Conventional Multiplex PCR for Identifying Soil Transmitted Helminthes (Ascaris Lumbricoides, Trichuris Trichura and Ancylostoma Duodenale) in Fecal Specimens
Ghada Basi Ali Alomashi1, Hasan Raheem Khudhur2*, Layla Safar Jebur3
1Department of Microbiology and Parasitology, College of Medicine, University of Al-Qadisiyah, Diwaniya, Iraq
2College of Health and Medical Techniques, Sawa University, Samawah, Iraq
3Department of Clinical Laboratory Sciences, College of Pharmacy, University of Al-Qadisiyah, Diwaniya, Iraq
*Corresponding author. E-mail: hasan.raheem.k@sawa-un.edu.iq
Received: Jan. 10, 2022; Revised: Jun. 12, 2022; Accepted: Dec. 29, 2022; Published: Dec. 31, 2022
Citation: G.B.A. Alomashi, H.R. Khudhur, L.S. Jebur. Conventional multiplex PCR for identifying soil transmitted helminthes (Ascaris lumbricoides, Trichuris trichura and Ancylostoma duodenale) in fecal specimens. Nano Biomedicine and Engineering, 2022, 14(4): 289–294.
DOI: 10.5101/nbe.v14i4.p289-294
Abstract
Ascaris lumbricoides, Trichuris trichiura, and Ancylostoma duodenale are soil-transmitted helminths (STHs) and medically neglected in Iraq country in spite of their effect on the public health. A cross sectional study was performed in the Maternity and Childhood Teaching Hospital and General Education Hospital in Al-Dewanyia Province, included 850 tool samples collected from patients who attended to the O&P Lab. General stool examination (GSE), direct wet mount method DWMM and Kato-Katz were using for diagnosis of STH infections through detecting the adult and the ovum of the helminthes. A conventional multiplex PCR assay was used for detection of STHs in fecal samples. Based on microscopic examination, the results showed that 275/850 range among triple, double and single infection on other hand was 365/850 range among triple, double and single infection. In conclusions, the investigative sensitivity of DWMM is notable for STH; in exception, it is capable to identify patients with the intention of highest requirement of management, and therefore contributes to the universal target to reduce STH as a community healthiness trouble.
Keywords: Multiplex PCR; Ascais lumbricoides; Trichuris trichura; Ancylostoma duodenale; Fecal specimens
Introduction
Soil transmitted helminthes (STH) are a group related to nematode helminthes responsible for high
morbidity in many countries with low economically status. With growing stress and a new strain on disease mapping and eradication, infections mostly remain undiagnosed due to lack of trained personnel and appropriate technologies [1]. Intermittent shedding of eggs or larvae further makes the diagnosis difficult. Thus, there is a dire need of rapid and accurate tests for the diagnosis of STHs [2]. The diagnostic methods include conventional and molecular methods for the accessibility of the exact, and low coast diagnostic measure is of dominant importance to worldwide control and suppression efforts [3, 4]. The soil transmitted helminthes are recurrently occurred in distant and lack distress areas where lowest hygiene, soil transmitted helminthes Ascaris lumbricoides, Trichuris trichiura, and hookworms (Necator americanus and Ancylostoma duodenale) are the most helminthes infestations worldwide [5, 6].
Material and Methods
Samples
Eight hindered and fifty stool samples were collected from patient who attended to the O&P Lab in the Maternity and Childhood Teaching Hospital and General Education Hospital in Al-Dewanyia Province from the beginning of May 2019 to the ending of May 2021. Ethical agreement of the project was obtained from the Medical Ethics Committee.
General stool examination (GSE) was done and also the Kato-Katz approach was used for detecting the egg and adult of the worms [7]. Formal-ether concentration (FEC) method has lower specific gravity than the helminthes, thus concentrating the worms’ eggs in the sediment [8]. About 2 g of all samples were conserved in 5 mL of 70% ethanol alcohol.
Diagnosis of Ascaris lumbricoides, Trichuris trichiura, and Ancylostoma duodenale was conducted by detecting an egg in a wet mount through making a smooth and thin preparation (with saline and iodine) and covering with a cover glass [9].
For extracting the DNA from stool samples, 0.5 mL of conserved ethanol alcohol stool samples were twice washed with deionizer sterile distal water and were freezing rapidly at –20 °C. DNA was extracted using stool DNA separation Mini-Kit (Bioneer. Korea). Concentrations of DNA were resoluted via NanoDrop® spectrophotometer ND-1000 (NanoDrop Technologies, Wilington, DE), and the extracted DNA was situated at –20 ℃ for analysis.
Primers
Primers for detection Ascaris lumbricoides, Trichuris trichiura, and Ancylostoma duodenale [10, 11] were used as listed in Table 1.
Table 1 Specific primers for detection of Ascaris lumbricoides, Trichuris trichiura and Ancylostoma duodenale using in conventional multiplex PCR [11]
Primer of helminthes spp. target DNA | Sequence 5'-3' | Accession number | Length (nt) | Amplification (bp) | |
A. lumbricoides COI | F | 5’ GGAGGTTTTTGGGTCTTTGG 3 | EU582499 | 20 | 192 |
R | 5’ CCAAACAAGGTAGCCAACCA 3’ | 20 | |||
A. duodenale ITS2 r DNA | F | 5´-GAT GAG CAT TGC WTG AAT GCC G-3´ | KC896800 | 20 | 330 |
R | 5´-GCA AGT RCC GTT CGA CAA ACA G-3 | 20 | |||
T. trichiura 18rS DNA | F | 5’ CTGCGAGGATTGACAGATCA 3’ | GQ352548 | 20 | 498 |
R | 'GTACAAAGGGCAGGGACGTA 3'5 | 20 | |||
hGPDH |
| 5’ GCATCCTGGGCTACACTGAG 3’ | XM005253678 | 20 | 150 |
| 5’’ TGCTGTAGCCAAATTCGTTG 3’ | 20 | |||
Statistical Analysis
Data were translated into a computerized database structure. An expert statistical advice was sought for. Statistical analyses were computer assisted using SPSS version 21 (Statistical Package for Social Sciences). Frequency distribution for the selected variables was done first. The statistical significance, direction and strength of linear correlation were between 2 quantitative variables, one of which being non normally distributed was measured by Spearman’s rank linear correlation coefficient. P value less than the 0.05 level of significance was considered statistically significant [12].
Results
The studied cases were classified into three categories based on results of microscope and conventional multiplex PCR into single, double and triple infection.
The microscopically result shows that a total patients infected with STH (Ascais lumbricoides, Trichuris trichura and Ancylostoma duodenale) was 275/850 range among triple, double and single infection as listed in Table 2 and Fig. 1.
As shown in Table 2 and Fig. 1, the single infection of Ascais lumbricoides was the highest among those with Trichuris trichiura, and Ancylostoma duodenale, while the lowest was Ancylostoma duodenale. The percent of double infection was lower than single infection while the triple infection was the lowest. The difference in P value among three helminthes was no significant while among three categories (single, double and triple), the infection was statistically significant.
Table 2 Percent of single, double and triple infection of STH based on microscopical examination
Microscopical examination |
Positive results |
Percent (%) |
P value |
Ascais lumbricoides | 64 | 0.07 |
NS |
Trichuris trichura | 55 | 0.06 | |
Ancylostoma duodenale | 35 | 0.04 | |
Total single infection | 154 | 18.11 | P <0.05 |
Ascais lumbricoides and Trichuris trichura | 43 | 0.05 |
NS |
Ascais lumbricoides, and Ancylostoma duodenale | 33 | 0.03 | |
Trichuris trichura and Ancylostoma duodenale | 30 | 0.03 | |
Total double infection | 106 | 12.47 | P <0.05 |
Total of triple infection Ascaris lumbricoides, Trichuris trichiura, and Ancylostoma duodenale | 15 | 1.17 |
|
Total infection | 275 | 32.35% | P<0.01HS |
Note: HS: High significant association (P <0.01); NS: no significant.
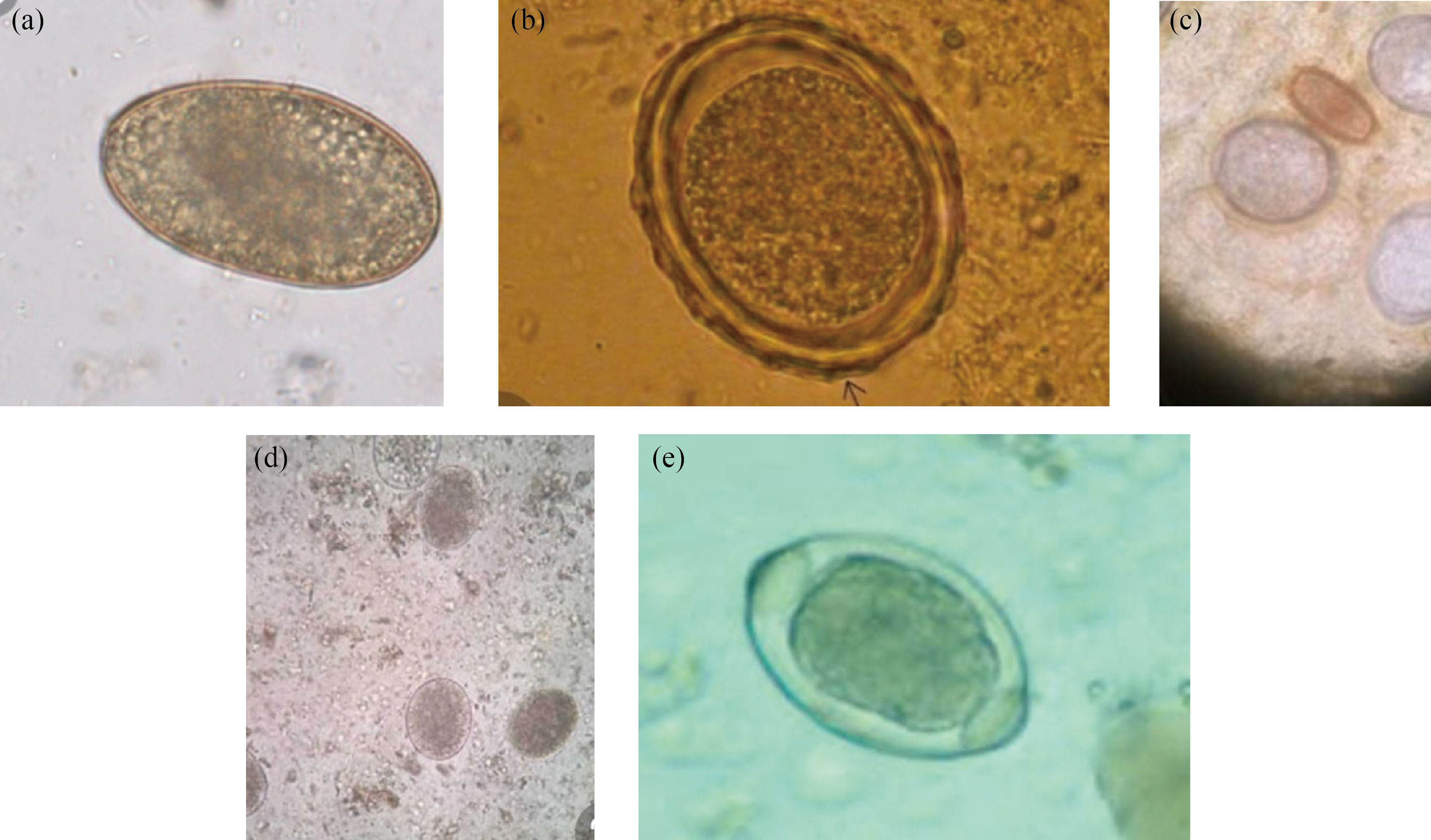
Fig. 1 (a) Ancylostoma douodenale ova; (b) Ascaris lumbrecoides ova; (c) double infection Ascais lumbricoides and Trichuris trichura ovum; (d) double infection Ascais lumbricoides, and Ancylostoma duodenale ovum; (e) Trichuris trichura ova. X = 400
The result shows that a total patients infected with STH (Ascais lumbricoides, Trichuris trichura and Ancylostoma duodenale) were 365/850 range among triple, double and single infection as listed in Table 3.
Table 3 Percent of single, double and triple infection of STH based on multiplex PCR examination
Multiplex PCR methode |
Positive results |
Percent(%) |
P value |
Ascais lumbricoides | 81 | 0.09 |
NS |
Trichuris trichura | 70 | 0.08 | |
Ancylostoma duodenale | 48 | 0.05 | |
Total single infection | 199 | 23.41 | P <0.05 |
Ascais lumbricoides and Trichuris trichura | 58 | 0.06 |
NS |
Ascais lumbricoides, and Ancylostoma duodenale | 49 | 0.05 | |
Trichuris trichura and Ancylostoma duodenale | 39 | 0.04 | |
Total double infection | 146 | 19 | P <0.05 |
Total of triple infection Ascaris lumbricoides, Trichuris trichiura, and Ancylostoma duodenale | 20 | 0.02 |
|
Total infection | 365 | 42.41 | P<0.01HS |
Note: HS: High significant association (P < 0.01).
The multiplex PCR was detected in the form of A. lumbricoides COI, T. trichiura 18rS DNA and A. duodenale ITS2 rDNA. The results showed that a single infection was 0.09%, 0.08% and 0.05% respectively, while a double and triple infection was 19% and 0.02%, as listed in Table 4.
Table 4 Validity parameters for conventional multiplex PCR and microscopically examination(%)
Multiplex PCR | Sensitivity | Specificity | Accuracy | PPV at pretest probability = | NPV at pretest probability = | ||
Positive if ≥ cut-off value |
|
|
| 10% | 50% | 10% | 50% |
Single infection Ascaris lumbricoides, | 95.0 | 87.0 | 78.9 | 64.9 | 97.3 | 76.5 | 98.4 |
Single infection Trichuris trichiura, | 90.0 | 85.0 | 89.3 | 56.8 | 92.2 | 100.0 | 100.0 |
Single infection Ancylostoma duodenale | 94.5 | 100.0 | 97.6 | 89.0 | 100.0 | 88.9 | 47.1 |
double infection Ascaris lumbricoides, Trichuris trichiura |
89.0 |
87.8 |
93.5 |
|
|
|
|
Double infection Ascaris lumbricoides, Ancylostoma duodenale | 100.0 | 98.5 | 98.8 | 98.5 | 99.8 | 100.0 | 100.0 |
Double infection Trichuris trichiura, and Ancylostoma duodenale | 81.3 | 100.0 | 96.4 | 100.0 | 100.0 | 84.2 | 37.3 |
Total of triple infection Ascaris lumbricoides, Trichuris trichiura, and Ancylostoma duodenale |
87.0 |
100 |
96.0 |
91.0 |
100.0 |
86.3 |
38.8 |
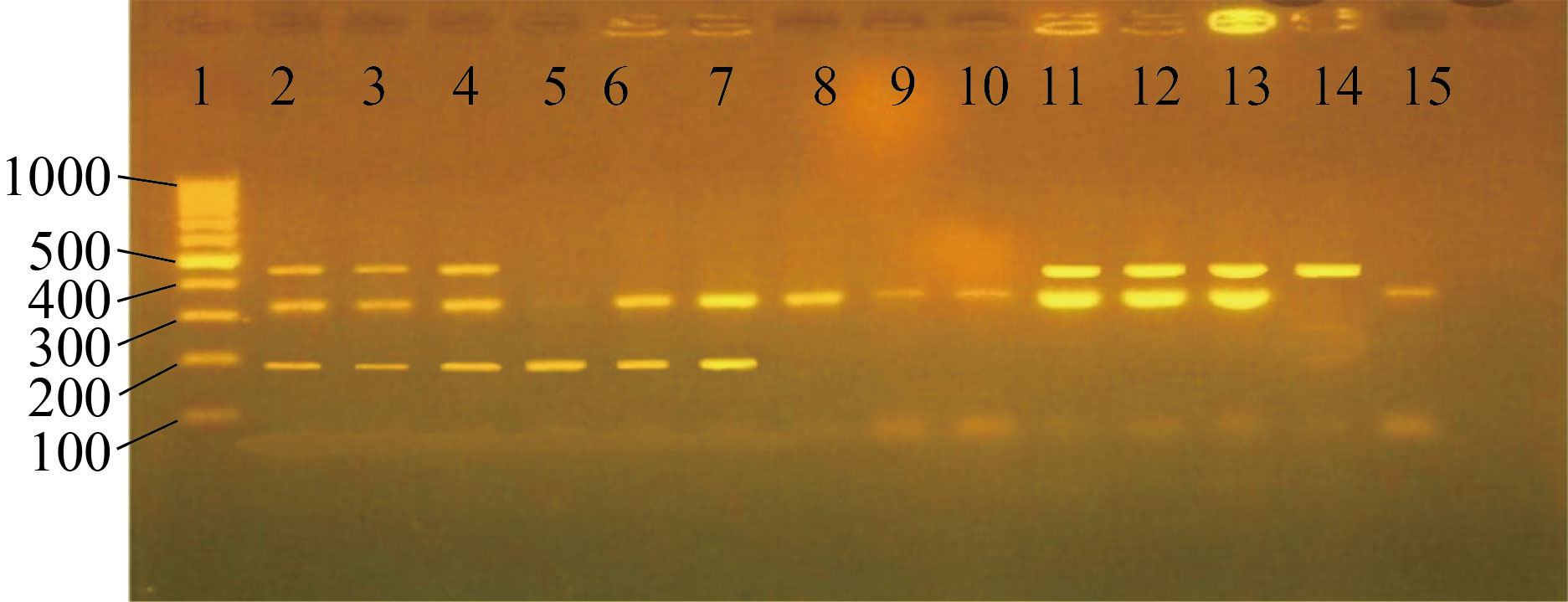
Fig. 2 Multiplex PCR detection of STHs. Staining by ethidium bromide stain-separated by 2% agrose. Lane 1 = DNA marker (100—1000), Lane 2, 3, and 4 = triple infection (192 bp Ascais lumbricoides, 330 bp Ancylostoma duodenale, and 498 bp Trichuris trichura), Lane 5 = single infection (192 bp Ascais lumbricoides), Lane 6, 7 = double infection (192 bp Ascais lumbricoides and 330 bp Ancylostoma duodenale), Lane 8, 9, 10, 15 = single infection (330 bp Ancylostoma duodenale), Lane 11, 12, 13 = double infection (330 bp Ancylostoma duodenale, and 498 bp Trichuris trichura), Lane 14 = single infection (498 bp Trichuris trichura)
The test was highly specific with a single infection (100) while in the double and triple infection were (87 and 98) respectively, resulting in low false positive test results (7.1%). Testing positive productive value (PPV) would establish the diagnosis of soil transmitted helminths with a single infection of Ascaris lumbricoides, Trichuris trichiura, and Ancylostoma duodenale (97.3, 92.2, and 100.0) respectively confidence at the highest pretest probability of 50%, while it would be unreliable at the low pretest probability of 10% (37% confidence). Testing negative productive value (NPV) on the other hand would exclude a possible diagnosis of with 93% confidence a single infection of Ascaris lumbricoides, Trichuris trichiura, and Ancylostoma duodenale at the lowest pretest probability, while it would of low usefulness at the 50% pretest probability (NPV = 59.8%).
To test the validity of the molecular technique in the presence of the infection STH, two receiver operating characteristics (ROCs) were obtained for the four genes, as shown in Fig. 3. All the 4 gens were highly useful (of high validity) in predicting pathogenic STH (ROC area > 0.9) among cases group.
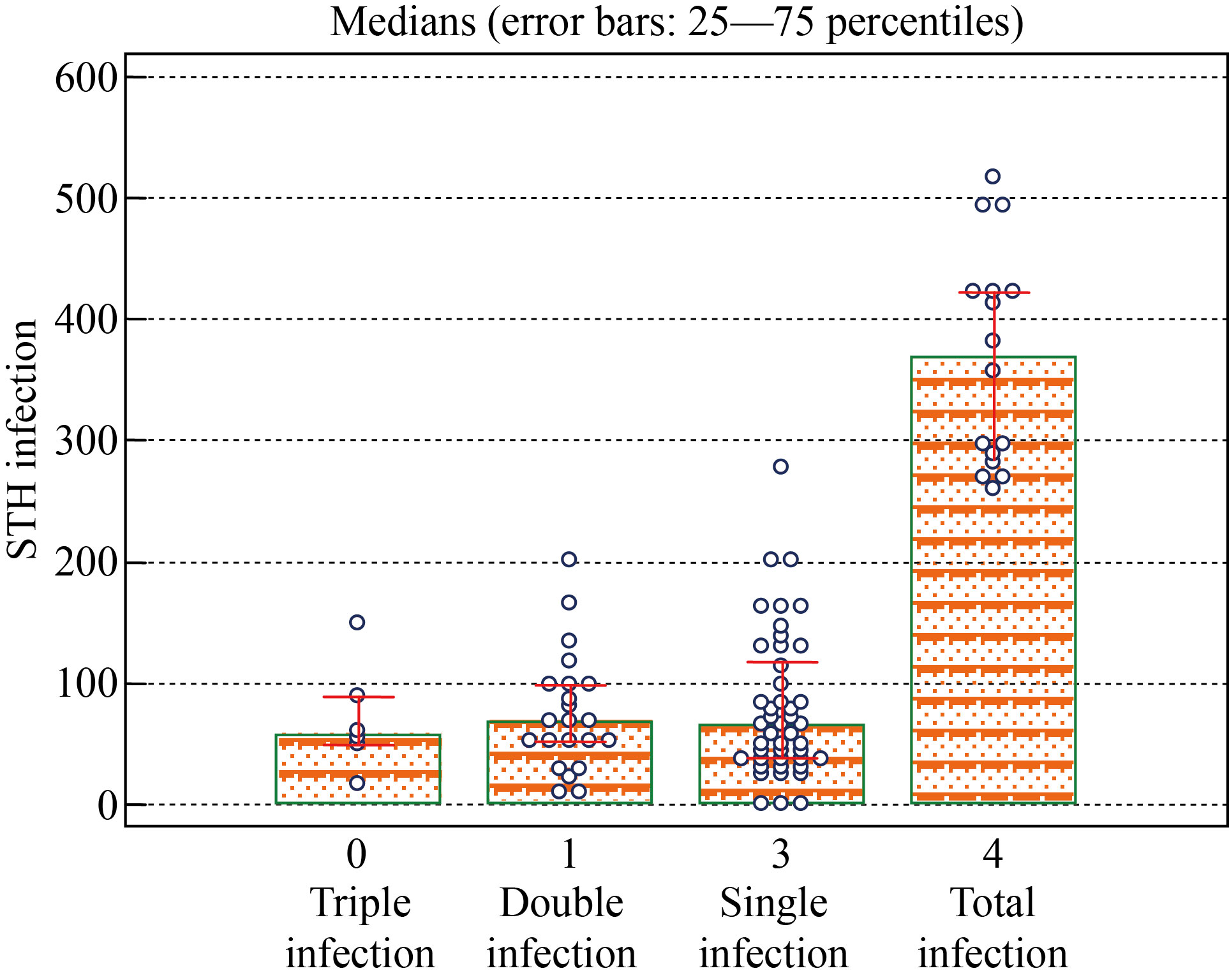
Fig. 3 Dot diagram with error bars showing the median (with its inter-quartile range) of multiplex PCR among 4 groups as classified study designed
Discussion
It could not find a field consisting of a triple infection and also could not find an image consisting
of a double infection with Trichuris trichura and Ancylostoma duodenale.
The standard method for STH diagnosis in these programs is the Kato-Katz thick smear. Beyond these programs, Kato-Katz is rarely used. Instead, the direct wet mount microscopy (DWMM) is the most frequently used routine diagnostic method for STH and other intestinal parasitic infections in health care facilities in Iraq country
Approximately, the health care services in developing countries utilize DWMM owing to its lowest cost and simple methods and that can be to identify a wide range of GIT parasites, including STH and protozoa. Even if its sensitivity is poor in general accepted, there are few researches had assessed the diagnostic sensitivity of DWMM for STH.
The present study confirmed the poor sensitivity, but it also painted significant differences both among soil-transmitted helminths. The diagnostic sensitivity of DWMM for Ascaris infection was significantly higher compared with that for both Trichuris and Ancylostoma, and the sensitivity was better with heavy infections.
The speedy, high sensitive of molecular techniques, predominantly multiplex PCR make it suitable for diagnosing STH. Until now, molecular revealing of STH was mostly limited to the research and study; on other hand, there is an agreement of adopting molecular tests in the World Health Organization STH elimination programs. Thus, STH infections are important public health problems and should be correctly diagnosed and treated to reduce the mortality and morbidity significantly.
The microscopic examination is still used as a usual method for diagnosing STH infections in Iraq country hospitals, even if this technique is easy and cheap but it is low sensitivity, particularly in patients who are carrier. One option is PCR assay, which it’s a sensitive and specific and has an elevated throughput ability that is able to be used for finding STH.
Conclusion
In conclusion, sensitivity and specificity of DWMM are poor for STH, especially when the infection was low intensity. On the other hand, DWMM is capable to distinguish those subjects that were in the heavy infection and needed a management, and therefore contributes to the universal aim to eradicate STH as a community problem.
Conflict of Interests
The authors declare that no competing interest exists.
References
[1] J. Bethony, S. Brooker, M. Albonico, et al. Soil transmitted helminth infections: Ascariasis, trichuriasis, and hookworm. The Lancet, 2006, 367(9521): 1521–1532. http://dx.doi.org/10.1016/S0140-6736(06)68653-4
[2] World Health Organization. Preventive chemotherapy in human helminthiasis: Coordinated use of anthelminthic drugs in control interventions: A manual for health professionals and programme managers. Genewa: WHO, 2006.
[3] World Health Organization. Soil-transmitted helminthiases: Eliminating as public health problem soiltransmitted helminthiases in children: Progress report 2001–2010 and strategic plan 2011–2020. Genewa: WHO, 2012. https://apps.who.int/iris/bitstream/handle/10665/44804/9789241503129_eng.pdf
[4] World Health Organization. Report of the eleventh meeting of the WHO strategic and technical advisory group for neglected tropical diseases. Geneva: WHO, 2011.
[5] G. Cringoli, M.P. Maurelli, B. Levecke, et al. The Mini-FLOTAC technique for the diagnosis of helminth and protozoan infections in humans and animals. Nature Protocols, 2017, 12(9): 1723–1732. https://doi.org/10.1038/nprot.2017.067
[6] P. Cools, J. Vlaminck, M. Albonico, et al. Diagnostic performance of a single and duplicate Kato-Katz, MiniFLOTAC, FECPAKG2 and qPCR for the detection and quantification of soil-transmitted helminths in three endemic countries. PLoS Neglected Tropical Diseases, 2019, 13(8): e0007446. https://doi.org/10.1371/journal.pntd.0007446
[7] E.P. Hansen, A.M. Tejedor, S.M. Thamsborg, et al. Faecal egg counts and expulsion dynamics of the whipworm, Trichuris trichiura following self-infection. Journal of Helminthology, 2016, 90(3): 298–302. https://doi.org/10.1017/s0022149x1500019x
[8] R.L. Pullan, J.L. Smith, R. Jasrasaria, et al. Global numbers of infection and disease burden of soil transmitted helminth infections in 2010. Parasites & Vectors, 2014, 7(1): 37. https://doi.org/10.1186/1756-3305-7-37
[9] B. Levecke, N. de Wilde, E. Vandenhoute, et al. Field validity and feasibility of four techniques for the detection of Trichuris in simians: A model for monitoring drug efficacy in public health? PLoS Neglected Tropical Diseases, 2009, 3(1): e366. https://doi.org/10.1371/journal.pntd.0000366
[10] D. Dana, J. Vlaminck, Z. Mekonnen, et al. Diagnostic sensitivity of direct wet mount microscopy for soiltransmitted helminth infections in Jimma Town, Ethiopia. The Journal of Infection in Developing Countries, 2020, 14(06.1): 66S–71S. https://doi.org/10.3855/jidc.11733
[11] O. Phuphisut, T. Yoonuan, S. Sanguankiat, et al. Triplex polymerase chain reaction assay for detection of major soil-transmitted helminths, Ascaris lumbricoides, Trichuris trichiura, Necator americanus, in fecal samples. Southeast Asian Journal of Tropical Medicine and Public Health, 2014, 45(2): 267–275. https://www.thaiscience.info/Journals/Article/TMPH/10959852.pdf
[12] J.B. Cornelissen, C.P. Gaasenbeek, F.H. Borgsteede, et al. Early immunodiagnosis of fasciolosis in ruminants usingrecombinant Fasciola hepatica cathepsin L-like protease. International Journal for Parasitology, 2001, 31(7): 728–737. https://doi.org/10.1016/s0020-7519(01)00175-8
Copyright© Ghada Basi Ali Alomashi, Hasan Raheem Khudhur and Layla Safar Jebur. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.