Study the Exposure of Low-Level Laser on rGO/Ag NPs to Improve the Antibacterial Activity of Different Types of Bacteria
Noora Al-Janabi1*, Amer Al-Nafiey2*
1Department of Laser Physics, Collage of Science for Woman, University of Babylon, Babylon, Iraq
2Department of Basic Science, Faculty of Density, University of Babylon, Babylon, Iraq
*Corresponding authors. E-mail: amer76z@yahoo.com; nooraaljanabi97@gmail.com
Received: Aug. 24, 2022; Revised: Sep. 30, 2022; Accepted: Dec. 08, 2022; Published: Dec. 31, 2022
Citation: N. Al-Janabi, A. Al-Nafiey. Study the exposure of low-level laser on rGO/Ag NPs to improve the antibacterial activity of different types of bacteria. Nano Biomedicine and Engineering, 2022, 14(4): 367‒374.
DOI: 10.5101/nbe.v14i4.p367-374
Abstract
The rGO-Ag NPs nanocomposite was synthesized by an easy, low-cost, one-step chemical method. The nanocomposite was analyzed by UV-Vis spectroscopy and atomic force microscope (AFM) to verify the properties of the nanoparticles. The study of the optical properties showed that there are two peaks, one of which belongs to silver and is 400 nm, and the other is related to graphene oxide at 268 nm. The AFM examination of the rGO-Ag NPs revealed that the average grain size is 71.26 nm, and this indicates that the material is within the nanoscale. The rGO-Ag NPs nanocomposite was applied as an antagonist against various types of bacteria that cause gum and tongue infections, and the results showed that with the increase in the nanocomposite concentration, the killing rate of bacteria increases. This means that the killing increases with increasing volumes of rGO-Ag NPs, and the best one is 40 μL. The result shows that the rGO-Ag NPs show a relationship between adding volumes of the nanomaterial and the percentage killing of bacteria, which is directly proportional. Additionally, through UV-Vis spectroscopy, the absorbance of the rGO-Ag NPs was determined, so that the laser with a wavelength of 405 nm was chosen to be applied to the same kinds of bacteria and for different periods of time with the use of a 40 μL volume of the rGO-Ag NPs, and it was found that with increasing the irradiation period, the percentage of bacteria killed increases. It showed that increasing the irradiation period with volume stability of 40 μL causes a higher killing rate. The study indicated that using nanocomposite and laser is a method to remove the infection from the gums and tongue. These results are promising and can be applied in the treatment of gum and tongue infections after clinical studies.
Keywords: Nanocomposite; Optical properties; rGO-Ag NPs; Laser; Antibacterial applications
Introduction
Harmful bacteria cause many diseases, including infections in various parts of the human body, such as acute and chronic infections, blood poisoning, food poisoning, infections of the gums, tongue, caries, infections of burns and wounds, pneumonia and many other diseases that can lead to death in the injured [1]. On the other hand, beneficial bacteria help produce many essential vitamins for the body, help digest complex molecules found in some types of food, stimulate microorganisms inside the body to form immunity, improve metabolism, and reduce excessive weight gain. In fact, some bacteria have developed resistance to antibiotics that were once commonly used to treat them, and these bacteria are able to cause serious disease, so this is a major public health problem [2, 3].
On the other hand, nanoscience and nanotechnology have attracted great interest over the last decade due to their potential impact on many scientific areas, such as energy, medicine, pharmaceutical industries, electronics, and space industries. One of these nanomaterials is nanoparticles (NPs) that show unique and considerably changed chemical, physical, and biological properties due to their high surface-to-volume ratio, compared with the bulk of the same chemical composition. Thus, nanomaterial-based therapies are promising tools to combat bacterial infections that are difficult to treat, featuring the capacity to evade existing mechanisms associated with acquired drug resistance. In addition, the unique size and physical properties of nanomaterials give them the capability to target biofilms, overcoming recalcitrant infections.
Graphene is an amazing nanomaterial, and detailed information on their structures and properties can be obtained from several studies. The graphene nanomaterials have attracted wide interest due to their excellent properties that arise from a flat monolayer of carbon atoms packed in a two-dimensional honeycomb lattice. High chemical stability, extreme mechanical strength, and exceptional electronic and thermal conductivity are just a few examples of unique properties. As a result, it has a wide range of applications, including supercapacitors [4‒6], solar cells [7], sensors [8], and catalysts [9]. It is still a brilliant star on the horizon of materials science [10]. To take advantage of the remarkable properties and unique structure of graphene nanomaterials, great efforts have been made to develop hybrid graphene nanocomposites. So far, graphene sheets have been developed as nanostructures to disperse and stabilize various metallic and metal oxide nanoparticles (Ag, Au, SnO2, TiO2, Pt, Pd, Cu, and ZnO) [11]. More importantly, some new properties of the nanocomposites can be obtained after hybridization [12]. Therefore, graphene sheets as support for nanoparticles open a new avenue for material development. Silver is among those nanoparticles. Due to its optical properties depending on the size of the nanoparticles of the noble metals in liquids, silver is a noble metal of great importance. Silver nanoparticles (Ag NPs) have attracted increased interest due to their optical and antibacterial properties [13, 14]. Decorating reduced graphene oxide rGO with Ag NPs in different methods will obtain a nanocomposite which is rGO-Ag NPs [15].
The effectiveness of antibiotics against different types of bacteria can also be increased through the use of a laser whose wavelength is proportional to the absorption of the nanocomposite used, which is obtained after conducting UV-Vis spectroscopy for the nanocomposite. On the other hand, the diode laser is important and has a distinct role in systems such as systems with high penetration for processing because it is characterized by unique and important characteristics, including its small size, narrow beam width, high efficiency, and low-power and high-power quality [16, 17].
In fact, the bacteria have shown resistance whether they used laser only or nanomaterials only, so we will use a nanocomposite with a laser as an antibacterial for different types of bacteria that cause gum and tongue infections.
There are several types of bacteria that have been identified, including Proteus mirabilis, Escherichia coli, Pseudomonas aeruginosa, Porphomnas. Streptococcus mutans, Enterococcus faecalis, Lactobacillus, Streptococcus pyogenes, and Staphylococcus epidermidis. There are a wide number of nanomaterials that can be used as antibacterial for different types of bacteria [18].
In this study, we will synthesize rGO-Ag NPs nanocomposite by an easy, low-cost, one-step chemical method, and study their optical and structural properties. They will be utilized as an antibacterial for four different types of bacteria that cause gum and tongue infections, and we will study the improvement of the effectiveness of the antibacterial by exposure to a low-level laser to the rGO-Ag NPs nanocomposite that absorbs the laser wavelength.
Materials and Methods
Silver nitrate (AgNO3), Sodium Borohydride (NaBH4) was purchased from market commercial, and Graphene Oxide (GO) has been synthesized by the hummer method [19]. The four types of bacteria were obtained from the microbiology laboratory and were activated and then put in dishes. Different concentrations of the nanocomposite were added and then irradiated [20, 21].
Method of synthesis of silver nanoparticles with graphene oxide
The rGO-Ag NPs were synthesized using the same chemical method as in our previous work [22]. In detail, the concentration of GO was 1 mg/mL by adding 10 mg of GO to 10 mL of distilled water, which was gradually added to 0.3 mmol/L of AgNO3 aqueous solution under vigorous stirring at room temperature, followed by the slow gradual addition of 0.1 mol/L of NaBH4 to the mixture solution. The color of the mixture turns from light yellow to dark yellow, then purple, and then dark brown. Then the rGO-Ag NPs nanocomposites were obtained by centrifugation at 8000 r/min and washed with distilled water to get rid of impurities and reduce toxicity. Finally, the rGO-Ag NPs are ready for analysis.
On the other hand, silver nanoparticles were prepared without graphene oxide by adding gradually 0.1 mol/L of NaBH4 to 0.3 mmol/L of AgNO3, in the same way.
Antibacterial activity assay of rGO-Ag NPs
The antibacterial activity of rGO-Ag NPs has been examined against four clinical isolates: Lactobacillus, Escherichia coli (E. coli) (gram-negative), Porphomnas, and Streptococcus mutans (gram-positive). The stock cultures for these four bacterial isolates were transferred into Mueller Hinton agar medium, incubated overnight at 37 °C, and stored in the refrigerator at 4 °C until used. The antibacterial activity of rGO-Ag NPs was inspected by utilizing a healthy dissemination procedure. Wells with diameters of about 6 mm was made at the agar media surface by micropipette tips, then NPs suspensions with different concentrations were added to the wells. These plates were incubated for 24 h. The antibacterial effectiveness of rGO-Ag NPs was recorded by measuring inhibition zone diameters from different directions using a ruler more than once. All tests were duplicates, and refined water was utilized as a control treatment.
Results and Discussion
The optical properties of rGO-Ag NPs and Ag NPs were obtained by using a UV-Vis spectroscopy and the result is shown in Fig. 1.
Figure 1(a) showed a curve for Ag NPs only and appeared with one peak at (392 nm) referring to the Ag NPs, and Fig. 1(b) for rGO-Ag NPs showed two peaks, one at 400 nm referring to Ag NPs and the second at 268 nm referring to reduced graphene oxide. rGO represented the transitions between π = π* in C==C bound. The two peaks have a redshift that indicates the nanoparticles of silver seemed to be getting smaller and the graphene oxide has been reduced. Also it indicates the removal of the oxygen groups and the close proximity to graphene. The curve in Fig. 1(b) indicates that the silver nanoparticles have an anchor on the reduced graphene sheets. This result is compatible with other reports [23].
From the wide absorbance at 400 nm, the diode laser (GaN) with a wavelength of 405 nm was chosen for radiation of the nanomaterials and study of the effect of the laser on the killing rate of different types of bacteria.
The atomic force microscopy (AFM) images show the structure of the sample in 2D and 3D and the statistical distribution as shown in Fig. 2.
Figures 2(a) and 2(b) are images of the rGO-Ag NPs nanocomposite, and in Fig. 2(c), we note that the average particle size is 71.26 nm, which indicates that the material is within the nanoscale.
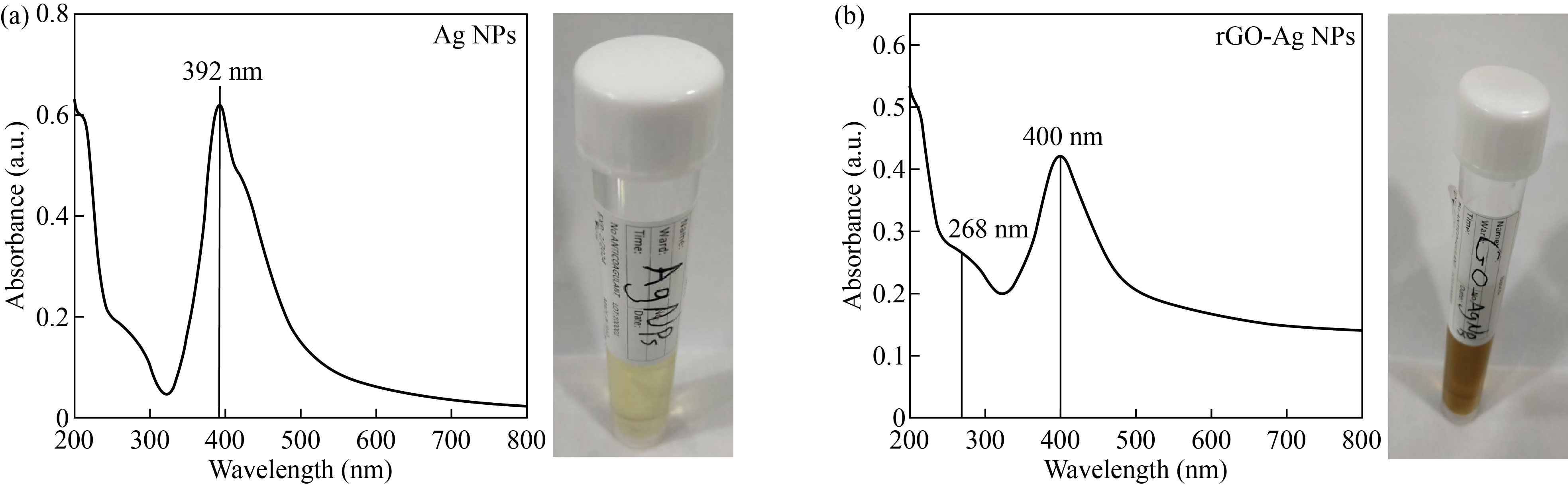
Fig. 1 UV-Vis absorption for (a) Ag NPs and (b) rGO-Ag NPs
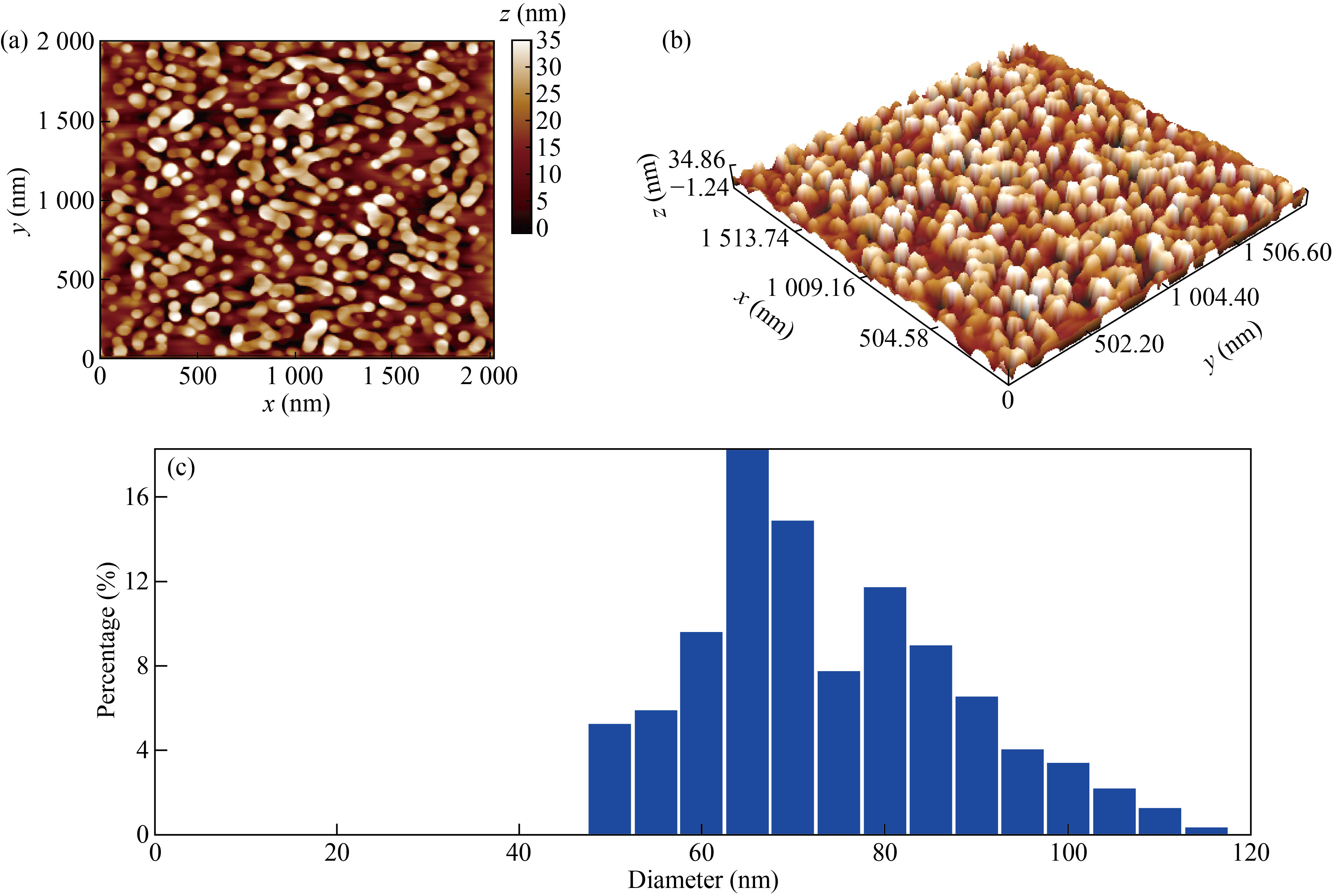
Fig. 2 The AFM images show the structure of the sample in (a) 2D, (b) 3D and (c) the statistical distribution
Effect of rGO-Ag NPs nanocomposite on bacteria
Figures 3‒6 show the percentage of bacterial killing after being injected individually with different volumes of 10, 20, 30, and 40 μL of distilled water, graphene oxide, silver nanoparticles, and the rGO-Ag NPs nanocomposite. Furthermore, a 24 h incubation period at 37°C with increasing volumes of nanomaterials killed a large percentage of bacteria for bacterial species (E. coli) (Lactobacillus-Porphomnas and Streptococcus).
We note that there is a relationship between adding volumes of the nanomaterial and the percentage killing of bacteria, which is directly proportional. This means that the killing increases with increasing volumes of rGO-Ag NPs and the best one is 40 μL. This result is compatible with other reports [24, 25].

Fig. 3 The killing of percentage of Lactobacillus bacteria with different amounts of nanomaterials (10, 20, 30, 40 µL)
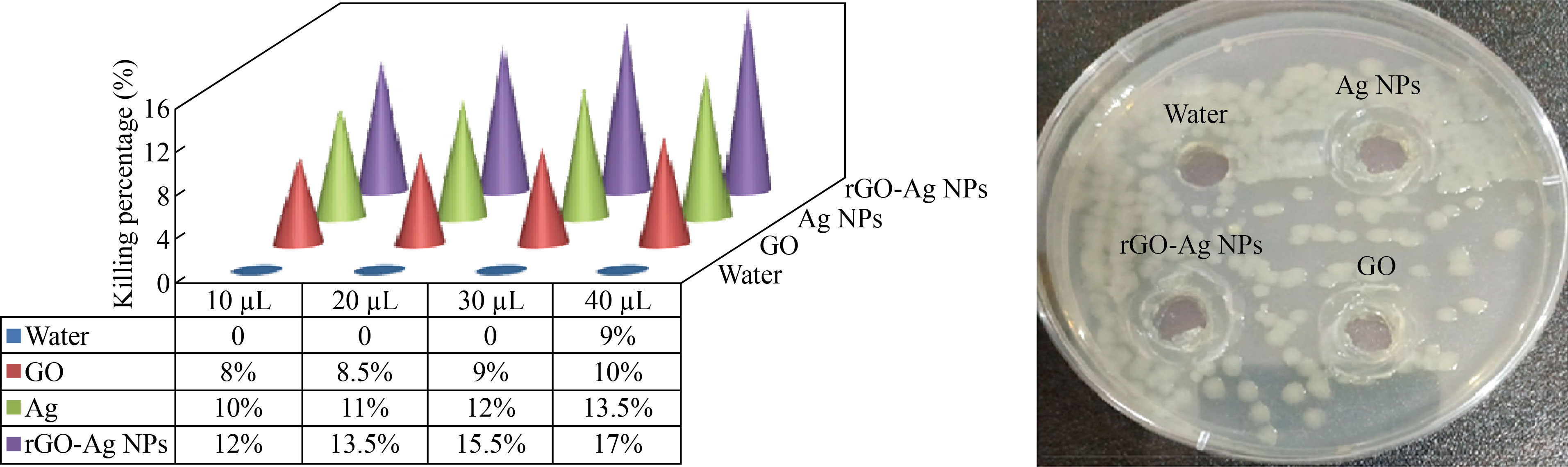
Fig. 4 The killing percentage of E. coli bacteria with different amounts of nanomaterials (10, 20, 30, 40 µL)

Fig. 5 The killing percentage of Porphomnas bacteria with different amounts of nanomaterials (10, 20, 30, 40 μL)

Fig. 6 The killing percentage of Streptococcus bacteria with different amounts of nanomaterials (10, 20, 30, 40 μL)
Effect of nanocomposite and laser in killing bacteria
When irradiating samples with a diode laser of wavelength 405 nm at a distance of 20 cm for different time periods (1, 2, 3, 4 min), for both types of bacteria (gram-negative and positive), using the same volume for all samples, which is 40 μL of each of silver Ag, GO, and nanocomposite rGO-Ag NPs, after an incubation period that lasted 24 h at a temperature of 37 °C, we found that the killing percentage in bacteria increased with the increase in the time irradiation periods, and the highest killing percentage obtained was an irradiation period of 4 min.
In addition, it was found that the killing percentage increases when using the laser with the nanocomposite; the reason is that the nanocomposite has a high absorption capacity for the laser, and the laser used will be absorbed by the nanocomposite and thus become a movement of the nanoparticles in a state of irritation, which helps in their penetration into the cell wall to contribute to the increasing bacteria killing percentage, as shown in Figs. 7‒10.

Fig. 7 The killing percentage of Streptococcus bacteria when adding 40 μL of nanomaterials with diode laser irradiation with a wavelength of 405 nm (1, 2, 3, 4 min)
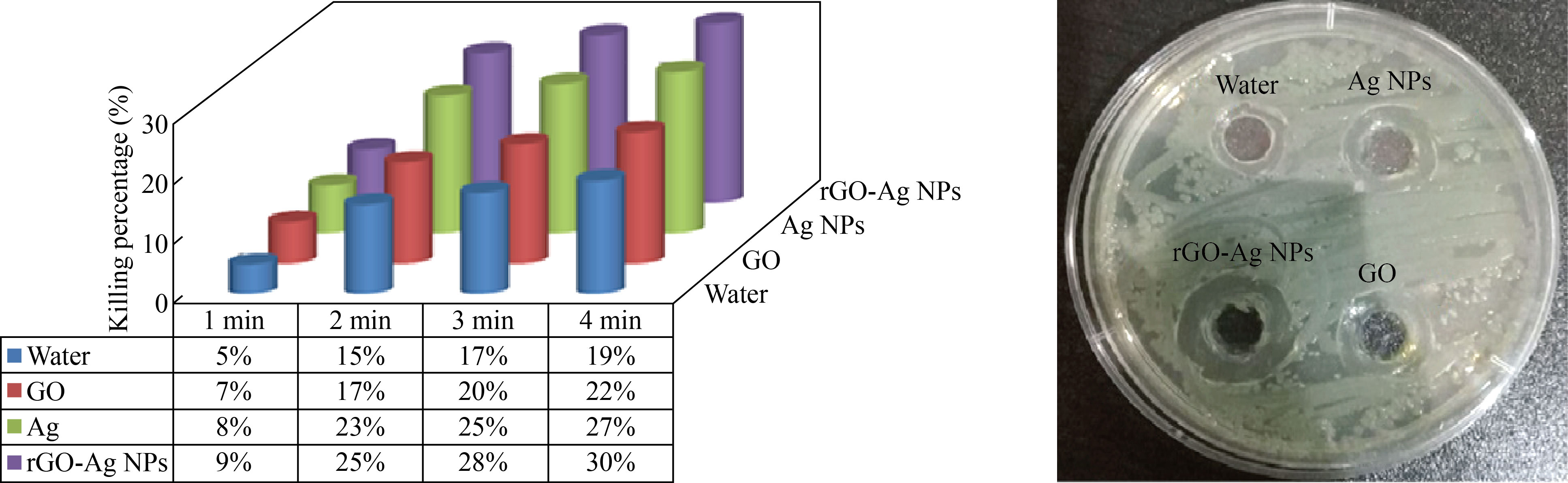
Fig. 8 The killing percentage of Porphomnas bacteria when adding 40 μL of baking materials with diode laser irradiation of 405 nm wavelength (1, 2, 3, 4 min)

Fig. 9 The killing percentage of E. coli bacteria when adding 40 μL of nanomaterials with diode laser irradiation with 405 nm wavelength (1, 2, 3, 4 min)
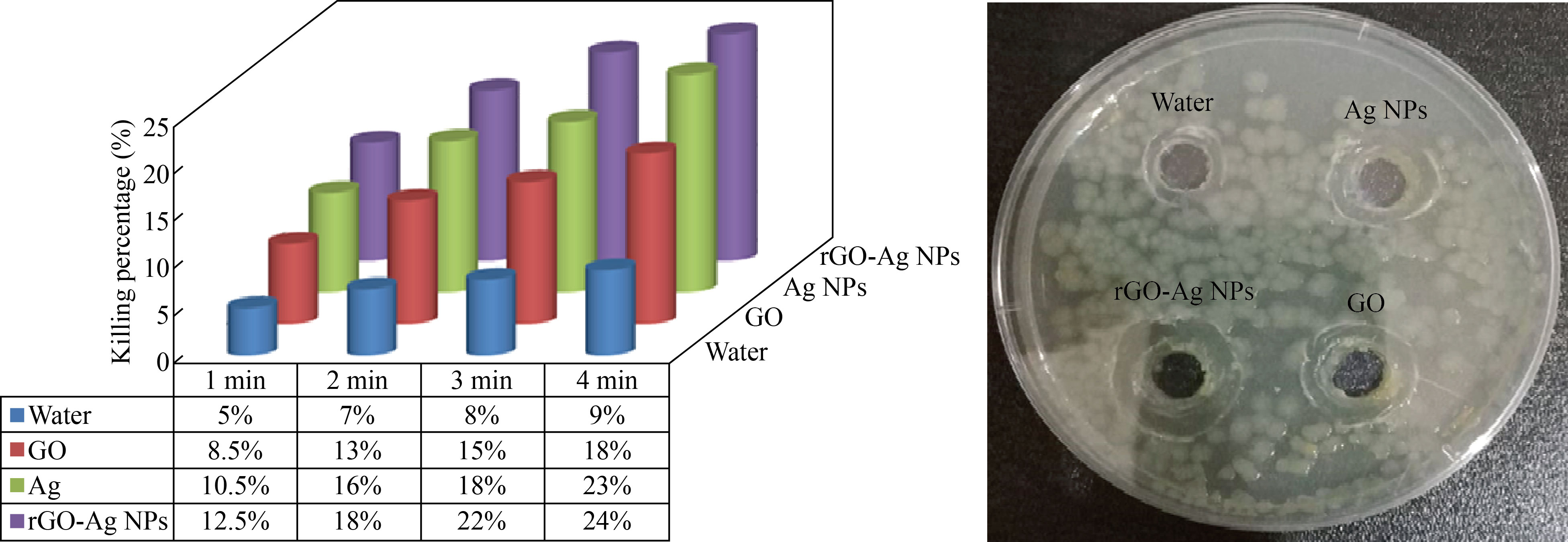
Fig. 10 The killing percentage of Lactobacillus bacteria when adding 40 μL of nanomaterials and 405 nm diode laser radiation (1, 2, 3, 4 min)
Disruption of bacterial cells is the main mechanism against gram-negative bacteria, while inhibition of cell division can explain the lysis of gram-positive cells.
The nanocomposites cause stronger damage towards the gram-negative membrane compared to gram-positive bacteria, and the antimicrobial potential of this nanocomposite is affected by the thickness of the cell wall of the microorganisms. The gram positive contains a thick layer (20–80 nm) of peptidoglycan bound to teichoic acid. Whereas in gram-negative bacteria, the cell wall is of a thin (7–8 nm) peptidoglycan layer. Their bactericidal action can be portrayed as releasing metal ions, penetrating through the cell membrane, and generating reactive oxygen species (ROS), DNA, protein, mitochondria, lipids and membrane damage, leading to cell death.
Through a comparison with the results of researchers on the use of nanomaterials in killing and inhibiting bacteria as shown in Table 1, it was found that the rGO-Ag NPs nanocomposite with laser caused a high rate of killing for different types of gram-negative and gram-positive bacteria when adding a small volume (40 μL) of the rGO-Ag NPs nanocomposite, which is quick to prepare, is not financially expensive and does not require the availability of special laboratory conditions.
Table 1 Comparison of the previous works with this study
Compound | Nanomaterials | Type of bacteria | Volumes/ concentrations | Ref. |
rGO-AgNPs |
GO, AgNO3, sodium citrate, 1 h, 50 °C |
E. coli |
40 µL |
[26] |
rGO-AgNPs |
AgNO3, rGO, L.AA, NaNO3, H2O2, KMnO4, HCL 60 °C, 1 h | E. coli S. aureus P. aeruginosa C. albicans |
80–85 µL/mg |
[27] |
rGO-AgNPs | H2SO4, H3PO4, graphite flakes, HCL, KMnO4, pure silver plate 99.9% | E. coli S. aureus |
104–105 CFU/mL |
[28] |
rGO-AgNPs |
GO, AgNO3, KMnO4, H2SO4, NaBH4, Hydrochloric acid 4 h, 50 °C |
E. coli S. aureus |
10 µL |
[29] |
rGO-Ag NPs |
AgNO3, NaBH4, rGO, Diode laser 405 nm 3 min, room temperature | E. coli S. aureus Lactobacallus Porphomnas |
10–40 µL |
This study |
Conclusion
It turns out that the proposed method for preparing the reduced graphene oxide compound with silver nanoparticles, rGO-Ag NPs, was an easy and inexpensive method. The study of the optical and structural properties of the rGO-Ag NPs nanocomposite showed that there are two peaks at 400 nm and 268 nm, and from the AFM examination, it was shown from the knowledge of the surface properties that the average particle size is 71.26 nm.
Also, the result shows that the rGO-Ag NPs show a relationship between adding volumes of the nanomaterial and the percentage killing of bacteria, which is directly proportional. This means that the killing increases with increasing volumes of rGO-Ag NPs, and the best one is 40 μL. Through the absorbance, a diode laser of wavelength 405 nm was selected and applied with the rGO-Ag NPs nanocomposite for the same kinds of bacteria and for different periods of time, and it showed that increasing the irradiation period with volume stability of 40 μL causes a higher killing percentage.
These results are promising and can be applied in the treatment of gum and tongue infections after clinical studies.
References
[1] C. Pendyala, R.V.C. Tiwari, H. Dixit, et al. Contemporary apprise on LASERS and its applications in dentistry. International Journal of Oral Health and Medical Research, 2017, 4: 47–51. https://begol.ir/book/wp-content/uploads/2022/03/LASERS-and-its-Applications-in-Dentistry.pdf
[2] G. Mancuso, A. Midiri, E. Gerace, et al. Bacterial antibiotic resistance: The most critical pathogens. Pathogens, 2021, 10: 1310. https://doi.org/10.3390/pathogens10101310
[3] T.M. Uddin, A.J. Chakraborty, A. Khusro, et al. Antibiotic resistance in microbes: History, mechanisms, therapeutic strategies and future prospects. Journal of Infection and Public Health, 2021, 14: 1750–1766. https://doi.org/10.1016/j.jiph.2021.10.020
[4] J.W. Xu, K. Yao, Z.K. Xu. Nanomaterials with a photothermal effect for antibacterial activities: An overview. Nanoscale, 2019, 11: 8680–8691. http://dx.doi.org/10.1039/C9NR01833F
[5] G. Guisbiers, S. Mejía-Rosales, F.L. Deepak. Nanomaterial properties: Size and shape dependencies. Journal of Nanomaterials, 2012, 2012: 180976. https://doi.org/10.1155/2012/180976
[6] L. Stander, L. Theodore. Environmental implications of nanotechnology—An update. International Journal of Environmental Research and Public Health, 2011, 8: 470–479. https://doi.org/10.3390/ijerph8020470
[7] A. Kokai, A. Iles. Materials sovereignty: Pathways for shaping nanotechnology design. Elementa: Science of the Anthropocene, 2020, 8: 14. https://doi.org/10.1525/elementa.410
[8] L. Filipponi, D. Sutherland, I.N. Center. Introduction to nanoscience and nanotechnologies. NANOYOU Teachers Training Kit in Nanoscience and Nanotechnologies, 2010: 1–29. https://www.nanoyou.eu/attachments/188_Module-1-chapter-1.pdf
[9] S.M. Bhagyaraj, O.S. Oluwafemi. Nanotechnology: The Science of the Invisible. Synthesis of Inorganic Nanomaterials. Woodhead Publishing, 2018: 1–18. https://doi.org/10.1016/B978-0-08-101975-7.00001-4
[10] R. Kumar, K.R. Aadil, S. Ranjan, et al. Advances of nanotechnology and nanomaterials based strategies for neural tissue engineering. Journal of Drug Delivery Science and Technology, 2020, 57: 101617. https://doi.org/10.1016/j.jddst.2020.101617
[11] V. Mangematin, S. Walsh. The future of nanotechnologies. Technovation, 2012, 32(3-4): 157–160. https://doi.org/10.1016/j.technovation.2012.01.003
[12] A. Al-sharani, W. Al-Hajj, A. Madfa. Clinical efficacy of nanosilver and chlorhexidine in the treatment of plaque-induced gingivitis: Randomized controlled clinical trial. Journal of Oral Research, 2018, 7(7): 238–244.
[13] F. Carrouel, S. Viennot, L. Ottolenghi, et al. Nanoparticles as anti-microbial, anti-inflammatory, and remineralizing agents in oral care cosmetics: A review of the current situation. Nanomaterials (Basel), 2020, 10: 140. https://doi.org/10.3390/nano10010140
[14] M. Dhanalakshmi, S. Thenmozhi, K.M. Devi, et al. Silver nanoparticles and its antibacterial activity. Int J Pharm Biol Arch, 2013, 4: 819–826.
[15] R. Al-Assaly, A. Al-Nafiey. Synthesize rGO-Ag NPs nanocomposite by a simple physical method and applying in water treatment. AIP Conference Proceedings, 2022, 2386: 080030. http://dx.doi.org/10.1063/5.0067431
[16] X.Y. Dong, Z.W. Gao, K.F. Yang, et al. Nanosilver as a new generation of silver catalysts in organic transformations for efficient synthesis of fine chemicals. Catalysis Science & Technology, 2015, 5: 2554–2574. http://dx.doi.org/10.1039/C5CY00285K
[17] H.H. Bahjat, R.A. Ismail, G.M. Sulaiman, et al. Magnetic field-assisted laser ablation of titanium dioxide nanoparticles in water for anti-bacterial applications. Journal of Inorganic and Organometallic Polymers and Materials, 2021, 31: 3649–3656. http://dx.doi.org/10.1007/s10904-021-01973-8
[18] X. Chen, H.J. Schluesener. Nanosilver: A nanoproduct in medical application. Toxicology Letters, 2008, 176: 1–12. https://doi.org/10.1016/j.toxlet.2007.10.004
[19] A.K.H. Al-Nafiey. Reduced graphene oxide-based nanocomposites: Synthesis, characterization and applications. Lille 1, 2016. https://www.theses.fr/2016LIL10009
[20] M.S. Hu, H.L. Chen, C.H. Shen, et al. Photosensitive gold-nanoparticle-embedded dielectric nanowires. Nature Materials, 2006, 5: 102–106. https://doi.org/10.1038/nmat1564
[21] M. Kauranen, A.V. Zayats. Nonlinear plasmonics. Nature Photonics, 2012, 6: 737–748. https://doi.org/10.1038/nphoton.2012.244
[22] N. Al-Janabi, A.K. Al-Nafiey. One-step synthesis and characterization of reduced graphene oxide/silver nanoparticles using chemical reaction. Journal of Physics: Conference Series, 2021, 1818(1): 012209. https://doi.org/10.1088/1742-6596/1818/1/012209
[23] P.D. Howes, R. Chandrawati, M.M. Stevens. Bionanotechnology. Colloidal nanoparticles as advanced biological sensors. Science, 2014, 346: 1247390. https://doi.org/10.1126/science.1247390
[24] M.S. Jabir, T.M. Rashid, U.M. Nayef, et al. Inhibition of Staphylococcus aureus α-hemolysin production using nanocurcumin capped Au@ZnO nanocomposite. Bioinorganic Chemistry and Applications, 2022, 2022: 2663812. https://doi.org/10.1155/2022/2663812
[25] A. Younus, S. Al-Ahmer, M. Jabir. Evaluation of some immunological markers in children with bacterial meningitis caused by Streptococcus pneumoniae. Research Journal of Biotechnology, 2019, 14: 131–133.
[26] M. Cobos, I. De-La-Pinta, G. Quindós, et al. Graphene oxide-silver nanoparticle nanohybrids: Synthesis, characterization, and antimicrobial properties. Nanomaterials (Basel), 2020, 10: 376. https://doi.org/10.3390/nano10020376
[27] J.Chen, B. Yao, C. Li. An improved Hummers method for eco-friendly synthesis of graphene oxide. Carbon, 2013, 64: 225–229. http://dx.doi.org/10.1016/j.carbon.2013.07.055
[28] L.S. Yang, M.C. Zou, S.T. Wu, et al. Graphene oxide glue-electrode for fabrication of vertical, elastic, conductive columns. ACS Nano, 2017, 11: 2944–2951. https://doi.org/10.1021/acsnano.6b08323
[29] L. Huang, H.T. Yang, Y.H. Zhang, et al. Study on synthesis and antibacterial properties of Ag NPs/GO nanocomposites. Journal of Nanomaterials, 2016, 2016: 1–9. https://doi.org/10.1155/2016/5685967
Copyright© Noora Al-Janabi and Amer Al-Nafiey. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.