Research Article
Saffron in KCl Mediated by Glassy Carbon Electrode Using Cyclic Voltammetry
Muhammed Mizher Radhi 1*, Lamyaa Abd Alrahman Jawad 2, Emad Abbas Jaffar Al-Mulla 3
1 Radiological Techniques Department, Health and Medical Technology College-Baghdad, Middle Technology University, Baghdad, Iraq.
2 Physiotherapy Techniques Department, Health and Medical Technology College-Baghdad, Middle Technology University, Baghdad, Iraq.
3 College of Health and Medical Techniques, Al-Furat Al-Awsat Technical University, 54003 Al-Kufa, Iraq.
* Corresponding author. E-mail: mmradhi@yahoo.com
Received: Feb. 22, 2018; Accepted: Apr. 10, 2018; Published: Jun. 21, 2018
Citation: Muhammed Mizher Radhi, Lamyaa Abd Alrahman Jawad, and Emad Abbas Jaffar Al-Mulla, Saffron in KCl Mediated by Glassy Carbon Electrode Using Cyclic Voltammetry. Nano Biomed. Eng., 2018, 10(2): 181-185.
DOI: 10.5101/nbe.v10i2.p181-185.
Abstract
Saffron in aqueous solution was studied via electrochemical analysis with KCl as a supporting electrolyte using cyclic voltammetric technique to determine the redox current peaks at different concentration, pH and scan rate (SR). It was found that an oxidation current peak appeared at potential +268 mV and two reduction current peaks at -282 and -850 mV. The oxidation current peak of saffron disappeared in alkaline medium at pH = 12. An enhancement was observed in acidic medium at pH = 6. Based on Randel equations and by using different scan rates, the diffusion coefficient (Df) for oxidation-reduction current peaks of saffron in KCl solution was determined with nearly equal values as of 1.87×10-5 and 1.12×10-5 cm2/sec, respectively. The results indicate a low detection limit of the different concentrations of saffron in KCl solution as determined from the calibration graph, and a high reliability revealed by the relative standard deviation (RSD). Stability of the GCE for oxidation-reduction current peaks was recorded as ± 7.12% and ± 1.04%, respectively.
Keywords: Saffron; Cyclic voltammetry; KCl; Glassy carbon electrode; Randel equation
Introduction
Scientists have done much research studying the electrochemical behavior of chemical compounds in aqueous electrolytes by cyclic voltammetry (CV) [1-5]. Saffron in aqueous solution is a compound instruction from saffron crocus flower; picrocrocin is responsible for saffron's flavour. The chemical formula of picrocrocin is C16H26O7; the chemical name is 4-(β-D-glucopyranosyloxy)-2, 6, 6-trimethylcyclohex-1-ene-1-carboxaldehyde); and the chemical structure is shown in Scheme 1 [6]. The electrochemical properties of saffron were investigated by CV and square wave voltammetry (SWV) at plane and multiwalled carbon nanotubes modified carbon electrode (MWCNT/CE). Saffron produces an anodic peak at MWCNTs modified CPE with a quasi-reversible nature in buffer solution. The application for the quantitative analysis of saffron (Crocus sativus) was determined by the detection limit with low value [7, 8]. Saffron was characterized by electrochemical analysis using voltammetric method; different concentrations with calibration model were optimized in fast response [9]. I-motif and anti-cancer drugs were studied by electrochemical techniques by using CV and differential pulse voltammetry (DPV) methods to determine and characterize saffron carotenoids on the modified electrode for treatment of the drugs [10, 11]. Three natural drugs of crocin, kaempferol and podophyllotoxin were evaluated as antioxidant medicine by cyclic voltammetric technique using multi-walled carbon nanotube paste electrode (MWCNTPE) at acidic pH 4.5. It was found that the scavenging ability of these drugs with oxidation property produced by electrochemical reduction of oxygen [11]. The electrochemical performance of m-AgSAE for the reduction of tetrachlorvinphos was evaluated using CV, DPV and SWV, respectively. It was applied for the detection of organophosphate pesticide tetrachlorvinphos in pH 7 buffer solution; it also offers new possibilities for fast and sensitive analysis of tetrachlorvinphos and its structural analogs in environmental samples [12]. In this study, cyclic voltammetric method was used to analyze the saffron compound in KCl electrolyte by glassy carbon electrode (GCE) to determine the electrochemical properties of redox current peaks for saffron compound.
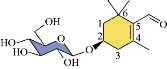
Scheme 1 Structure of picrocrocin.
Experimental
Materials and method
Saffron was supplied from EDMAN Company, Iran as solid material which dissolved in deionized water after filtering by filter paper to be used in the experiments. KCl as powder was from SCRC, China. And NaOH was from BDH Company, Germany.
Instrument: Potentiostat
An instrument of EZstat series (Potentiostat/Galvanostat) from NuVant Systems Inc., USA was used in the experiments. The electrochemical bio-analytical cell was connected to a potentio-stat device and was monitored by special software to perform CV. Silver-silver chloride reference electrode (Ag/AgCl in 3 M NaCl) and platinum wire (1 mm diameter) were used as a reference and counter electrode, respectively. The GCE was used in this study after being cleaned with alumina solution and treated with ultrasonic path water for 10 min. The three electrodes were immersed in cyclic voltammetric cell (10 mL) with the solution.
Results and Discussion
Study of different concentrations of saffron in KCl solution (calibration graph)
Fig. 1 illustrates the cyclic voltammogram of saffron solution in supporting electrolyte (0.1 M KCl) at different concentrations (0.1 - 0.5 mM). The oxidation-reduction current peaks of saffron at potential +125 and -200 mV, respectively. An enhancement was observed to higher current against the increasing in concentration and the shifting to higher potential [13]. As shown in Fig. 2, a low detection limit of calibration graph was found with high sensetivity of the graph R2 = 1, by the equation y = 50x + 66. The results confirmed that the electrochemical method by CV is very sensitive to saffron in KCl solution and can be considered in CV techinque [11-14].
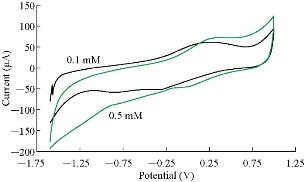
Fig. 1 Cyclic voltammogram of saffron at different concentrations in 0.1 M KCl solution at pH = 6 on GCE against Ag/AgCl as reference electrode (scan rate 100 mV/sec).
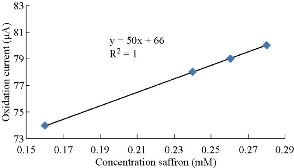
Fig. 2 Relationship between oxidation current peak and different concentrations of saffron in 0.1 M KCl solution on GCE.
Effect of different pH
Saffron compound has especial electrochemical behavior for oxidation-reduction current peaks at different pH, which is shown in Table 1 and discussed in Fig. 3. The oxidation peak was enhanced in acidic medium and disappeared in alkaline medium, while the reduction peak remained in both media (acid and base). The relationship of oxidation-reduction current peaks of saffron in acidic and alkaline media is shown in Fig. 4 and 5, respectively [15].
Table 1 Redox current peaks of saffron in 0.1 M KCl at different pH
|
pH |
Ipa (uA) |
Ipc (uA) |
|
6 |
73 |
48 |
|
7 |
28 |
33 |
|
11 |
21 |
21 |
|
12 |
19 |
24 |
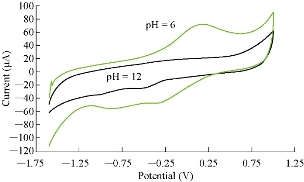
Fig. 3 Cyclic voltammogram of 0.5 mM saffron in 0.1 M KCl solution at different pH (acidic and alkaline) on GCE against Ag/AgCl as reference electrode and scan rate of 100 mV/sec.
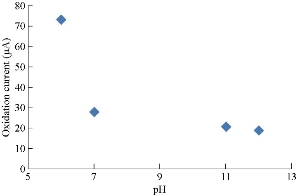
Fig. 4 Relationship between the oxidation current peak of saffron in 0.1M KCl solution and different pH on GCE.
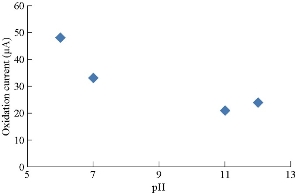
Fig. 5 Relationship between the reduction current peak of saffron in 0.1 M KCl solution and different pH on GCE.
Effect of different scan rate
One of the electrochemical properties which was used to characterization saffron was different scan rates, so as to confirm the redox current peaks and also to calculate the diffusion coefficient by Randel equation [16]. Fig. 6 shows the effect of different scan rates on the oxidation-reduction current peaks. It is revealed that the current was enhanced with increasing the scan rate (Table 2), and there was a good relationship between the oxidation-reduction current peaks and the scan rate (Fig. 7 and 8). The equation for oxidation peak is y = 685.61x + 0.4067, with high sensitivity as R2 = 0.9916, and for reduction peak is y = 411.94 + 7.5933, with sensitivity as R2 = 0.9979 [17].
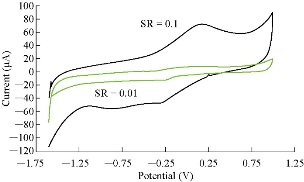
Fig. 6 Cyclic voltammogram of 0.5 mM saffron in 0.1 M KCl solution at different scan rates (0.01 to 0.1 mV/sec)on GCE against Ag/AgCl as reference electrode.
Table 2 Redox current peaks of saffron in 0.1 M KCl solution at different scan rates
|
Scan rate |
Ipa (uA) |
Ipc (uA) |
|
0.01 |
8.85 |
11.9 |
|
0.02 |
14.2 |
14.9 |
|
0.03 |
19.5 |
19.7 |
|
0.04 |
28.1 |
24.3 |
|
0.05 |
34.9 |
28.5 |
|
0.06 |
41.4 |
33 |
|
0.07 |
47.9 |
37.1 |
|
0.08 |
54.2 |
40.2 |
|
0.09 |
59 |
45.2 |
|
0.1 |
73.1 |
47.7 |
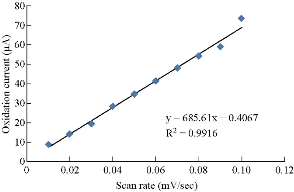
Fig. 7 Relationship between oxidation current peak of saffron in 0.1M KCl solution and different scan rates on GCE.
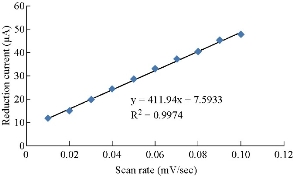
Fig. 8 Relationship between reduction current peak of saffron in 0.1M KCl solution and different scan rates on GCE.
Diffusion coefficient (Df)
The usual of mathematical method can be used in finding the Df of redox process for the saffron compound in KCl solution from Randles-Seveik equation which describes reversible redox couple and the peak current [18, 19]:
Ip = (2.69 × 105) n3/2 AC Df1/2 v1/2, (1)
where, Ip is the current peak; n is the number of moles of electrons transferred in the reaction; A is area of the electrode; Df is the diffusion coefficient; and V is scan rate of the applied potential. It was found that the Df of oxidation-reduction process of saffron in KCl solution was 1.87 × 10-5 and 1.12 × 10-5 cm2/sec, respectively [19-21].
Reliability and stability study
Saffron compound in KCl solution was studied by GCE in CV. To ensure that the results were reliable in this study, ten times of scanning was used for the voltammogram (Fig. 9), and the relative standard deviation (RSD) was determined for both oxidation-reduction current peaks of saffron as of ± 7.12% and ± 1.04%, respectively [21-23].
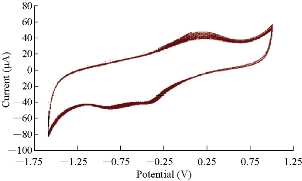
Fig. 9 Cyclic voltammogram of 0.5 mM saffron in 0.1 M KCl solution on GCE at ten times against Ag/AgCl as reference electrode and scan rate of 100 mV/sec.
Conclusions
Saffron compound was studied by cyclic voltammetric technique to find its electrochemical behavior in KCl as supporting electrolyte. It was found that saffron compound was oxidative reagent in acidic pH and antioxidant in alkaline medium. Diffusion coefficient values of oxidation-reduction current peaks were also found by Randel equation from different scan rates, which were 1.87 × 10-5 and 1.12 × 10-5 cm2/sec, respectively, indicating that the redox process was reversible. In addition, a low detection limit value was determined from the calibration graph at different concentrations of saffron in KCl solution by the equation y = 50x + 66, with high sensitivity as R2 = 1.
Conflict of Interests
The authors declare that no competing interest exists.
References
1. Z. Nasri, E. Shams, Application of silica gel as an effective modifier for the voltammetric determination of dopamine in the presence of ascorbic acid and uric acid. Electrochim. Acta, 2009, 54: 7416-7421.
2. F. Teng, W. Yao, Y. Zheng, et al., Synthesis of flower-like CuO nanostructures as a sensitive sensor for catalysis. Sens. Actuators B Chemical, 2008, 134: 761-768.
3. X. Wang, N. Yang, and Q. Wan, Cyclic voltammetric response of nicotinic acid and nicotinamide on a polycrystalline gold electrode. Electrochim. Acta, 2006, 52: 361-370.
4. P. Kalimuthu, S.A. John, Electropolymerized film of functionalized thiadiazole on glassy carbon electrode for the simultaneous determination of ascorbic acid, dopamine and uric acid. Bioelectrochemistry, 2009, 77: 13-21.
5. M.M. Radhi, W.T. Tan, M.Z.B.A. Rahman, et al., Electrochemical redox of Hg2+ mediated by activated carbon modified glassy carbon electrode. Int. J. Electrochem. Sci., 2010, 5: 615-622.
6. A.L. Lopresti, P.D. Drummond, Saffron (Crocus sativus) for depression: a systematic review of clinical studies and examination of underlying antidepressant mechanisms of action. Human Psychopharmacology: Clinical and Experimental, 2014, 29: 517-527.
7. R. Dara, G. Ahmad, and N. Israr, Electrochemical behavior of kaempferol and its determination in presence of quercetin employing multi-walled carbon nanotube modified carbon paste electrode. Analytical Chemistry Research, 2016, 7: 1-8.
8. A. Riyaz, K. Brahmana, and N. Khuranab, Evaluation of antioxidant activity of crocin, podophyllotoxin and kaempferol by chemical, biochemical and electrochemical assays. Arabian Journal of Chemistry, 2017, 10: 1119-1128.
9. K. Heidarbeigi, S.S. Mohtasebi, J. Serrano-Diaz, et al., Flavour characteristics of Spanish and Iranian saffron analysed by electronic tongue. Quality Assurance and Safety of Crops and Foods, 2016, 8(3): 359-368.
10. E.A.J. Al-Mulla, Nanoparticles of TiO2-ZnO modified polystyrene-acrylonitrile characterization using glassy carbon electrode. Nano Biomed. Eng., 2018, 10(1): 34-39.
11. R.A. Dar, P.K. Brahman, N. Khurana, et al., Evaluation of antioxidant activity of crocin, podophyllotoxin and kaempferol by chemical, biochemical and electrochemical assays. Arabian Journal of Chemistry, 2017, 10: 1119-1128.
12. N. Al-Qasmi, A. Hameedac, A.N. Khan, et al., Mercury meniscus on solid silver amalgam electrode as a sensitive electrochemical sensor for tetrachlorvinphos. Journal of Saudi Chemical Society, 2018, 22(4): 496-507.
13. E.A. Sutter, P.W. Sutter, Determination of redox reaction rates and orders by in situ liquid cell electron microscopy of Pd and Au solution growth. J. Am. Chem. Soc., 2014, 136(48): 16865-16870.
14. F.H. Jabbar, Z.J. Kadhim, A.A. Abdullah, et al., Epoxidized palm oil plasticized polycaprolactone nanocomposites preparation. Nano Biomed. Eng., 2017, 9(3): 214-220.
15. M.D.T. Rahman, M.D.E. Hossain, and M.Q. Ehsan, Spectrophotometric and cyclic voltammetric study of interaction of Fe(III) with vitamin B3 and vitamin B6. Journal of Bangladesh Academy of Sciences, 2014, 38(2): 143-153.
16. G.J. Islam, H.M.N. Akhtar, M.A. Mamun, et al., Investigations on the redox behaviour of manganese in manganese(II)–saccharin and manganese(II)–saccharin–1,10-phenanthroline complexes.Journal of Saudi Chemical Society, 2009, 13: 177-183.
17. F. Haque, M.S. Rahman, E. Ahmed, et al., A cyclic voltammetric study of the redox reaction of Cu(II) in presence of ascorbic acid in different pH media. Dhaka Univ. J. Sci., 2013, 61(2): 161-166.
18. P. Zanello, Inorganic electrochemistry: Theory, practice and application. Royal Society of Chemistry, 2003: 120-130.
19. S.R. Crouch, D.A. Skoog, Principles of instrumental analysis. Cengage Learning, 2006: 213-239.
20. M.M. Radhi, E.A.J. Al-Mulla, Voltammetric characterization of grafted polymer electrode self-modification with carbon nanotubes (GPESMCNT). Portug. Electrochim. Acta., 2016, 34(2): 97-103.
21. L.D. Torbeck, Statistical solutions: %RSD: Friend or foe. Pharmaceutical Technology, 2010, 34: 133-139.
22. A.A. Abdullah, Electrochemical studies of copper fatty amides complex in organic medium. Res. Chem. Intermed., 2013, 39(6): 2817-2823.
23. Y.K. Abdul-Amir, M.M. Radhi, and E.A.J. Al-Mulla, Use of nano-sensors of the interferences between Pb((II) with each of Mg(II), Zn(II), Mn(II), Ca(II), Co(II) and PO4 -3 in blood medium: An electrochemical study. Nano Biomed. Eng., 2017, 9(3): 199-207.
Copyright© Muhammed Mizher Radhi, Lamyaa Abd Alrahman Jawad, and Emad Abbas Jaffar Al-Mulla. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.