. Worden, L. Bergquist, T. Hegmann. A quick and easy synthesis of fluorescent iron oxide nanoparticles featuring a luminescent carbonaceous coating via in situ pyrolysis of organosilane ligands. RSC Advances, 2015, 5(121): 100384–100389. http://dx.doi.org/10.1039/C5RA18382K
[32] S. Prabhu, T. Daniel Thangadurai, P. Vijai Bharathy, et al. Synthesis and characterization of nickel oxide nanoparticles using Clitoria ternatea flower extract: Photocatalytic dye degradation under sunlight and antibacterial activity applications. Results in Chemistry, 2022, 4: 100285. http://dx.doi.org/10.1016/j.rechem.2022.100285
[33] S. Banerjee, M.C. Chattopadhyaya. Adsorption characteristics for the removal of a toxic dye, tartrazine from aqueous solutions by a low cost agricultural by-product. Arabian Journal of Chemistry, 2017, 10(S2): S1629–S1638. http://dx.doi.org/10.1016/j.arabjc.2013.06.005
Copyright© T. Indumathi, N. Krishnamoorthy, R. Valarmathy, K. Saraswathi, S. Dilwyn and S. Prabhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.