Green Synthesis of Selenium Nanoparticles in the Presence of Mycobacterium Bovis Cell Lysate: a Novel Fabrication Approach and its Immune-Modulatory Effects
Mohammad Hossein Yazdi1,2* , Seyed Mehdi Hassanzade3, Ramak Ajideh4, Maryam Mozafar4, Faranak Mavandadnejad4, Sara Rahimzadeh4, Mehdi Mahdavi2, Ahmad Reza Shahverdi4*
1Biotechnology Research Center, Faculty of Pharmacy, Tehran University of Medical Sciences, Tehran, Iran
2Recombinant Vaccine Research Center, Tehran University of Medical Sciences, Tehran, Iran
3BCG Vaccine Department, Research and Production Complex, Pasteur Institute of Iran, Tehran, Iran
4Department of Pharmaceutical Biotechnology, Faculty of Pharmacy, Tehran University of Medical Sciences, Tehran, Iran
*Corresponding authors. E-mail: mh-yazdi@sina.tums.ac.ir; shahverd@sina.tums.ac.ir
Received: Jul. 22, 2022; Revised: Nov. 20, 2022; Accepted: Dec. 30, 2022; Published: Dec. 31, 2022
Citation: M.H. Yazdi, S.M. Hassanzade, R. Ajideh, et al. Green synthesis of selenium nanoparticles in the presence of mycobacterium bovis cell lysate: A novel fabrication approachand its immune-modulatory effects. Nano Biomedicine and Engineering, 2022, 14(4): 308–316.
DOI: 10.5101/nbe.v14i4.p308-316
Abstract
Mycobacterium bovis (M. bovis) is a slow-growing bacteria that can intracellularly reduce selenium ions to elemental selenium nanoparticles (SeNPs). We used bacterial lysates along with vitamin C to help the synthesis of SeNPs coated with M. bovis Bacille Calmette-Guérin (BCG) crude hydrophobic materials. However, the large-scale fabrication, separation, extraction, and purification of intercellular SeNPs which are prepared by using M. bovis, have many complexities. So, we developed a simple method for preparation of above BCG-coated nanoparticles and tested its potential immune-modulatory effects. In the current investigation, we cultivated the M. bovis in conventional media and prepared total cell lysates from this bacterium by just applying freeze and thaw and ultra-sonication. The resulting cell lysates were added to the solution containing selenium ions before adding the ascorbic acid as a reducing agent. At the end of the process, the fabricated selenium nanoparticles were separated by centrifugation and characterized by different instrumentation methods. In the next step, to evaluate the immune-modulatory effects of the hepatitis B surface antigen (HBsAg) vaccine alone, and in combination with plain SeNPs or SeNPs-BCG lysate, the serum level of interferon-gamma (IFN-γ) was determined in different groups by enzyme-linked immunosorbent assay (ELISA). This study showed adjuvant effects of prepared nanoparticles (in both 10 µg/300 µL and 100 µg/300 µL doses) in increasing the level of interferon-gamma (IFN-γ) in comparison with vaccine alone. Moreover, in both doses of SeNPs-BCG lysate, the level of interferon-gamma (IFN-γ) was remarkably higher than the same doses of plain SeNPs. As a result, synthesized SeNPs in the presence of whole-cell lysates of M. bovis indicated a greater ability to induce the interferon-gamma (IFN-γ) compared with other groups. Additionally, its easy fabrication procedure can be considered its superiority.
Keywords: Green synthesis; Hepatitis B surface antigen; Immune-modulatory effects; Mycobacterium bovis; Selenium nanoparticles
Introduction
Nanotechnology has gained tremendous attention during these centuries. Nanoparticles are a crucial component of nanotechnology [1]. In comparison with respective particles on higher scales, nanoparticles exhibit distinct physical, chemical, and biological properties. Due to their relatively larger surface area to the volume and increased reactivity or stability in a chemical process, nanoparticles possess the potential to be applied in various applications [1]. There are several approaches to the fabrication of nanoparticles. These methods are categorized into bottom-up or topdown methods. On the other side, numerous synthesis methods are either being developed or improved to advance the properties and decrease the production costs. Some methods are modified to achieve processspecific nanoparticles to enhance their optical, mechanical, physical, and chemical properties [2].
Elemental Se is an insoluble metalloid compound, which can be chemically or biologically produced at the nanoscale [3]. Since nanosized elemental Se (Se0) presents superior bioavailability and less toxicity compared with selenate (Se2+) or selenite (Se4+) ions, this nanoparticle can be a desirable alternative for other forms of Se in clinical practice [4]. The main effects of the selenium nanoparticles (SeNPs) are found in various scientific studies, such as their precious roles in immune-modulating, antioxidant and antimicrobial effects, acting as a cancer preventive agent, etc [5]. Selenium nanoparticles are usually produced by chemical or biological methods. Although SeNPs harbor the potential to modulate immune responses, the biological synthesis of these nanoparticles has a great attraction due to the presence of different functional groups or in better words pathogen-associated molecules (PAMPs) from producing microorganisms on the surface of nanoparticles. In this regard, our previous research demonstrated that the administration of biogenic SeNPs of Lactobacillus to the mice increased Th1 immune responses. The results showed that the biogenic or biologic form of SeNPs can be used as a modulator to boost the immune response [6, 7]. Meanwhile, the potential of biogenic SeNPs as an immunomodulatory agent besides the conventional adjuvant in the formulation of the hepatitis B surface antigen (HBsAg) vaccine model has been studied in our previous research, and results showed a promising potential for these nanoparticles to be used in the vaccine formulation [8].
Hepatitis B (HB) is considered the most probable leading cause of viral hepatitis in humans and involves three-quarters of the world’s population. Many types of research that develop appropriate vaccines to control this viral infection show promising results [9–12]. However, there are still some non-responder individuals among the vaccinated population, which have to be considered as a healthcare system challenge. This population should be treated differently, or with different vaccine formulations [13–15]. This is very well known that the most common adjuvant for human use is aluminum salts such as hydroxide and phosphate [16]. Although alum is used as a common adjuvant in hepatitis B surface antigen (HBsAg) vaccine formulation to induce a humoral immune response, it is less effective in stimulating a Th1 response [17, 18]. For instance, in some non-responder patients who get the alum alone vaccine formulation, the virus interferes with cytokine production, leading to a defect in interferon (IFN) production and weakening cellular immune response [7]. To handle this challenge, many under investigational vaccines are mixed with a potent adjuvant as an immune modulator agent in parallel with HBs antigen [19]. As stated above, microbial components that activate the host immune system have been designated as adjuvants [20]. Currently, monophosphoryl lipid A (MPL) from salmonella besides aluminium hydroxide is being used in the formulation of the Fenderix vaccine produced by GlaxoSmithKline. On the other hand, the cell wall skeleton of Mycobacterium bovis (M. bovis) has been investigated and well accepted as an immune potentiator element to help the alum adjuvant for better Th1 response stimulation. The cell wall skeleton (CWS) of M. bovis (BCG-CWS) is known as a unique and potent adjuvant for innate immunity and has been used in many clinical studies [21].
In our previous study, SeNPs were prepared intracellularly using M. bovis, isolated and characterized (hereafter, biogenic myco@selenium nanocomposites or BMSN). The mentioned nanoparticles were covered with some molecules related to this bacteria and could stimulate the humoral immune system considerably [22]. In synthesizing BMSN, the growth rate of M. bovis was a limiting factor. In this process, selenium, which is a toxic element, is added to the bacterial culture media and M. bovis, converting the Se into SeNPs to detoxify it. Extraction of these SeNPs from M. bovis is quite a difficult and time-consuming process in which the cytoskeleton components of M. bovis are damaged due to applying the solvent to make two phasic milieu and capture the SeNPs in the water phase. In the current research, we develop a novel synthetic method using ascorbic acid and total cell lysate of M. bovis which is achieved only by freeze and thaw and ultra-sonication, to fabricate synthetic myco@selenium nanocomposites in a test tube (hereafter, synthetic myco@selenium nanocomposites or SMSN). Moreover, we evaluate its potential as an immunomodulator in HBs-Ag vaccine formulation besides the alum adjuvant.
Experiment
Bacterial strain and growth conditions
Maycobacterium bovis Bacille Calmette-Guérin (BCG) (Pasteur Institute strain 1173P2) was obtained from Pasteur Institute in Karaj. Conventional HBV vaccine (HBs-Ag formulated in alum adjuvant) and hepatitis B surface antigen (HBsAg) were supplied by Pasteur Institute of Iran (Karaj, Iran) and were stored at 4 ℃ until use.
Green synthesis and characterization of plain SeNPs
5.2 mmol/L selenium dioxide solution (Merck, Germany) was prepared, and an aqueous ascorbic acid solution (5.2 mmol/L) was slowly added to the mixture with continuous stirring at 300 r/min using a laboratory magnetic stirrer. Later, the mixture was centrifuged and washed three times with double distilled water.
Preparation of SMSN
To synthesize these nanoparticles, first, the M. bovis strain BCG (Pasteur Institute strain 1173P2) was subcultured and grown as a pellicle on Sauton liquid medium. Then, M. bovis was inoculated in 250 mL of Sauton liquid medium (106 cells) for 18 days at 37 ℃. After that, the culture medium was centrifuged and the BCG cells were washed twice with 0.9% sodium chloride solution (4000 r/min, 15 min). In the next step, washed cells (3×109 cells/mL) were disrupted by the freeze and thaw process with liquid nitrogen in a mortar and pestle. This was done five times. The cell lysate mixture was ultrasonicated at 100 W for 5 min and centrifuged at 2800 g for 15 min. The supernatant was collected and added (2.5 mL) to the 5.2 mmol/L of selenium oxide (SeO2) solution (50 mL). In the next step, an aqueous ascorbic acid solution (50 mL, 5.2 mmol/L) was slowly added to the above mixture with continuous stirring at 300 r/min. Finally, the suspension was centrifuged (15000 r/min, 30 min) at 4 ℃, and settled nanoparticles were washed three times with double distilled water.
Characterization of fabricated nanoparticles
A field emission scanning electron microscope (FESEM) equipped with energy-dispersive X-ray spectroscopy (EDS) was employed to observe the NPs’ surface features and determine the elemental composition. For FESEM observation, NPs were mounted on specimen stubs and coated with gold. Samples were analyzed with an MIRA 3 FESEM (MIRA 3 TESCAN, USA) operated at 15 kV. In EDS, the elemental composition was recorded by focusing
on a cluster of NPs.
To characterize the surface chemistry of SeNPs, freeze-dried powder of synthesized SeNPs in presence of BCG lysate was ground with potassium bromide pellets and subjected to Fourier transform infrared spectroscopy (FTIR) analysis using a BOMEM FTIR spectrophotometer (ABB Bomen Inc., St-Laurent, Canada). FTIR studies were performed in the range of 500–4000 cm−1 to determine the functional groups on the NPs.
The particle size distribution patterns of generated NPs were determined by dynamic light scattering (DLS) equipment (Zetasizer MS2000, Malvern Instruments Inc.). The size of the SeNPs prepared in the presence of BCG lysate was further studied using atomic force microscopy (AFM). It was observed under an AFM microscope (Model-Nanowizard II, German). AFM imaging was done in tapping mode.
Thermogravimetric analysis (TGA) experiments were performed using NETZSCH STG 209F3 equipment (Netzsch GmbH, Germany). Dried samples were placed in the TGA furnace. The samples were heated from room temperature to 800 ℃ with a heating rate of 10 ℃/min under nitrogen. From the TGA curve, percent weight loss was noted.
Animal study and immunization profile
To evaluate the possible immune-modulatory effects of SMSN and plain SeNPs, 36 female BALB/c mice, aged 6–8 weeks, were purchased from the Pasture Institute of Iran (Tehran, Iran). The experimental mice were randomly divided into four tests and two control groups, each containing 6 mice (Table 1). They were housed at a temperature of (23 ± 1) ℃ and (55 ± 10)% with a 12 h light/dark cycle. All mice were fed via a standard mouse pellet diet. Mice were immunized subcutaneously with 5 µg of HBs-Ag vaccine alone in the positive control group and in combination with SMSN or plain SeNPs (at concentrations of 10 and 100 µg in a total volume of 300 µL) in test groups. The immunization of mice was scheduled for the 1st, 14th, and 28th days of the in-vivo study. Mice were not immunized in the negative control group.
Table 1 Six categorized groups received different formulations
Groups No. | HBs-Ag vaccine 5 µg | Phosphate buffered saline (PBS) | Plain SeNPs | Synthetic myco@selenium nanocomposites (SMSN) | Administration dose (µg) |
1 | + | – | + | – | 100 |
2 | + | – | + | – | 10 |
3 | + | – | – | + | 100 |
4 | + | – | – | + | 10 |
5 | + | – | – | – | – |
6 | – | + | – | – | – |
Delayed-type hypersensitivity (DTH) test
Mice in different groups received HBs-Ag at the right footpads and PBS at the left footpads to compare the corresponding induration. The thickness of the footpads was measured for up to 72 h after footpad injection, and DTH responses were represented as the percentage increase of footpad swelling at the injection site.
Serum sample collection for IFN-γ
About 1 week following the last immunization, under deep anaesthesia, blood was drawn from the heart of all mice in each group. The blood was allowed to clot, and the samples were centrifuged for 15 min at 10000 r/min. The serum aliquots were stored at –70 ℃. The serum level of IFN-γ was determined in different groups by enzyme-linked immunosorbent assay (ELISA).
Ethics approval
All experimental procedures using animals in this study were carried out according to the guidelines of the Tehran University of Medical Science (Tehran, Iran) for the care and use of laboratory animals.
Statistical analysis
In all statistical analyses, the variance was calculated. These tests were performed with Sigma Plot. The values were reported as mean ± standard deviation.
Results
Preparation of nanoparticles
Plain SeNPs were chemically synthesized using ascorbic acid, and their shape and size were confirmed with FESEM. The upper illustration in Fig. 1 is a representative FESEM image of the synthesized SeNPs using ascorbic acid in the absence of BCG cell lysate. The FESEM image showed that the particles range in sizes below 100 nm with an average size of about 60 nm. In addition, SMSN was synthesized using BCG cell lysate and ascorbic acid. The FESEM image of the SMSN (upper illustration in Fig. 2) indicated that the particles have a well-dispersed spherical shape, and the size of most of them is less than 200 nm. The lower illustrations in Figs. 1 and 2 respectively depicted the EDS spectra of the plain SeNPs and SMSN. These EDS peaks confirm the existence of elemental selenium in both types of prepared NPs. Additional peaks related to copper, carbon, oxygen, and nitrogen elements are attributions of the grid used for FESEM imaging, or organic materials originated substances.
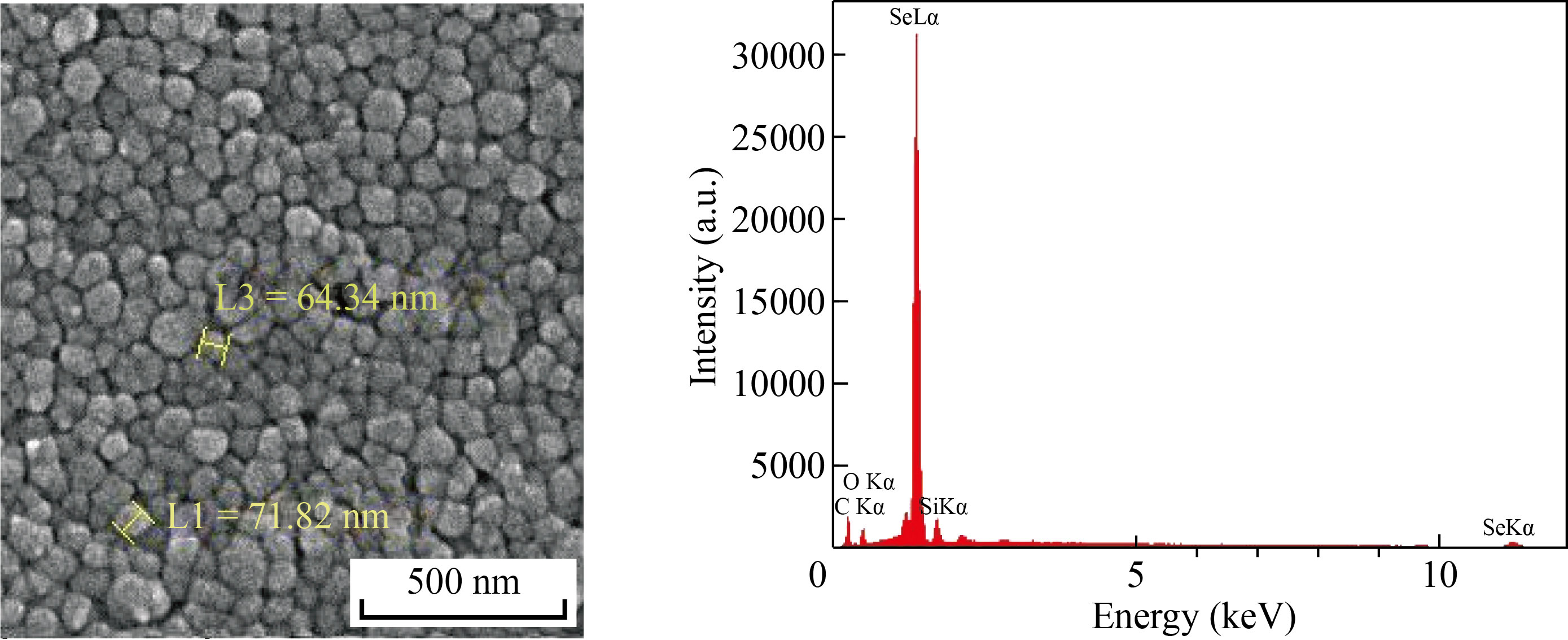
Fig. 1 FESEM image of plain SeNPs, which was prepared using ascorbic acid in the absence of BCG cell lysate (upper illustration), indicates that the SeNPs are well below 100 nm in size. The lower illustration shows the EDS spectrum of SeNPs, confirming the presence of Se atoms and the existence of SeNPs. Additional peaks of copper and carbon elements are attributed to the grid used for FESEM imaging, or organic materials originated substance
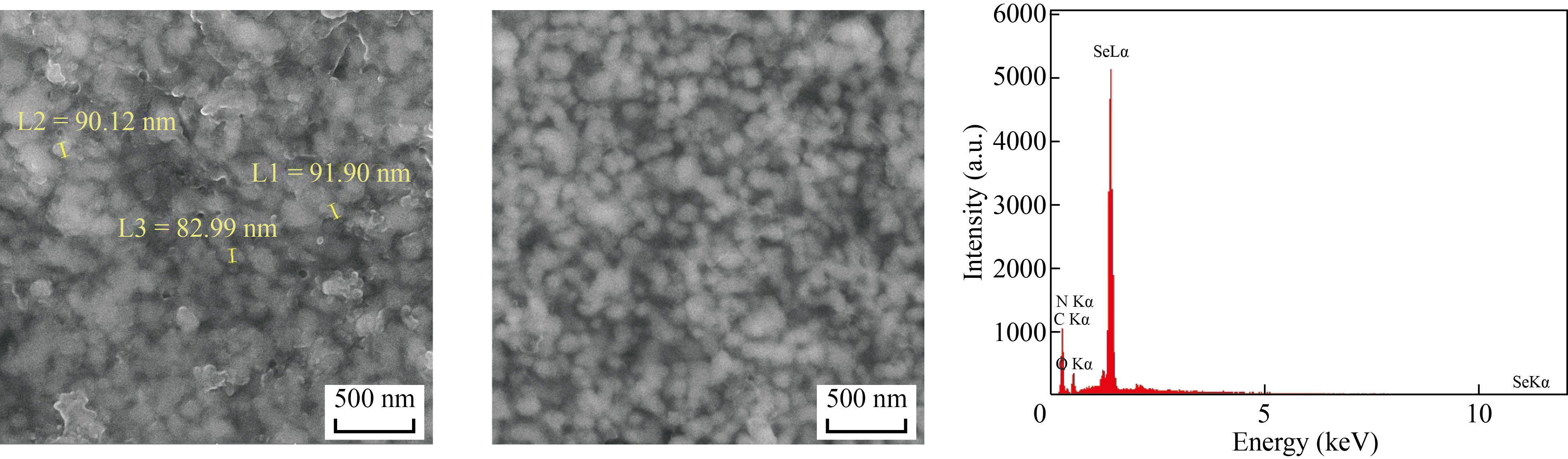
Fig. 2 FESEM image of synthetic myco@selenium nanocomposites (SMSN) (upper illustration) indicates that the SMSNs are well below 200 nm in size. The lower illustration shows the EDS spectrum of this type of SeNPs, which is prepared in the presence of BCG lysate, confirming the presence of Se atoms and the existence of SeNPs. Additional peaks of copper, carbon, oxygen and nitrogen elements are attributed to the organic materials originated substance or grid used for FESEM imaging
Characterization of SMSN by FTIR
The FTIR technique was used to show the surface of the nanocomposite. FTIR spectroscopy for SMSN lysate is shown in Fig. 3, which confirms some functional groups attached to the surfaces. The peaks at 2925 and 2852 cm−1 correspond to the C–H stretch; the peak at 3424 cm−1 represents the N–H or O–H stretch; the sharp bands at 1636 cm−1 were assigned to the amide bond, and the peak at 1020 cm−1 was assigned to the C–O stretch. All these peaks revealed that SeNPs were coated with the organic materials originating from M. bovis strain BCG and were not bare.
Fig. 3 FTIR analyses of synthetic myco@selenium nano composites (SMSN)
Characterization of SMSN by dynamic light scattering
The size distribution and surface charge for fabricated SMSN were measured in a suspension on a Zeta sizer Nano ZS particle analyzer (Malvern). As shown in Fig. 4, this analysis confirms that the mean size and surface charge of the nanocomposites were around 200 nm and –19.3 mV, respectively.
Fig. 4 (a) Size distribution and (b) zeta potential distribution of synthetic myco@selenium nanocomposites (SMSN) were shown as DLS patterns
Characterization of SMSN by AFM
The sizes of the SMSN were also analyzed by the AFM technique. Typical two-dimensional (2D) and 3D AFM images of the SMSN are demonstrated in Fig. 5. The sizes of SMSN in the 2D image were observed to be less than 200 nm, whereas the 3D image indicated the presence of individual spherical particles, with a maximum height of 70 nm in the z-sheet.
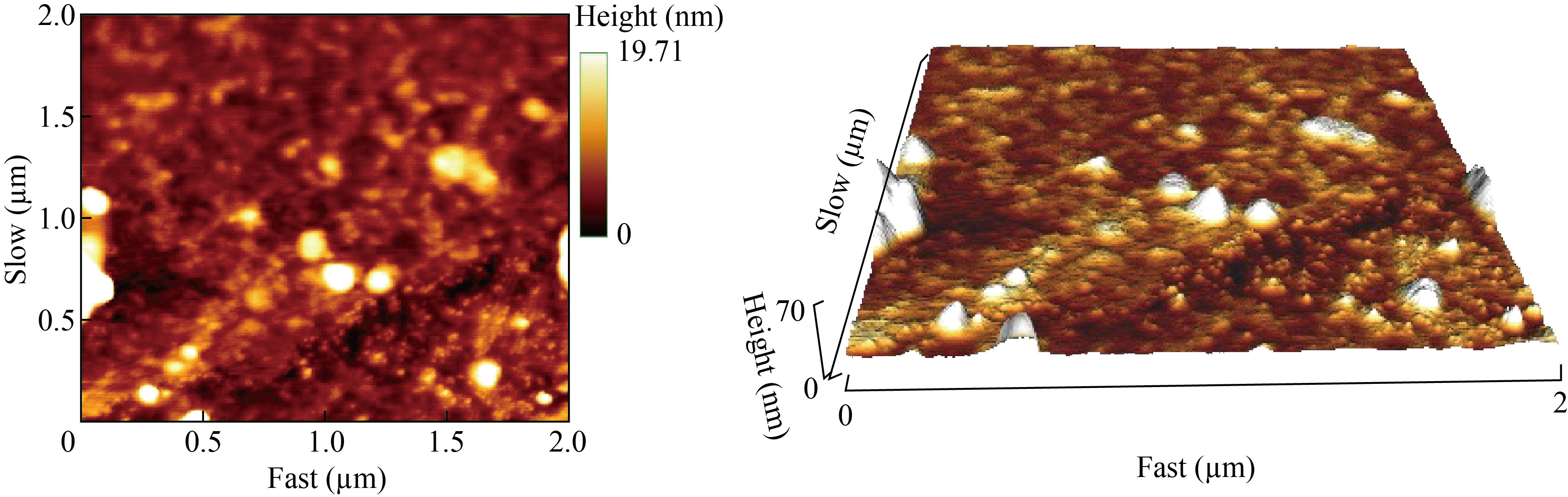
Fig. 5 AFM measurement of synthetic myco@selenium nanocomposites (SMSN): (a) 2D image; (b) 3D image
Characterization of SMSN by TGA
The TGA plot showed that about 75% of the NPs pertained to the organic groups attached to the core of selenium, and about 20% pertained to selenium (Fig. 6).
Fig. 6 TGA plot of the SMSN. The obtained result via TGA proved the thermal stability of the SMSN. About 73% pertained to organic material, which in turn pertained to cell lysate of M. bovis. The remainder about 26% was selenium
Delayed-type hypersensitivity test (DTH)
DTH assay was performed by using 10 µg and 100 µg of both features of SeNPs. According to the results of induration measurement in footpad of the mice, it revealed that SMSN could induce better Th1 immune response was compared with other test and control groups in doses of 10 and 100 µg, and in 24 h (Fig. 7).
Fig. 7 DTH responses in (a) 10 µg dose and (b) 100 µg dose of both feature SeNPs in the mice at 24, 48, and 72 h after HBs antigen re-challenge. The mice were challenged with the HBs antigen on the left footpad and with the PBS on the right footpad and the difference between induration size in these two footpads are stated in the graph
Cytokine assay
In this study, the level of IFN-γ in two major test groups was compared using sandwich ELISA; one received plain SeNPs and the other received SMSN with two doses (10 µg and 100 µg). The level of IFN-γ for all doses in both groups is significantly higher than the vaccine alone (Fig. 8). Also, the level of IFN-γ in the doses of 10 µg and 100 µg of SMSN in comparison with doses of 10 µg and 100 µg of plain SeNPs increased considerably. In other words, the level of IFN-γ between doses of 100 µg and 10 µg of the SMSN group was not statistically notable.
Fig. 8 Cytokine assay. The sandwich ELISA method was performed for evaluating IFN-γ. Data of each group was presented as mean ± SD (n = 6) (*P = < 0.001)
Discussion
Nanotechnology is improving our everyday lives to upgrade the performance and efficiency of everyday objects. Nanotechnology has been established to be an enriched field of science where various researches have been done to implement this technology. Many methods are being tested for extensive new applications to enhance the efficiency and performance of the nanocomposite or process, and finally reduce the cost [1]. Nanomedicine, the application of nanotechnology in medicine, draws on the natural scale of biological phenomena to produce precise solutions for disease prevention, diagnosis, and treatment. Nanotechnology researchers are working on several different therapeutics where nanoparticles can encapsulate or in other ways help deliver medication directly to cancer cells and minimize the risk of damage to healthy tissue.
Biosynthesis uses bacteria, plant extracts, fungi, etc. along with the precursors to produce nanoparticles instead of convention chemicals for bioreduction and capping purposes. The biosynthesized nanoparticle has unique and enhanced properties that find its way into biomedical applications [1]. For example, in our previous research, SeNPs which were synthesized using different bacteria could heighten the immune response [6, 22]. On the other hand, many studies also reported that M. bovis strain BCG can be used as a potent stimulator of the immune system [9, 20- 23]. In addition, in our recent study BMSN were extracted from M. bovis strain BCG and were well characterized. The results indicated that the decorated SeNPs with BCG antigenic materials could be potent candidates to stimulate the immune system. However, the preparation of these nanoparticles was extremely challenging and time-consuming [22]. In addition, the process of BMSN extraction from M. bovis, a slow-growing bacterium, was troublesome due to its complexity.
So based on the results achieved in the previous research, we developed a synthetic method to prepare myco@selenium nanocomposites. In the current study, these nanocomposites synthesized in the presence of total lysate of M. bovis and ascorbic acid also lead to stimulating the immune system by increasing IFN-γ. IFN-γ is known as the first line of the immune response against cancer and viral infection. Meanwhile, due to skipping the solvent extraction step, which was
necessary for the extraction of biogenic SeNPs from M. bovis in our previous study, PAMPs have remained somehow intact. Therefore, SMSN will be more reliable for triggering immune responses by signaling through the toll-like receptor on the surface of immune cells. By using different analyzing methods, we showed that these nanocomposites are also covered with some organic materials which can be derived from M. bovis. Compared with our previous study, these nanocomposites (SMSNs) could stimulate IFN-γ more than the SeNPs extracted from M. bovis (BMSN) because more antigens could cover the nanoparticles. SMSN was synthesized in the presence of BCG lysate; this way could lead to producing the nanoparticles covered with more antigens of M. bovis and could enhance the immune response. Mahdavi et al. showed that plain SeNPs synthesized by ascorbic acid could increase the level of IFN-γ compared with the vaccine
group [7]. In the current study, also the level of IFN-γ was higher in SMSN and plain SeNPs groups compared with the vaccine group. This result showed that these nanomaterials in both features (SMSN or SeNPs) could be more potent in stimulating immune response via IFN-γ than vaccine alone. Moreover, the results represented that SeNPs synthesized in the presence of BCG lysate (SMSN) can stimulate IFN secretion remarkably in comparison with plain SeNPs in both doses of 10µg and 100 µg. In other words, this modified method not only smooths the synthetic approach but also increases the level of IFN-γ and DTH, which can be a good candidate for stimulation of the immune system.
Acknowledgment
This work was supported by the Tehran University of Medical Sciences (Project No. 32451).
Conflict of interest
The authors declare no conflicts of interest regarding this article.
References
[1] S. Hasan. A review on nanoparticles: Their synthesis and types. Research Journal of Recent Sciences, 2015, 4(SC-2014): 1–3. http://www.isca.in/rjrs/archive/specialISC-2014/3.ISCA-ISC-2014-Poster-3BS-63.pdf
[2] S. Anu Mary Ealia, M.P. Saravanakumar. A review on the classification, characterisation, synthesis of nanoparticles and their application. IOP Conference Series: Materials Science and Engineering, 2017, 263(3): 032019. https://doi.org/10.1088/1757-899x/263/3/032019
[3] S. Shirsat, A. Kadam, M. Naushad, et al. Selenium nanostructures: Microbial synthesis and applications. RSC Advances, 2015, 5: 92799-92811. http://dx.doi. org/10.1039/C5RA17921A
[4] S. Dwivedi, A.A. Alkhedhairy, M. Ahamed, et al. Biomimetic synthesis of selenium nanospheres by bacterial strain JS-11 and its role as a biosensor for nanotoxicity assessment: A novel se-bioassay. PLoS One, 2013, 8(3): e57404. https://doi.org/10.1371/journal. pone.0057404
[5] B. Hosnedlova, M. Kepinska, S. Skalickova, et al. Nanoselenium and its nanomedicine applications: A critical review. International Journal of Nanomedicine, 2018, 13: 2107–2128. https://doi.org/10.2147/ijn.s157541
[6] M.H. Yazdi, M. Mahdavi, B. Varastehmoradi, et al. The immunostimulatory effect of biogenic selenium nanoparticles on the 4T1 breast cancer model: An in vivo study. Biological Trace Element Research, 2012, 149: 22- 28. http://dx.doi.org/10.1007/s12011-012-9402-0
[7] M. Mahdavi, F. Mavandadnejad, M.H. Yazdi, et al. Oral administration of synthetic selenium nanoparticles induced robust Th1 cytokine pattern after HBs antigen vaccination in mouse model. Journal of Infection and Public Health, 2017, 10: 102–109. http://dx.doi. org/10.1016/j.jiph.2016.02.006
[8] N. Posuwan, N. Wanlapakorn, P. Sa-Nguanmoo, et al. The success of a universal hepatitis B immunization program as part of Thailand’s EPI after 22 years’ implementation. PLoS One, 2016, 11(3): e0150499. https://doi.org/10.1371/journal.pone.0150499
[9] M. Mendy, I. Peterson, S. Hossin, et al. Observational study of vaccine efficacy 24 years after the start of hepatitis B vaccination in two Gambian Villages: No need for a booster dose. PLoS One, 2013, 8(3): e58029. https://doi.org/10.1371/journal.pone.0058029
[10] S.M. Alavian. Networking for overcoming on viral hepatitis in middle east and central Asia: Asian hepatitis network. Hepatitis Monthly, 2007, 7(4): 181–182. https://www.sid.ir/paper/306148/en
[11] D. Bhattacharya, C.L. Thio. Review of hepatitis B therapeutics. Clinical Infectious Diseases, 2010, 51(10): 1201–1208. https://doi.org/10.1086/656624
[12] E.J. Aspinall, G. Hawkins, A. Fraser, et al. Hepatitis B prevention, diagnosis, treatment and care: A review. Occupational Medicine, 2011, 61(8): 531–540. https://doi.org/10.1093/occmed/kqr136
[13] L. Koumbi, P. Karayiannis. The epigenetic control of hepatitis B virus modulates the outcome of infection. Frontiers in Microbiology, 2015, 6: 1491. https://doi.org/10.3389/fmicb.2015.01491
[14] L.C. Meireles, R.T. Marinho, P. Van Damme. Three decades of hepatitis B control with vaccination. World Journal of Hepatology, 2015, 7: 2127–2132. https://doi.org/10.4254/wjh.v7.i18.2127
[15] K. Cardell. Studies on hepatitis B vaccination and factors associated with the vaccine response. Linköping: Linköping University Electronic Press, 2009.
[16] R.K. Gupta, E.H. Relyveld, E.B. Lindblad, et al. Adjuvants — a balance between toxicity and
adjuvanticity. Vaccine, 1993, 11(3): 293-306. http://dx.doi. org/10.1016/0264-410X(93)90190-9
[17] S.M. Lin, I.S. Sheen, R.N. Chien, et al. Long-term beneficial effect of interferon therapy in patients with chronic hepatitis B virus infection. Hepatology, 1999, 29(3): 971–975. https://doi.org/10.1002/hep.510290312
[18] L.G. Guidotti, F.V. Chisari. Noncytolytic control of viral infections by the innate and adaptive immune response. Annual Review of Immunology, 2001, 19: 65–91. https://doi.org/10.1146/annurev.immunol.19.1.65
[19] E. Oleszycka, E.C. Lavelle. Immunomodulatory properties of the vaccine adjuvant alum. Current Opinion in Immunology, 2014, 28: 1–5. https://doi.org/10.1016/j.coi.2013.12.007
[20] M. Kundi. New hepatitis B vaccine formulated with an improved adjuvant system. Expert Review of Vaccines, 2007, 6(2): 133–140. https://doi.org/10.1586/14760584.6.2.133
[21] T. Taniyama, T. Watanabe, I. Azuma, et al. Adjuvant activity of mycobacterial fractions. II. In vitro adjuvant activity of cell walls of mycobacteria, nocardia and corynebacteria. Japanese Journal of Microbiology, 1974, 18(6): 415–426. https://doi.org/10.1111/j.1348-0421.1974. tb00829.x
[22] F. Mavandadnejad, M.H. Yazdi, S.M. Hassanzadeh, et al. Biosynthesis of SeNPs by Mycobacterium bovis and their enhancing effect on the immune response against HBs antigens: an in vivo study. IET Nanobiotechnology, 2018, 12(1): 57–63. https://doi.org/10.1049/iet-nbt.2017.0006
[23] Y. Koike, Y.C. Yoo, M. Mitobe, et al. Enhancing activity of mycobacterial cell-derived adjuvants on immunogenicity of recombinant human hepatitis B virus vaccine. Vaccine, 1998, 16(20): 1982–1989. http://dx.doi.org/10.1016/S0264-410X(98)00084-X
Copyright© Mohammad Hossein Yazdi, Seyed Mehdi Hassanzade, Ramak Ajideh, Maryam Mozafar, Faranak Mavandadnejad, Sara Rahimzadeh, Mehdi Mahdavi and Ahmad Reza Shahverdi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.