Removal of Pollution with Eosin Yellow Dye from Waste Water by Using a Novel Nano Co-polymer
Sameer Kadhim B. Al_Zubaidy1,2*, Mohammad N. Al-Baiati1,2, Emad Salam Abood1,2
1Department of Chemistry, College of Education for Pure Sciences, University of Kerbala, Karbala, Iraq
2Medical Physics Department, Hilla University College, Babylon, Iraq
*Corresponding author. E-mail: sameer.k@s.uokerbala.edu.iq
Received: Oct. 26, 2022; Revised: Nov. 15, 2022; Accepted: Nov. 29, 2022; Published: Nov. 30, 2022
Citation: S.K.B. Al_Zubaidy, M.N. Al-Baiati , E.S. Abood. Removal of pollution with eosin yellow dye from waste water by using a novel nano co-polymer. Nano Biomedicine and Engineering, 2022, 14(3): 272–279.
DOI: 10.5101/nbe.v14i3.p272-279
Abstract
In this study, graft nano co-polymer was synthesized by esterification method of phthalic anhydride and glycerol. Nano polymer was diagnosed by infrared spectroscopy FT-IR, atomic force microscopy technique (AFM), X-ray diffraction analysis (XRD) and differential scanning calorimetry (DSC). Tendency of fabricated nano-polymer to adsorb Eosin yellow from aqueous solutions was evaluated. The average size of the height density of the nano-polymer was 22.04 nm. The effects of three different temperatures (298, 308 and 318 K) and concentrations (1, 3, 5 and 7 ppm) for nano polymer have been studied. The study’s findings made it abundantly evident that manufactured nano-polymers were highly effective in completely removing Eosin yellow from aqueous solutions.
Keywords: Nano co-polymer; Characterization; Adsorption; Eosin yellow
Introduction
A study on the design, installation, characterization and applications of materials, devices and systems controls the shape and size of the nanoscale. As the size of nanomaterials ranges from 1 to 100 nm according to the US National Nano Initiative, the presence of pollutants such as dyes, heavy metals, phenols, drugs and others is very dangerous to the health of human and aquatic life, and artificial dyes are used everywhere in the era of modern technology [1–3]. Since water-soluble dyes are used to colour papers, leather, hair, foods, cosmetics, and clothing, the removal of dyes from industrial wastewater is extremely necessary and has attracted great interest in the past few years due to its toxicity [4]. And the demand for clean water, which is expected to rise rapidly with an accelerating pace of industrialization, poses the disposal of industrial liquid pollutants threats to the environment and becomes a great matter of concern for the sustainable development of human society, and that the reclamation of polluted water and its recycling are among the objectives and improvement of environmental quality [5,6].
In the last few decades, great emphasis has been placed on the exploration of biodegradable polymers for specific applications such as controlled drug delivery, insecticides and pesticide vectors as well as sorbents to remove many harmful components from the aqueous system [7]. The adsorption method on a nano polymer's surface is one of the most crucial of these techniques. When we observe that most researchers have a propensity to making use of nanoparticles to create novel and high-quality materials, larger and more adsorbents are required because the particles on the interactions at the nanoscale differ from those at larger scales [8]. Although many commercial polymers are available, they are almost resistant to microbial attack, and survival of such polymers is a major problem and a potential source for creating an environment. Therefore, the aim of the study was to develop new nano-polymers of phthalic anhydride and glycerine specifically designed to absorb some pollutants such as dyes from wastewater. There are various ways that monomers can be arranged into a common polymer along the spine. Sequence-controlled polymers are co-polymers with carefully chosen monomer arrangements. Simple examples of sequentially managed polymers are mass copolymers, cyclic and co-rotating polymers [9].
Material and Methods
The chemicals employed in this investigation, including phthalic anhydride, glycerol, and others, were of analytical high quality chemical compounds.
Synthesis of nanoparticles co-polymers
About 200 mL beaker (3.0 mole, 444 g) of phthalic anhydride and (48 mL) of DMSO, were mixed together. This beaker was equipped with a thermometer. The mixture warmed carefully with a hot plate magnetic stirrer to 70℃ until clear liquor is formed and added (1.0 mole, 92 g) of glycerol to the solution. The mixture warmed carefully to 100℃ then 15 mL of xylene was added carefully to the reaction beaker, in the form of batch, withdrawal of water formed by the esterification process, and the beaker was gently heated. Heating was stopped after (71 min at 125℃), until no more water came off to prepare the nano co-polymer. Then add the cold distilled water, where the suspension solution is formed. Leave the suspension solution to precipitate overnight, then filtrate and wash with distilled water and leave to dry [10].
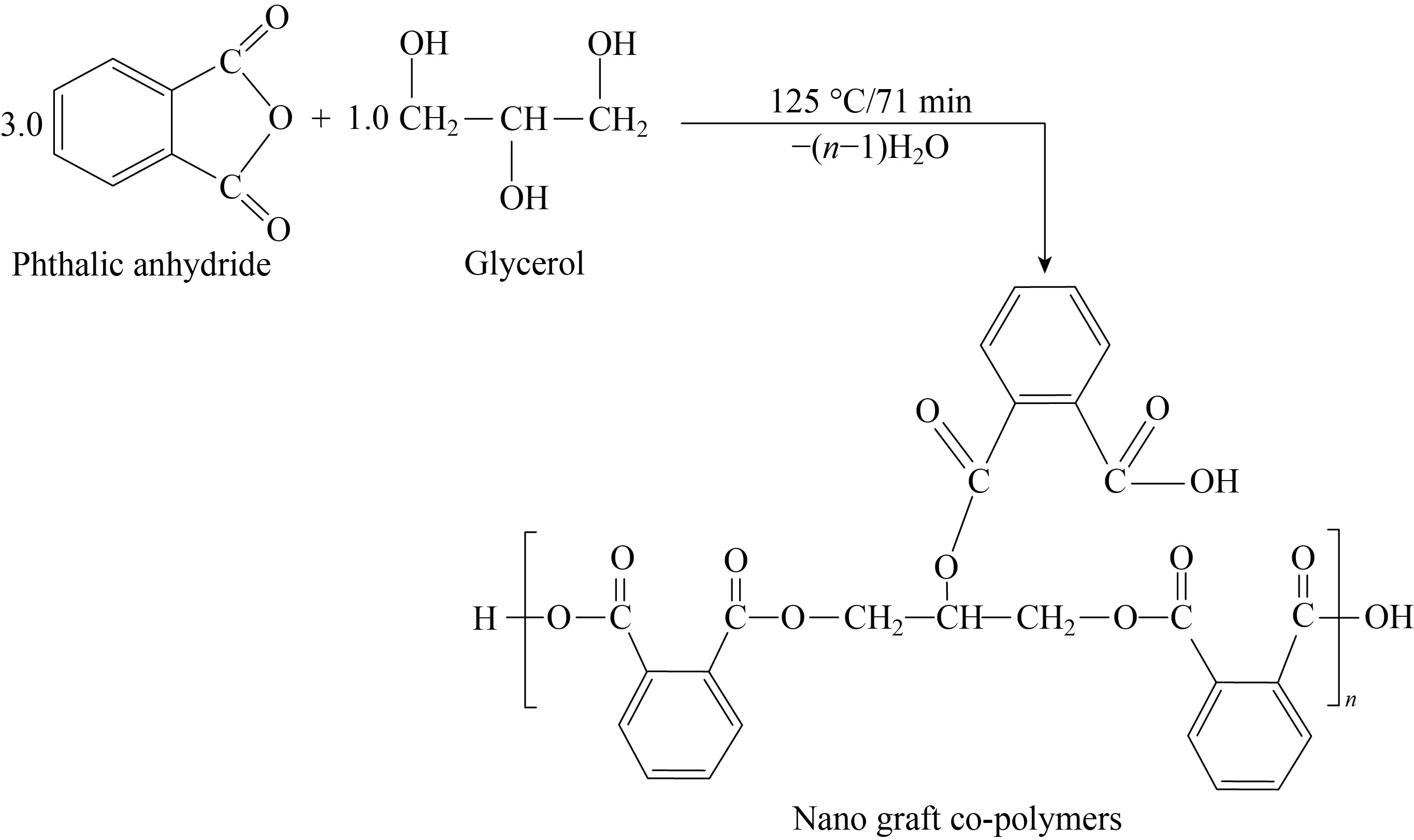
Fig. 1 Steps of polymer preparation
Purification of polymer
After isolation, purification is required because the generated polymer can contain traces of solvent, an unreacted monomer, or other impurities. The polymer was dissolved in the appropriate solvent, in accordance with the described procedure [10], and 5% concentration of non-solvent was added while being violently agitated.
The non-solvent was miscible with the polymer and the solvent. The solid polymer was separated from the solution as a result of precipitation. The entire re-dissolving and re-precipitating procedure was completed three times in order to improve the polymer's quality [11]. After being purified, the nano co-polymer was vacuum dried at 50℃ and maintained in a vacuum desiccator until it could be further characterized and used [12].
Adsorption behavior of grafting nano co-polymers
The adsorption of a new nano-polymer on dyes was examined using the following technique to generate dye solutions (Eosin yellow): A solution of Eosin yellow dye was made by immersing 0.5 g of the dye in a certain amount of distilled water and then diluted to 1000 mL to establish a concentration (500 parts per million). From this concentrated solution, the dilute solutions were prepared in concentrations 1, 3, 5 and 7 ppm by taking the appropriate volume of the concentrated solution and then diluting it with 100 mL distilled water. Its absorption was measured after 60 min of taking the vials containing the solution, which consists of a nano polymer adsorbent surface with a weight of 0.02 and the dye adsorbent (Eosin yellow), from being placed in the vibrator at a temperature of 298 K. Then samples were taken from them at successive times and the absorbance was measured through the spectral changes of visible and ultraviolet rays over time. The number of adsorbed dyes (Eosin yellow) on the surface of the new nano polymer was calculated from the next equation [13]:
![]() (1)
(1)
Results and Discussion
Characterization of grafting nano co-polymer
Figure 2 showed the FT-IR spectrum which appears a very weak broad band at (3093—3401 cm-1) attributed to the bond (O—H) carboxylic acid and H-bond, showed a stretching band at (3067 cm-1 and 2887 cm-1) attributed to the bond (C—H) aromatic and a asymmetric (C—H) aliphatic bond, also a strong stretching band at (1757 cm-1) attributed to the carbonyl bond (C==O), and stretching bands at (1402, 1468 and 1595 cm-1) attributed to (C—C) aromatic, showed strong sharp peak at (1107 cm-1) attributed to the bond (C—O) ester, and showed bands at (709 and 903 cm-1 ) attributed to disubstitution of aromatic ring.
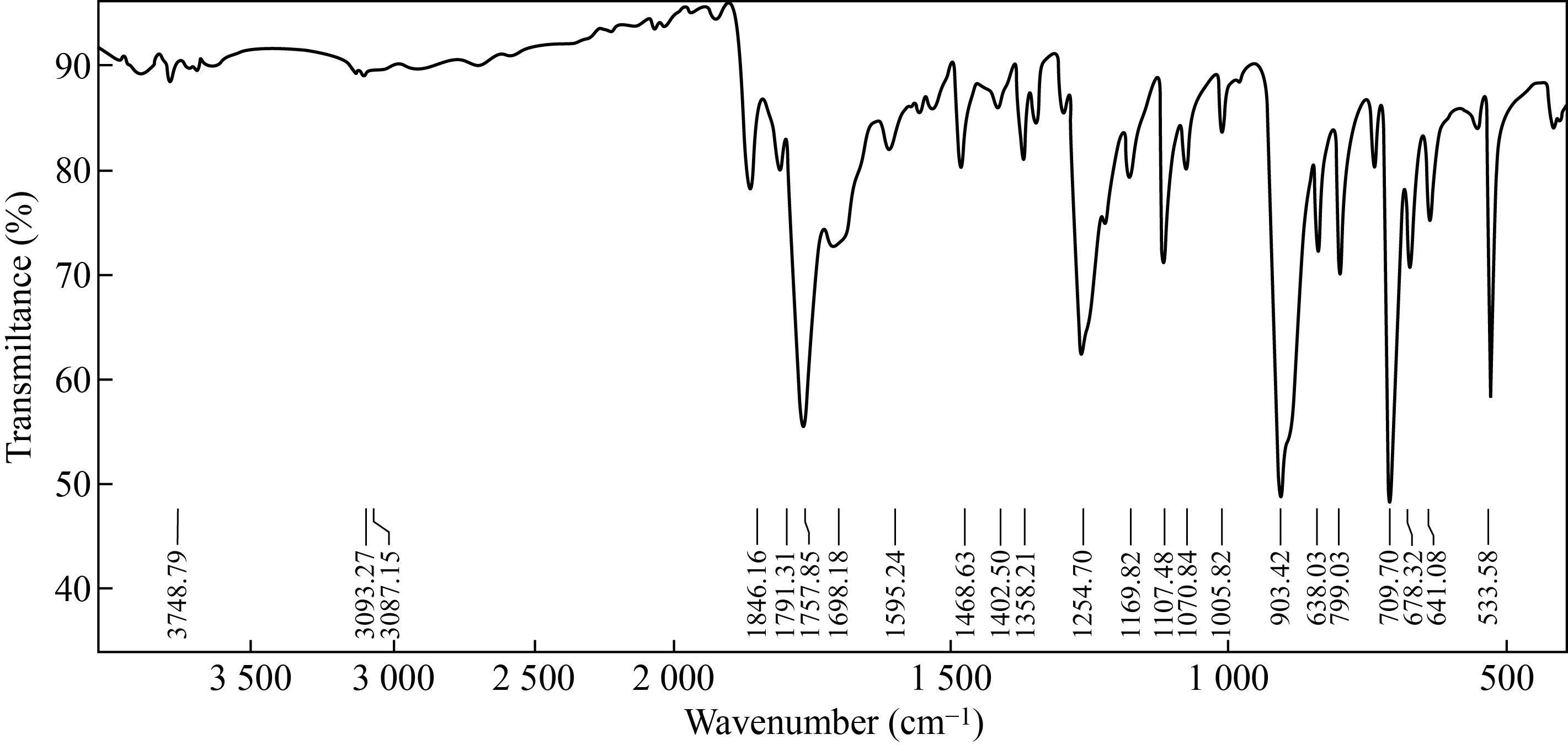
Fig.2 FT-IR of graft nano co-polymer
Figure 3 shows the 1H-NMR spectrum, which explains the singlet signal at 13.04 ppm of characteristic proton in the carboxylic acid group. Moreover, the multiple in the region 7.55—7.68 ppm attributed to all protons in the aromatic ring, the signals at 4.26—4.28 ppm for four methylene protons in the co-polymer structure, and multiple at 4.15 ppm of methyl protons, but aliphatic alcohol signal has disappeared indicating the formation of a graft co-polymer.
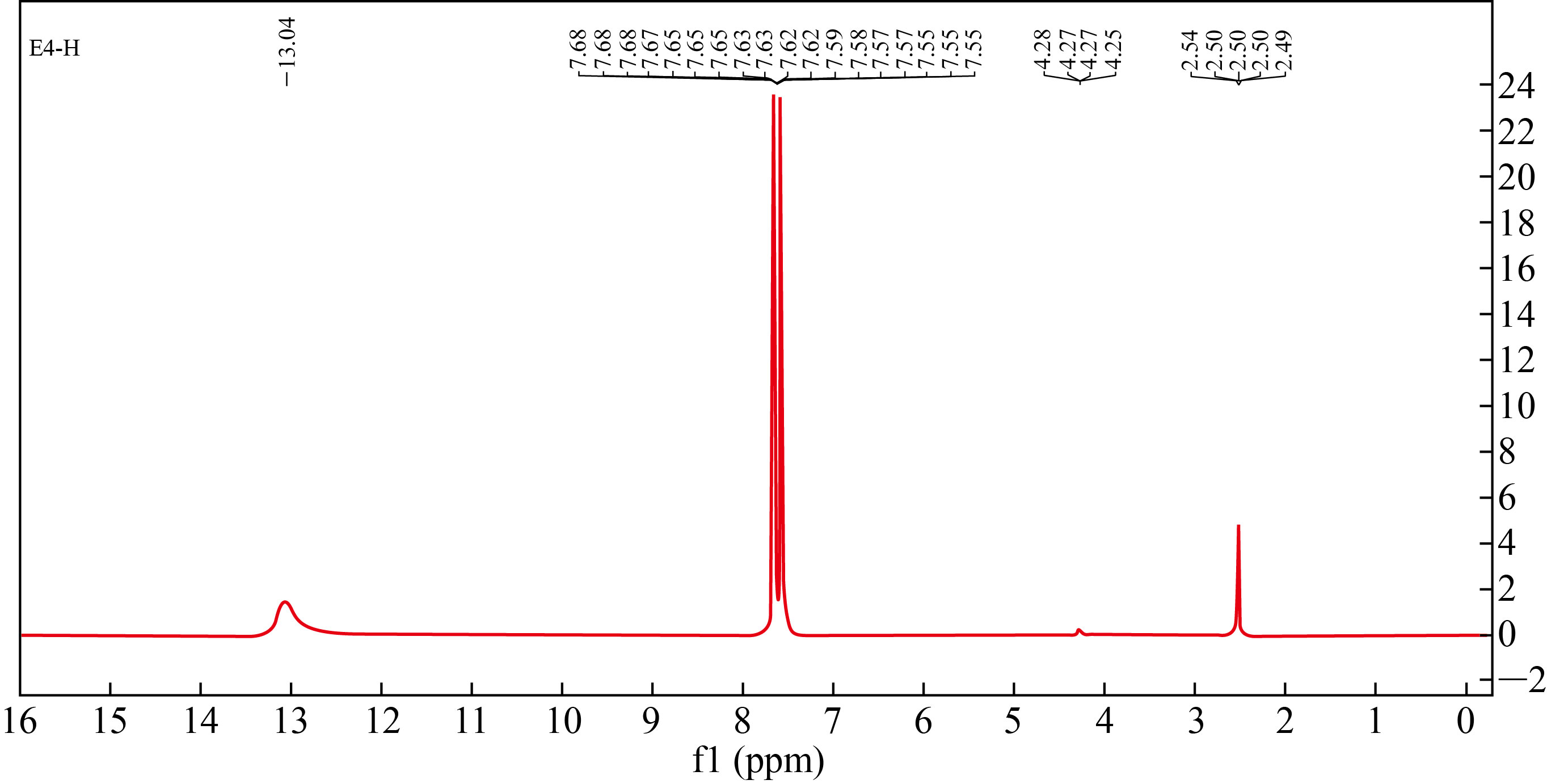
Fig. 3 1H-NMR spectrum of nano co-polymer
Figure 4 shows the outer surface of the nanoparticles of co-polymer. The roughness coefficient of co-polymer surface was 5.08 nm and the square root square was equal to 5.94 nm. This indicates that the bold size of the nanoparticles plays an important role in the roughness of the surface, its uniform crystalline system, and the surface homogeneity. Also, the average of height of the particles was equal to 22.04 nm, as observed in Fig. 3(a). Table 1 represents the total rate of the particle sizes of the common nanoparticle and the different proportions of these volumes; the results indicate that the molecular size of the co-polymer nanoparticle was 68.62 nm.
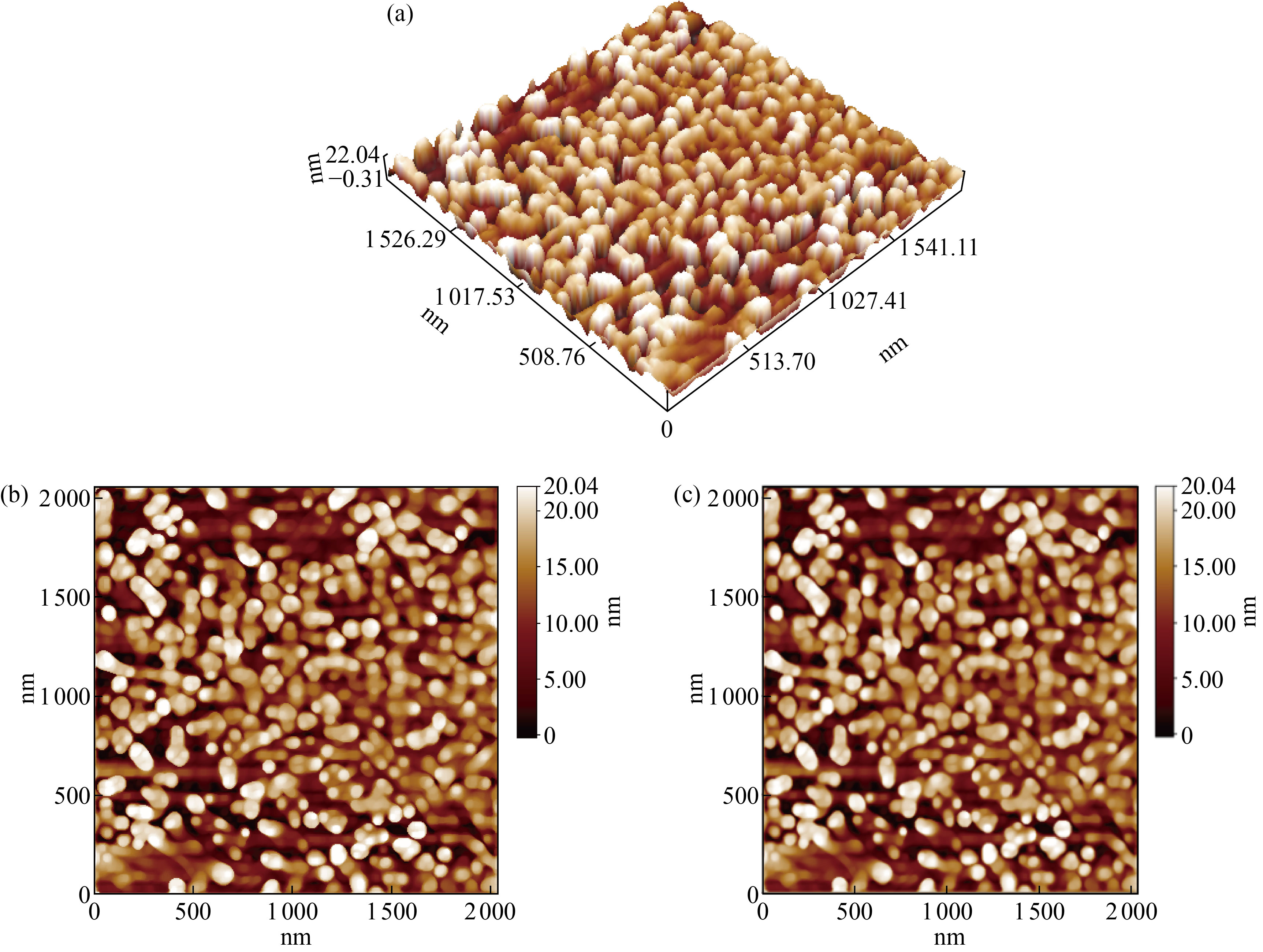
Fig. 4 Image of atomic force microscope for nano co-polymer: (a) 3D image, (b) 2D image, and (c) 2D image showing all details of particles
Table 1 Total rate of the particle sizes of the nanoparticle co-polymer and different proportions of these volumes
Diameter (nm) | Volume (%) | Cumulation (%) | Diameter (nm)< | Volume (%) | Cumulation (%) | Diameter (nm)< | Volume (%) | Cumulation (%) |
45.00 50.00 55.00 60.00 | 1.52 4.17 9.09 8.33 | 1.52 5.68 14.77 23.11 | 65.00 70.00 75.00 80.00 | 17.80 12.12 13.64 12.50 | 40.91 53.03 66.67 79.17 | 85.00 90.00 | 11.74 9.09 | 90.91 100.00 |
The X-ray diffraction (XRD) in the nanoparticle's co-polymer (Fig.5) shows peaks at 2θ values of 15.4°, 18.5°, 21.2°, 22.2°, 26.9° and 30.5°. These peaks indicated that the new co-polymer has been formed as a crystalline compound with less of amorphous carbon atoms. By using origin software, the average inters planer spacing between atoms (dhkl) was 0.415 nm according to Bragg's law:
nλ = 2dsinθ
The total average crystallites size was 68.48 nm relative to Scherrer's equation:
D=kλ/(βcosθ)
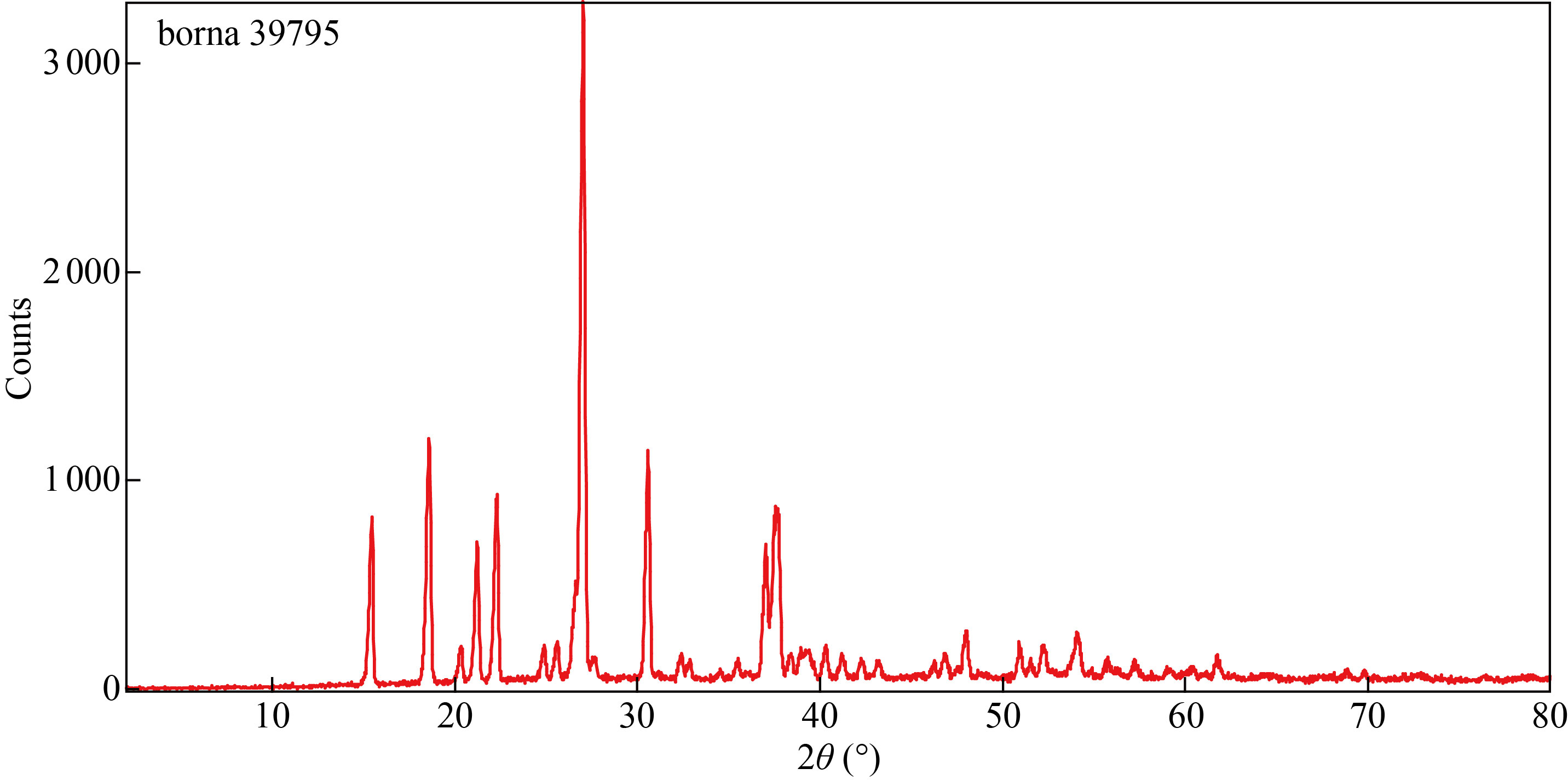
Fig. 5 X-ray diffraction in the nanoparticle’s co-polymer
The TEM micrographs of different sizes and shapes of the nanoparticle’s co-polymer (Fig. 6) were observed with irregular particles such as vertebras with different thicknesses shapes, semi-spherical in the form of layers and rods shapes. An average particle size of the co-polymer nanoparticle was found to be 68.08 nm. Table 3 shows the proportions diameters, angels and standard deviations of the nano co-polymer using image software and Fig. 7 represents the histogram for distribution of the different proportions of particle sizes of the nano co-polymer.
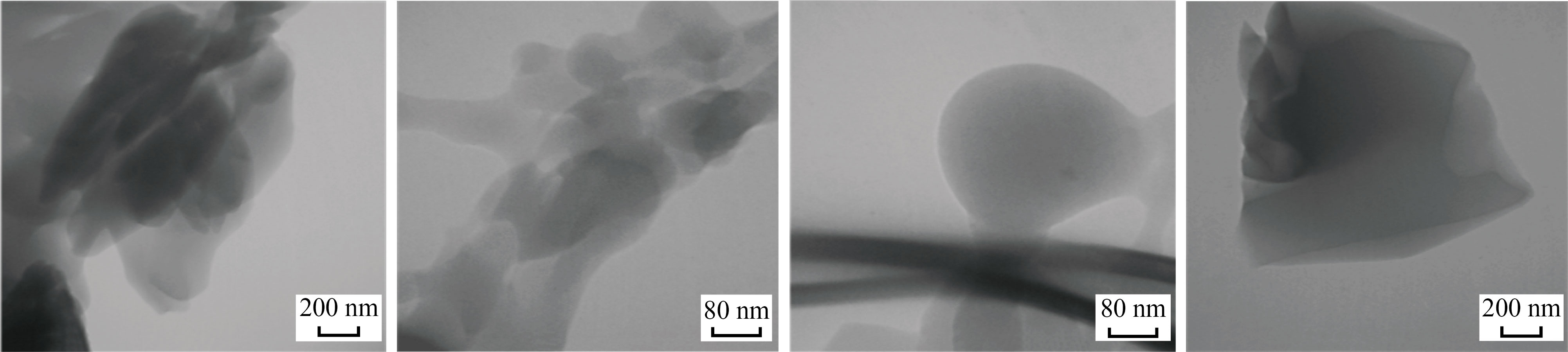
Fig. 6 TEM micrographs for the nanoparticle’s co-polymer
Table 2 Proportions crystallites sizes and the distances between atoms (d-spacing) in the nano co-polymer
hkl (nm) | (nm) | dhkl (nm) | D(nm) | FWHM | θ(°) | 2θ(°) |
0.4152 | 68.4874 | 0.57421 | 77.77159 | 0.10308071 | 7.70944 | 15.41888 |
|
| 0.47764 | 66.10961 | 0.12176233 | 9.28079 | 18.56158 |
|
| 0.41868 | 69.58317 | 0.11615246 | 10.60181 | 21.20363 |
|
| 0.39898 | 68.94823 | 0.11743049 | 11.13191 | 22.26382 |
|
| 0.32998 | 61.54072 | 0.13275784 | 13.49960 | 26.99920 |
|
| 0.29230 | 66.97155 | 0.12296861 | 15.27956 | 30.55913 |
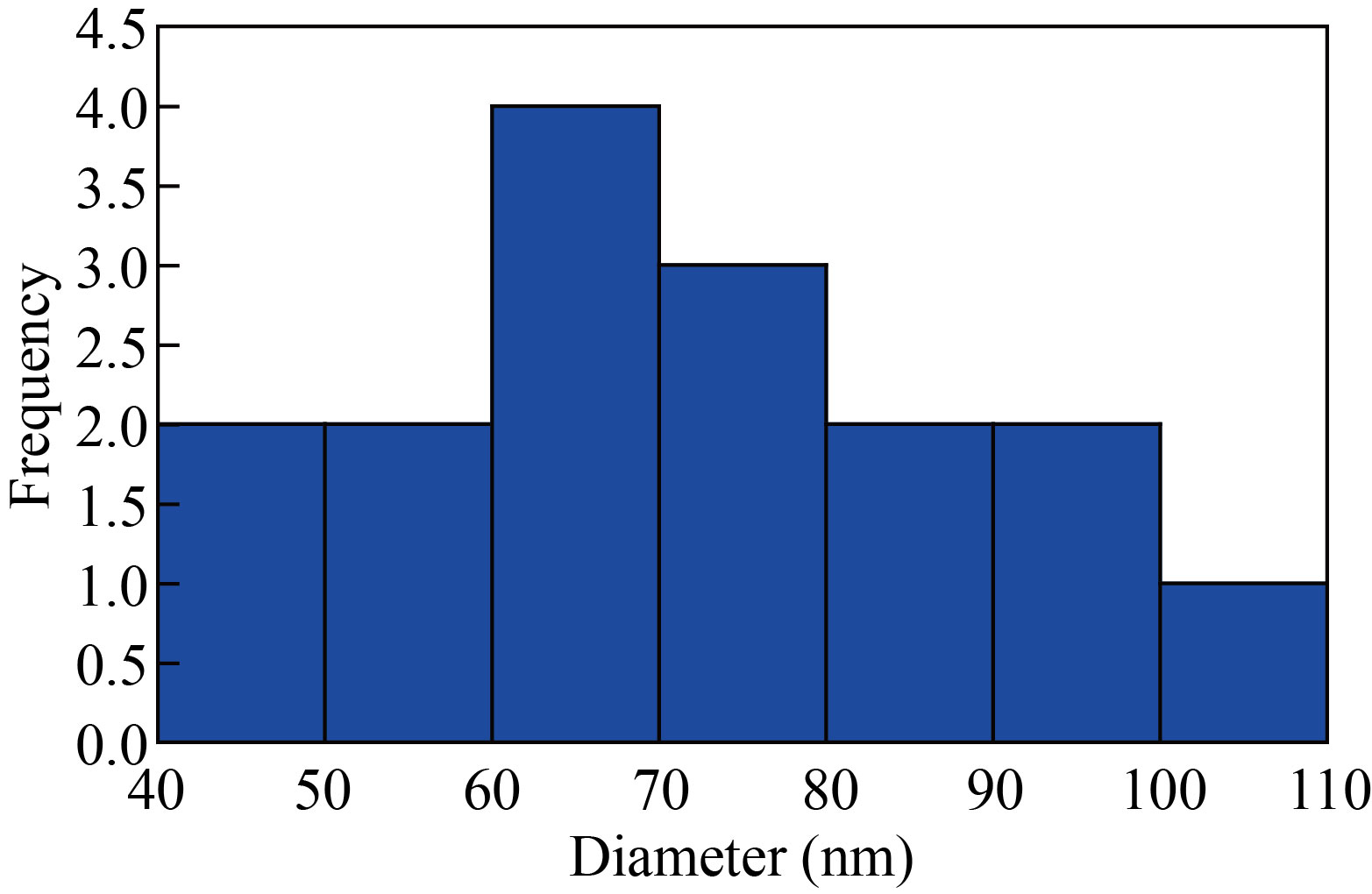
Fig. 7 Histogram for distribution of the different proportions of particle sizes of the nano co-polymer
Table 3 Proportions diameters, angels and standard deviations of the nano co-polymer
Area | StdDev | Angel(o) | D (nm) | D (nm) |
14.775 | 7.288 | 8.13 | 45.617 | 68.084 |
14.879 | 6.905 | -51.843 | 45.944 |
|
16.231 | 13.912 | -78.111 | 50.104 |
|
17.896 | 7.185 | 90 | 55.481 |
|
21.225 | 11.734 | 90 | 65.803 |
|
21.954 | 17.472 | -76.759 | 67.6 |
|
22.578 | 22.617 | -93.18 | 69.781 |
|
22.682 | 6.093 | 94.236 | 69.864 |
|
20.393 | 8.205 | -135.83 | 72.958 |
|
24.555 | 10.457 | -48.447 | 75.862 |
|
19.457 | 9.157 | -37.117 | 79.868 |
|
27.364 | 10.744 | 23.429 | 84.371 |
|
19.04 | 5.677 | -74.745 | 88.844 |
|
30.486 | 9.79 | -88.431 | 94.223 |
|
31.942 | 9.633 | 52.98 | 98.575 |
|
32.566 | 15.94 | -80.417 | 100.755 |
|
Figure 8 showed the DSC thermos grams for the nano co-polymer, the first thermal transition at the peak (48.68℃) represents the glass transition temperature (Tg), the second transition at the peak (134.10℃) represents the crystallization temperature (Tc), and the third and fourth transitions at the peaks (197.60℃ and 302.19℃) represents the melting temperature (Tm1 and Tm2) respectively for the nano co-polymer.
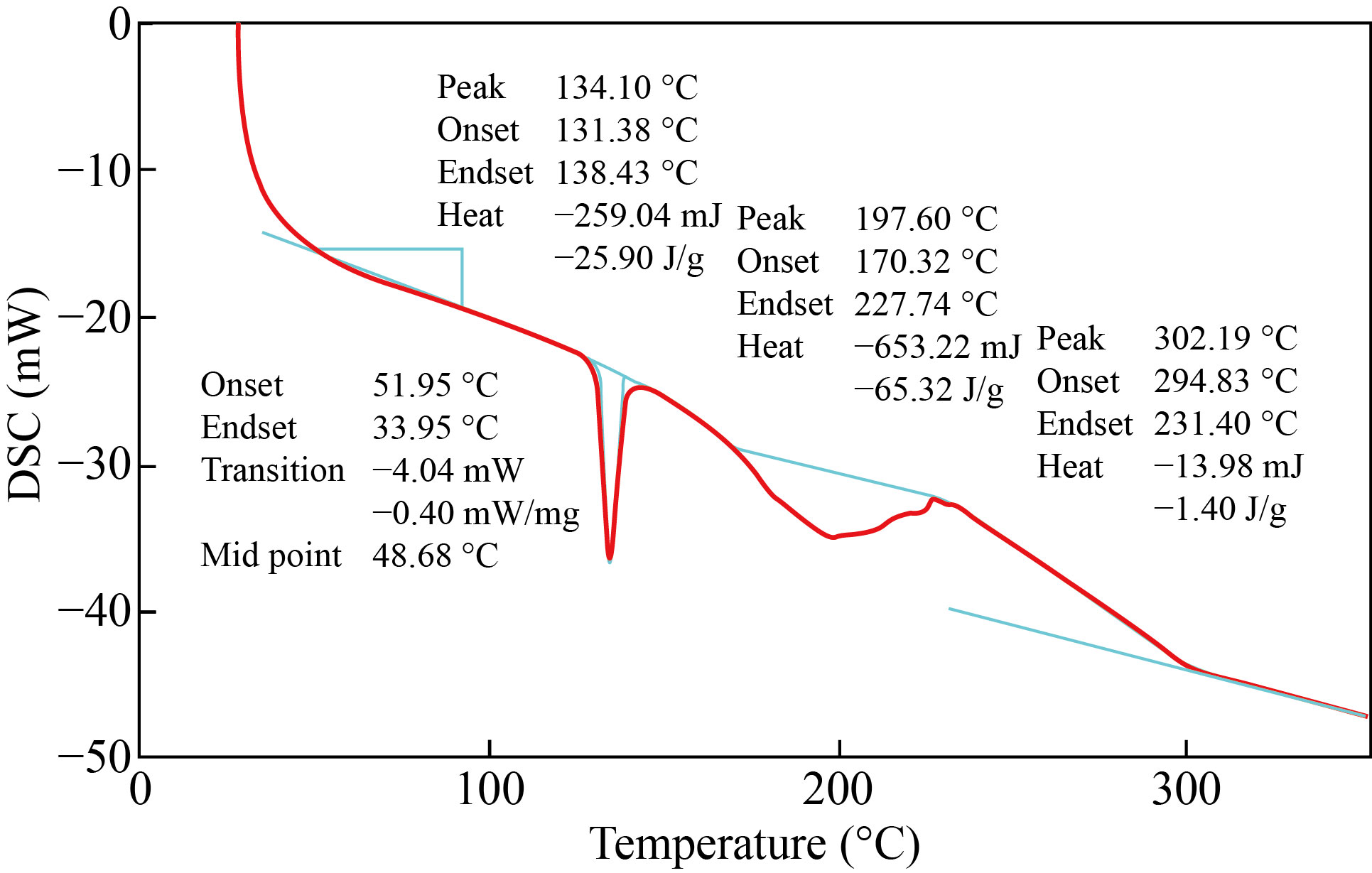
Fig. 8 DSC thermos grams of nano co-polymer
Adsorption of Eosin yellow
The titration curve represents the relationship between absorbance and concentration through the graph as shown in Fig. 9. Four generations (1, 3, 5 and 7 ppm) from the Eosin yellow solution utilized in the investigation were employed to identify it, and these concentrations’ absorbance was measured at their highest wavelength (520 nm).
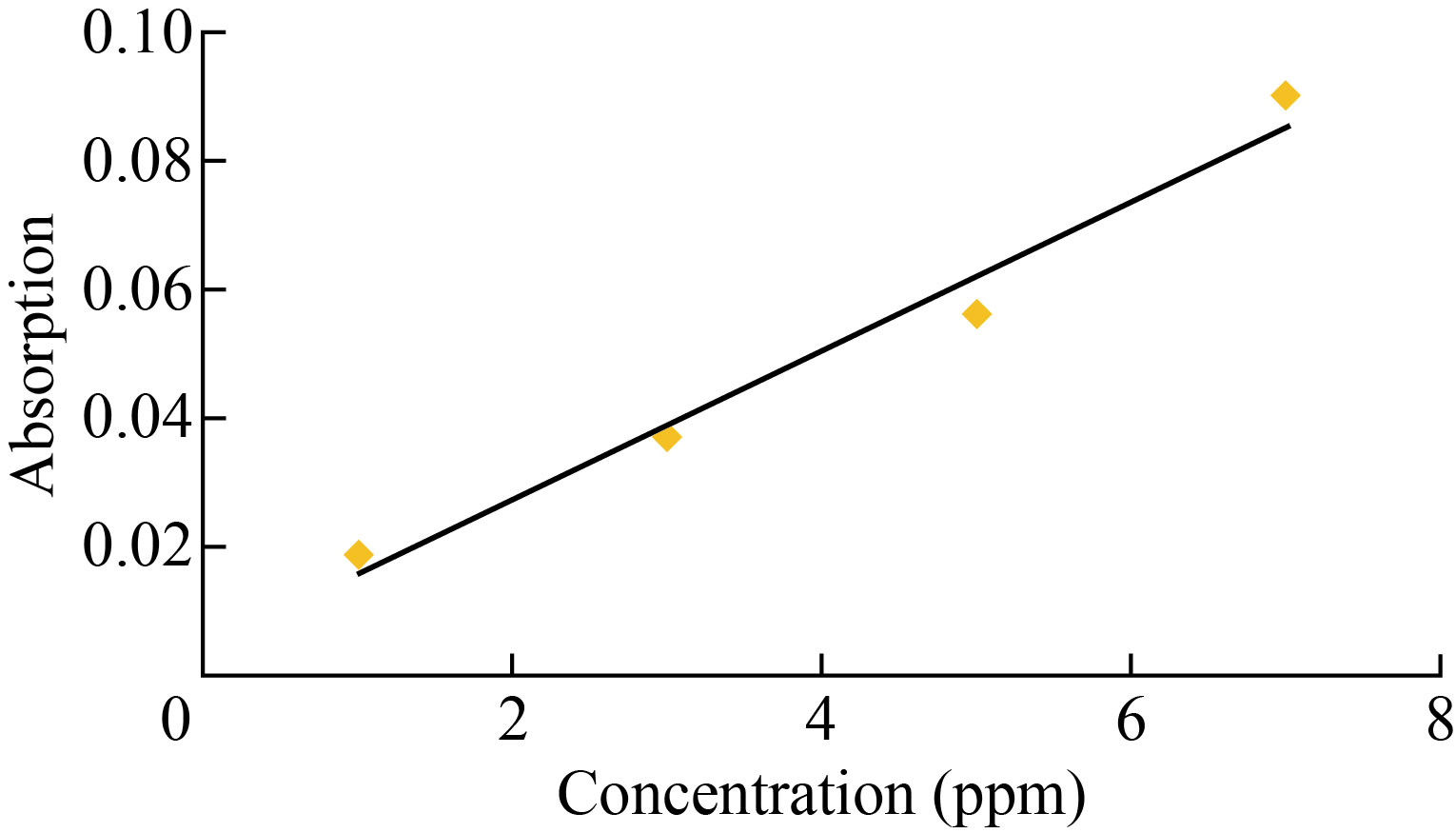
Fig. 9 Calibration curve between absorption and concentration of Eosin yellow
Table 4 displays the results of a study on the thermal range effects of temperature on the adsorption of Eosin yellow pigment on the surface of polymer nanoparticles (298, 308 and 318 K). The Eosin yellow pigment’s adsorption on the surface of these nanoparticles that were created decreased with increasing temperature, indicating that the process is exothermic, according to the experimental data [14]. This suggests the existence of a desorption process, which is the separation of the adsorbed particles on the adsorbent surface and their return to the solution [15], lowering the speed of molecular diffusion with an increase in temperature [16], as illustrated in Fig. 10.
Table 4 Effect of temperature on adsorption of Eosin yellow
Concentration(ppm) | 298K | 308K | 318K | ||||
Ce (mg/L) | Qe (mg/g) | Ce (mg/L) | Qe (mg/g) | Ce (mg/L) | Qe (mg/g) | ||
1 | 0.1637 | 0.6969 | 0.25 | 0.625 | 0.3362 | 0.5531 | |
3 | 0.3362 | 2.2198 | 0.5086 | 2.0761 | 0.681 | 1.9325 | |
5 | 0.4224 | 3.8146 | 0.5948 | 3.671 | 0.7672 | 3.5273 | |
7 | 0.5086 | 5.4095 | 0.681 | 5.2658 | 0.8534 | 5.1221 | |
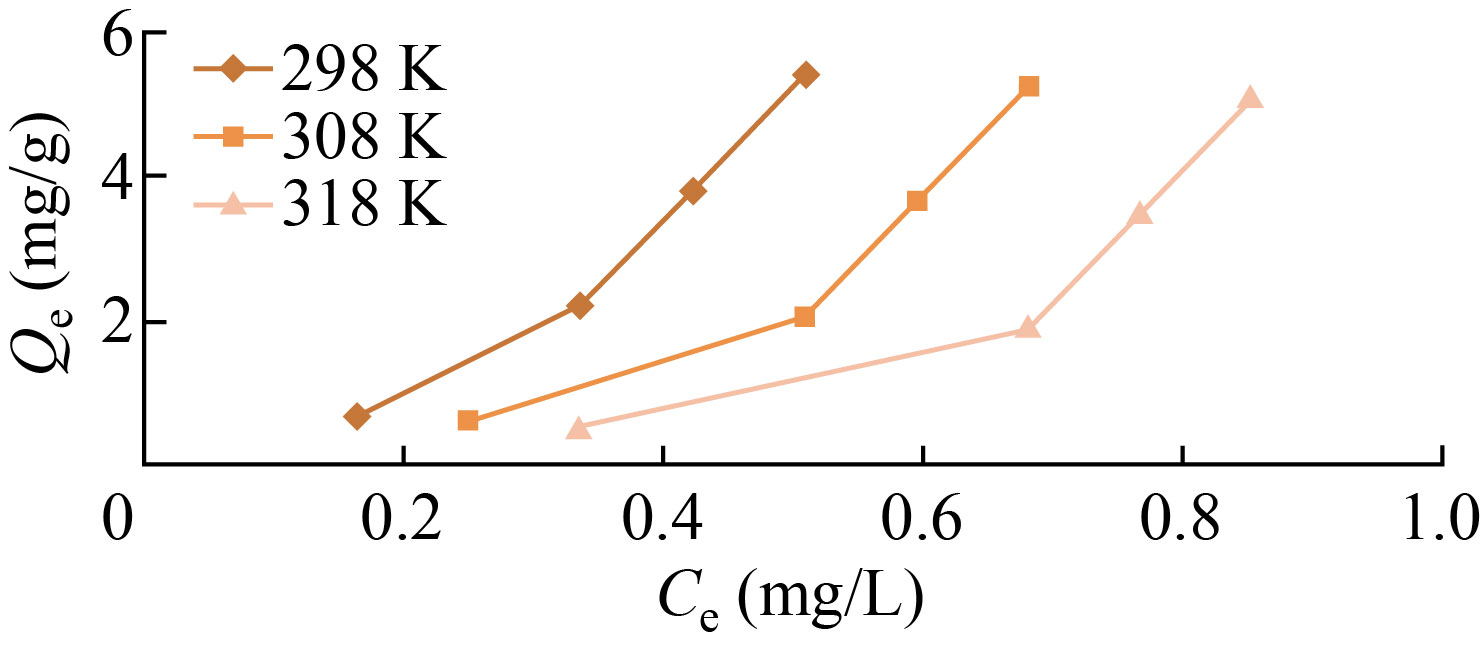
Fig. 10 Effect of temperature on adsorption of graft co-polymer at 1, 3, 5 and 7 ppm of Eosin yellow dye
Adsorption isotherms
Researchers looked at the adsorption of dyes (Eosin yellow) on the nano-copolymer and calculated adsorption isotherms at a temperature of 298 K, as shown in Fig. 11, and this suggests that the adsorbent’s surface is not uniform. Additionally, the overall shape of the adsorption isotherms, as determined by the Giles classification, is Type S1 [17].
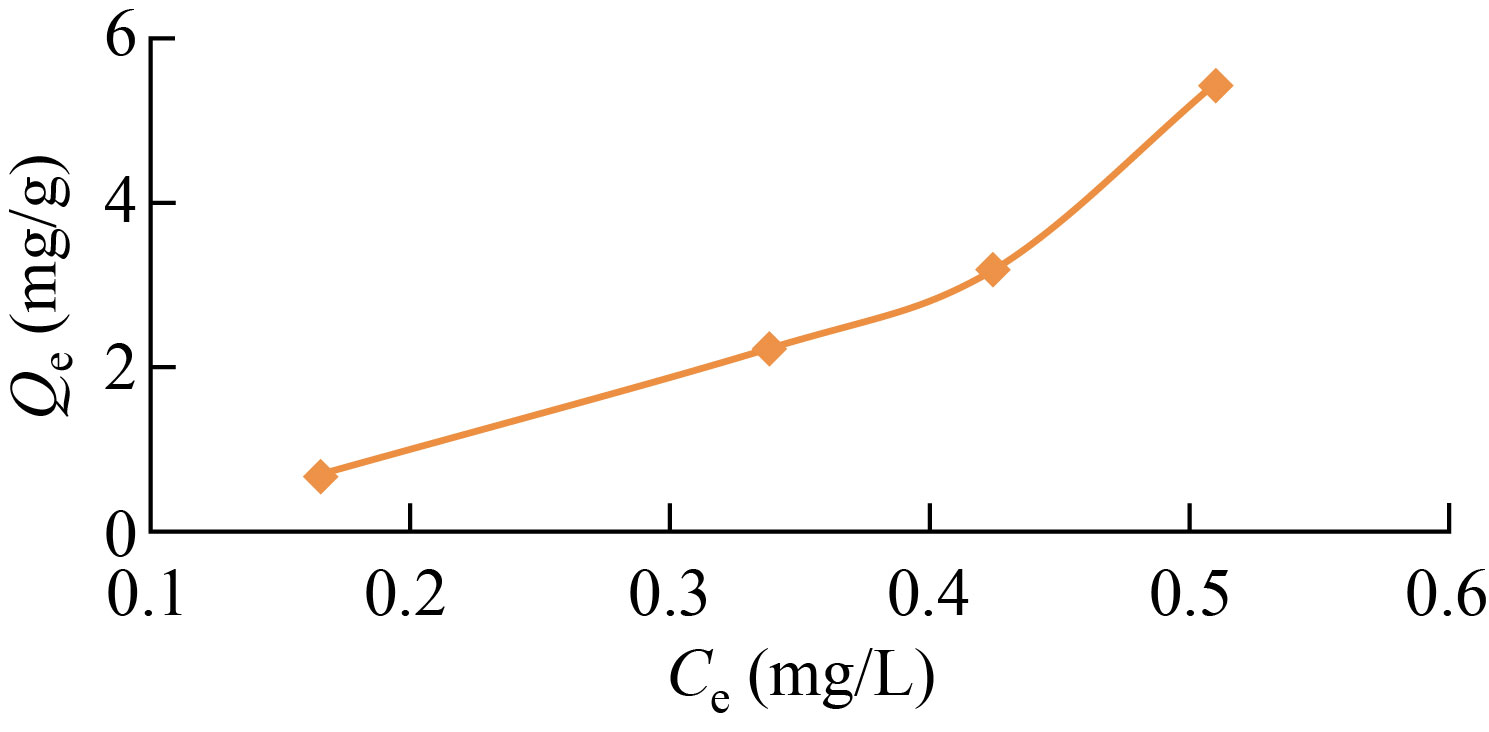
Fig. 11 Adsorption isotherm Eosin yellow dye on the surface of graft co-polymer
Freundlich equation for adsorption
One of the most significant isothermal equations was created by German scientist Freundlich to
describe the adsorption of solutions on the surfaces of heterogeneous materials [18]. The Freundlich equation has the following mathematical formula:
Qe=KfC1/n e
Figure 12 illustrates the result of depicting the link between logQe and logCe as a straight line. While Tables 6 and 7 provide the findings of the Freundlich equation for the adsorption of Eosin yellow dye on the surface of nano co-polymer at 298 K.
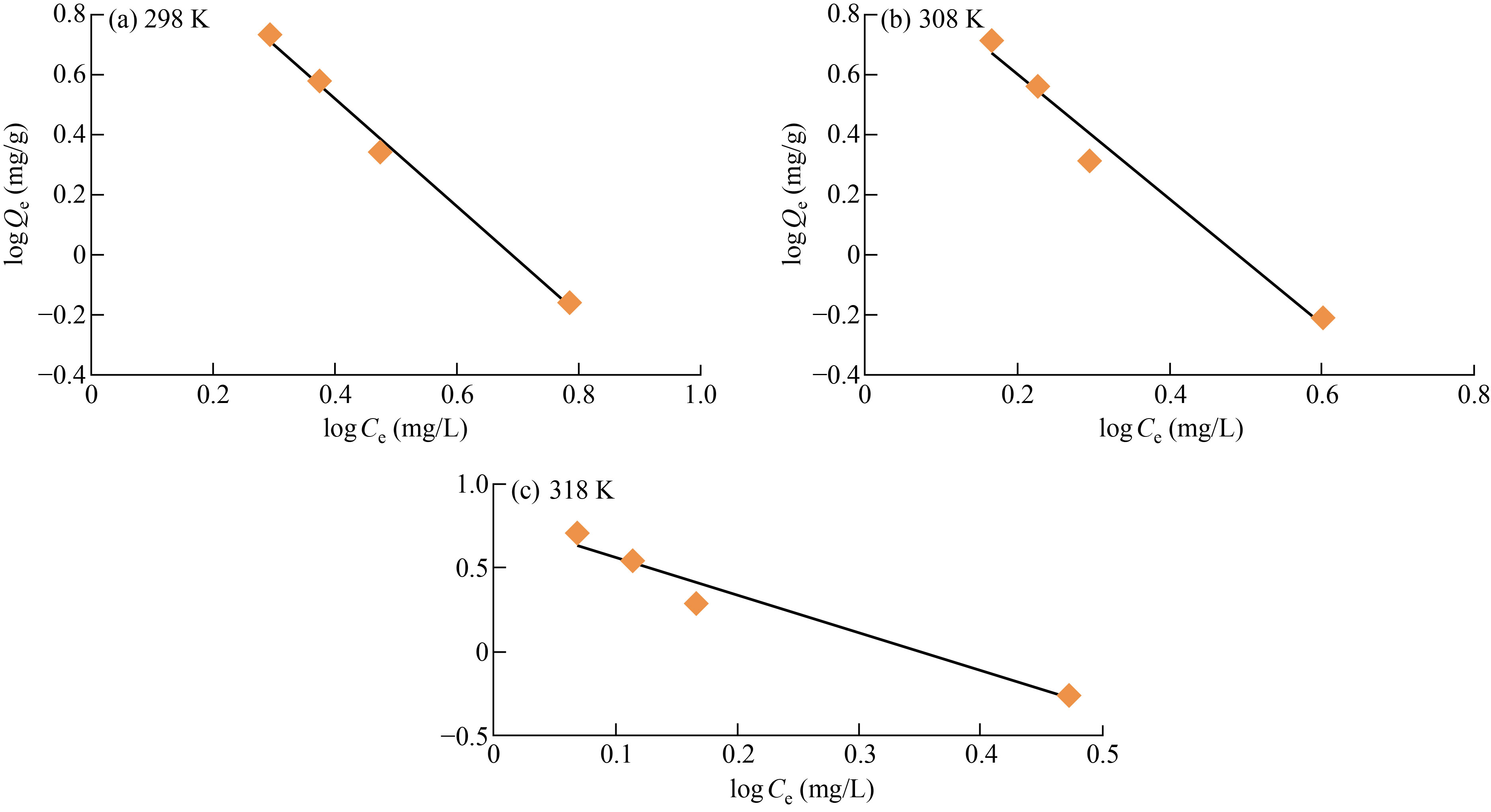
Fig. 12 Apply Freundlich equation on adsorption of Eosin yellow dye on the surface of graft co-polymer at (a) 298 K, (b) 308 K and (c) 318 K
Table 6 Adsorption of Eosin yellow dye on the surface of graft co-polymer at 298K,308K and 318K (by applying Freundlich equation)
Concentration(ppm) | 298K | 308K | 318K | |||
LogCe- | LogQe | LogCe- | LogQe | LogCe- | LogQe | |
1 | 0.7859 | -0.1568 | 0.602 | -0.2041 | 0.4734 | -0.2571 |
3 | 0.4734 | 0.3463 | 0.2936 | 0.3172 | 0.1668 | 0.2861 |
5 | 0.3742 | 0.5814 | 0.2256 | 0.5647 | 0.115 | 0.5474 |
7 | 0.2936 | 0.7331 | 0.1668 | 0.7214 | 0.0688 | 0.7094 |
Table 7 Values of Freundlich constants for adsorption of Eosin yellow dye on the surface of nano co-polymer at 298K
R2 | Kf | -N | Temperature (K) |
0.9941 | 17.49444 | 0.55577 | 298 |
0.9761 | 10.34904 | 0.48412 | 308 |
0.9581 | 6.146105 | 0.44096 | 318 |
Conclusion
The novel nano-copolymer between phthalic anhydride and glycerol was synthesized with a certain temperature. The XRD results indicated the presence of a crystal structure, while the AFM images confirmed the nanostructure of the polymer. The adsorption of Eosin yellow dye on the nano polymer was studied and the results showed that the adsorption of Eosin yellow dye was effective at normal temperature and neutral pH.
Reference
[1] M.F. Anad, H.E. Salman, M.N. Al-Baiati. Synthesis a novel nano graft co-polymer and studying the swelling behaviors using different molar ratios of acrylic acid monomer. IOP Conference Series: Materials Science and Engineering, 2019, 571: 012096. https://doi.org/10.1088/1757-899X/571/1/012096
[2] V. Selvaraj, T. Swarna Karthika, C. Mansiya, et al. An over review on recently developed techniques, mechanisms and intermediate involved in the advanced azo dye degradation for industrial applications. Journal of Molecular Structure, 2021, 1224: 129195. http://dx.doi.org/10.1016/j.molstruc.2020.129195
[3] F.A. Razzak Mageed, M.M. Kareem, M.N. Al-Baiati. Preparation and characterization of new carrier drug polymers based maleimide and its drug release behaviour. Asian Journal of Chemistry, 2019, 31(3): 569–574. https://doi.org/10.14233/ajchem.2019.21638
[4] Z.M. Abd Al-Aama, M.N. Al-Baiati. Synthesis of a new co-polymer and studying its ability as drug delivery system. Journal of Pharmaceutical Sciences and Research, 2018, 10(4): 723–732. http://www.jpsr.pharmainfo.in/Documents/Volumes/vol10Issue04/jpsr10041807.pdf
[5] M.N. Al-Baiati. Preparation of a new acrylonitrile copolymer and studying the flammability and mechanical properties of its composites. Journal of Global Pharma Technology, 2017, 9(5): 1–10. http://ceps.uokerbala.edu.iq/wp/wp-content/uploads/2018/07/Preparation-of-a-New-Acrylonitrile-Co-Polymer-and-Studying-the.pdf
[6] M.N. AL-Baiati, N. NA Jafar, R.H. Zaooly. Study the effect verifies of the number of moles of acrylic acid monomer on swelling of the new prepared modified copolymer. Research Journal of Pharmaceutical, Biological and Chemical Sciences, 2016, 7(5): 1452–1463. https://www.rjpbcs.com/pdf/2016_7(5)/[184].pdf
[7] H.M. Awwad, A.F. Alkaim, M.N. Al-Baiati. Adsorption of Maxilon Blue (GRL) from Aqueous Solutions by using a novel nano-composite polymer. IOP Conference Series: Materials Science and Engineering, 2019, 571: 012095. https://doi.org/10.1088/1757-899x/571/1/012095
[8] M. Sajid, M.K. Nazal, Ihsanullah, et al. Removal of heavy metals and organic pollutants from water using dendritic polymers based adsorbents: A critical review. Separation and Purification Technology, 2018, 191: 400–423. https://doi.org/10.1016/j.seppur.2017.09.011
[9] H. AL-Masoudi, M.N. AL-Baiati. Studying the flammability of some organic sulfur compounds on two types of the thermosetting polymers. International Journal of Pharmaceutical Research, 2018, 10(4): 466–474. https://doi.org/10.31838/ijpr/2018.10.04.076
[10] M. Parlapiano, Ç. Akyol, A. Foglia, et al. Selective removal of contaminants of emerging concern (CECs) from urban water cycle via Molecularly Imprinted Polymers (MIPs): Potential of upscaling and enabling reclaimed water reuse. Journal of Environmental Chemical Engineering, 2021, 9(1): 105051. http://dx.doi.org/10.1016/j.jece.2021.105051
[11] B. Jeong, M.R. Kibbey, J.C. Birnbaun, et al. Thermogelling biodegradable polymers with hydrophilic backbones: PEG-g-PLGA. Macromolecules, 2000, 33(22): 8317–8322. http://dx.doi.org/10.1021/ma000638v
[12] H. Yagoub, L. Zhu, M.H.M.A. Shibraen, et al. Complex aerogels generated from nano-polysaccharides and its derivatives for oil-water separation. Polymers, 2019, 11(10): E1593. https://doi.org/10.3390/polym11101593
[13] A.F. Hasan, M.M. Kareem, M.N. Al-Baiati. Synthesis a novel nano co-polymer and using as carrier drug system. International Journal of Pharmaceutical Research, 2020, 12(4): 850–859. https://doi.org/10.31838/ijpr/2020.12.04.120
[14] H.E. Salman, N.J. Hussein. Synthesis of zinc-aluminum layered double hydroxides and application of adsorption for nitrate from water. IOP Conference Series: Materials Science and Engineering, 2019, 571: 012070. https://doi.org/10.1088/1757-899X/571/1/012070
[15] H.E.Salman, M.A. Mohammed, M.A. Abdulzahra. Electronic structure, thermodynamics functions and physical properties for (E)-2-cyano-3-(2,4-dichlorophenyl) acrylic acidderivatives by using Ab intio calculations(DFT-model). European Academic Research, 2017, 5(2): 1421–1431. https://euacademic.org/UploadArticle/3193.pdf
[16] K.G. Akpomie, F.A. Dawodu, K.O. Adebowale. Mechanism on the sorption of heavy metals from binary solution by a low cost montmorillonite and its desorption
potential. Alexandria Engineering Journal, 2015, 54(3): 757-767. http://dx.doi.org/10.1016/j.aej.2015.03.025
[17] C.H. Giles, D. Smith, A. Huitson. A general treatment and classification of the solute adsorption isotherm. I. Theoretical. Journal of Colloid and Interface Science, 1974, 47(3): 755–765. http://dx.doi.org/10.1016/0021-9797(74)90252-5
[18] S. Gita, A. Hussan, T.G. Choudhury. Impact of textile dyes waste on aquatic environments and its treatment. Environment & Ecology, 2017, 35(3C): 2349–2353. https://www.researchgate.net/publication/321443064_Impact_of_Textile_Dyes_Waste_on_Aquatic_Environments_and_its_Treatment
Copyright© Sameer Kadhim B. Al_Zubaidy, Mohammad N. Al-Baiati and Emad Salam Abood. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.