Article
Physical-Chemical Properties and In Vitro Biocompatibility Assessment of Spider Silk, Collagen and Polyurethane Nanofiber Scaffolds for Vascular Tissue Engineering
Chuanglong He 1,2*, Lei Zhang 2, Hongsheng Wang 2, Fan Zhang 2, Xiumei Mo 1,2
1 State Key Laboratory for Modification of Chemical Fibers and Polymer Materials, Donghua University, Shanghai, 2016204.
2 College of Chemistry and Chemical Engineering and Biological Engineering, Donghua University,Shanghai, 201620.
* Corresponding authors. Email: hcl@dhu.edu.cn
Citation: C. He, et al., Physical-Chemical Properties and In Vitro Biocompatibility Assessment of Spider Silk, Collagen and Polyurethane Nanofiber Scaffolds for Vascular Tissue Engineering. Nano Biomed. Eng., 2009, 1(1): 80-88.
DOI: 10.5101/nbe.v1i1.p80-88
Abstract
In this study, three kinds of nanofiber scaffolds including spider silks (SS), collagen, and polyurethane (PU) were fabricated by electrospinning technique. Their physical chemical properties such as surface hydrophilicity, water stability, and porosity were investigated by water contact angle (CA) measurement, stabilization assay and scanning electron microscope (SEM). Results showed that SS scaffolds had stronger hydrophobic surface, superior water stability and higher porosity than other scaffolds. Furthermore, their in vitro biocompatibility including cell attachment, spreading, and proliferation were evaluated and compared by using porcine aorta endothelium cells (PIECs). The MTT results showed that the cell proliferation on SS nanofibers was significantly higher than that on collagen and PU scaffoldss, the SEM images demonstrated that the PIECs can migrate into SS nanofibers and maintain a spreading shape, and the RT-PCR results also indicated the SS nanofiber scaffolds promote better cell growth and proliferation. Thus, these results strongly suggest the potential application of SS nanofibers as vascular engineering scaffolds.
Keywords: Spider silk; In Vitro Biocompatibility; Electrospinning; Nanofibers; Vascular tissue engineering
1 Introduction
Vascular tissue engineering has emerged as one of the most promising approaches to developing ideal vascular substitutes with properties similar to that of native tissue [1-3]. In the last two decades, numerous efforts have been made to develop scaffolds for vascular tissue engineering by combining material synthesis and scaffold processing techniques. A variety of synthetic materials such as poly(caprolactone) (PCL) [4,5], poly (L-lactide) (PLA) [6], poly (glycolide) (PGA) [7] and naturally occurred materials such as collagen [8,9] and fibrin [10-12] can be employed for vascular substitutes and scaffolds for vascular tissue engineering. To engineer materials mimicking the physical structure and biological function of natural blood vessel, a few technologies have been exploited with varying degrees of success [10, 11, 13-15]. Electrospinning is an attractive technique to fabricate various tissue engineering scaffolds in that it permits fabrication of scaffolds that similar to the nanofibrous structure found in the native extracellular matrix (ECM), which is mainly composed of a three- dimensional network of nanofibrous proteins with fiber bundle diameter varying from 50 to 500nm [16, 17]. Nanofibrous materials have been shown to actively regulate cellular behaviors and cell matrix interactions because of their unique properties such as high surface to volume ratio and high porosity [18]. In addition, electrospinning is able to produce aligned and oriented fiber network, which provides special benefit for blood vessel tissue [19]. To construct an ideal scaffold for vascular regeneration, considerable efforts have been made to create different polymeric scaffolds using electrospinning technique, focusing on the processing parameters and physical-chemical properties of materials [14, 15, 19]. However, rather less attention has been paid to compare the biocompatibility of different nanofibrous materials for vascular tissue engineering. Spider silks (SS) have remarkable mechanical properties that make them attractive for vascular tissue engineering applications [20, 21]. The molecular struture and various characteristics of electrospun SS nanofibers have been studied widely [21, 22], little is known on their biocompatibility as tissue engineering scaffolds. The objective of this study is to seek suitable biomaterials for vascular tissue engineering by comparing the physical chemical properties and in vitro biocom patibility of SS, collagen and PU nanofibers. These nanofiber scaffolds were fabricated by electrospinning, the hydrophilicity, water stability, and porosity of resulting nanofiber scaffolds were tested, and their in vitro biocompatibility on Porcine Aorta Endothelium cells was assessed by cell adhesion, proliferation and expression of proliferating cell nuclear antigen (PCNA).
2 Materials and Methods
2.1 Materials
SS (Mw: 2.0-3.0×105) was kindly donated by Nanning Spider Research Center (Nanning, China). Collagen (Mw: 0.8-1.0 x 105) was purchased from Mingrang Biotechnology CO.,LTD. (Sichuan, China). PU ( Mw: 0.5-1.0 x 106 ) was purchased from Japan. 1,1,1,3,3,3,- hexafluoro-2-propanol(HFIP) was obtained from Dai- kin Industries Ltd (Japan). Endothelium cells from porcine aorta (PIEC) were obtained from Institute of Biochemistry and Cell Biology (Chinese Academy of Sciences, China). Unless stated otherwise, all culture media and reagents were purchased from Gibco Life Technologies CO.
2.2 Fabrication of nanofiber scaffolds
SS and Collagen solution for electrospinning were prepared by dissolving SS and Collagen in HFIP at a concentration of 8 wt%, respectively. PU was dissolved in a solvent mixture of DMF/THF (1:1 in volume) at a concentration of 20 wt%. The solutions for electrospinning were mixed using an electromagnetic mixer until transparent and homogeneous. In the electrospinning process, the as-prepared solution was respectively loaded into a 5 mL plastic syringe with a stainless steel needle (inner diameter, 0.21 mm) attached. The needle was connected to a high-voltage supply (BGG6-358, BMEI CO, LTD., China). The solution was fed at 0.8ml/h using a syringe pump (789100C, Cole Parmer Instruments Co., USA). A below the needle tip, and used to collect the nanofibers. The voltage for electrospinning was set at 10-15 kV. The thicknesses of all resulting nanofibers were controlled at around 0.1 mm. These fibers were then dried in a vacuum for 48 h to remove the residual solvents. The resulting Collagen nanofibers were then crosslinked by steam of 25 % (v/v) glutaraldehyde solution for 8h and dried under vacuum overnight at room temperature.
2.3 Scaffolds characterization
The surface hydrophilicity of nanofiber scaffolds was characterized by water contact angle (CA) measurement (OCA40, Dataphysics, Germany), and photographs were taken after distilled water dripping on them for 30 sec. Stabilization of various nanofiber scaffolds was evaluated by immersing the scaffolds (1 cm2 in size) into medium under cell culture conditions for 7 d. These nanofiber scaffolds were then washed three times with water and dried in vacuum. The weights of these nanofiber scaffolds before and after incubation with medium were measured. The morphologies of electrospun fibers were ob- served with scanning electron microscope (SEM, Hitachi S-2700, Japan) at voltage of 15 kV. The diameters of the nanofibers were measured based on SEM images using image visualization software (Image J 1.34s, NIH Image, USA). The thicknesses of the nanofiber scaffolds were accurately measured with a micrometer. The apparent density and porosity of nanofiber scaffolds (NFM) were calculated according to the method reported by reference [23].
2.4 Cell adhesion and proliferation assessment
For biocompatibility assessment, the coverslips with 14 mm in diameter were placed onto the aluminum foil to collect the nanofibers scaffolds, the scaffolds were then fixed in the 24 well plate with stainless steel rings and sterilized with 75 % alcohol solution, which was re- placed with phosphate-buffered saline solution (PBS) after 2 hours. PIECs were cultured in a 5 % CO2, humid atmosphere at 37 ℃ in DMEM medium containing 10 % fetal serum, 100 units/mL penicillin and 100 units/mL streptomycin, the culture medium was re- placed every 3 d. To study cell adhesion, migration, and proliferation, PIECs (0.5×105/mL) were seeded onto nanofiber scaffolds with tissue culture plates (TCP) as control. After 6 h, 2d, and 5 d of culture, unattached cells were washed out. The cell-scaffold composites were evaluated by Hematoxylin Eosin (HE) staining. MTT assay was also used to assess the cell proliferation ability at the absorbance of 492 nm by an enzyme labeled instrument (MK3,Thermo,U.S.). For further study, the morphologies of PIECs on various nanofiber scaffolds after 5 d of culture were observed by SEM (Hitachi S-2700, Japan). Cells cultured on scaffolds were washed with 1×PBS and then fixed with 4 % glutaraldehyde for 12h at 4 ℃ The samples were dehydrated in 50 %, 75 % and 100 % alcohol solutions and dried under vacuum. Afterwards, the samples were sputter coated with gold and observed with an SEM at voltage of 15 kV.
Table 1. CA measurement of surface of nanofibers using distilled water as solvent.
|
nanofibers |
CA(L)(°) |
CA(M) (°) |
CA(R) (°) |
Err(°) |
T(℃) |
|
SS |
114.8 |
114.8 |
0 |
1.06 |
16.4 |
|
Collagen |
63.5 |
63.5 |
0 |
1.39 |
15.5 |
|
PU |
130.4 |
130.4 |
0 |
1.07 |
15.8 |
Note: Data were representatives of three independent experiments and all data were given as means±SD (n=3).
2.5 RT-PCR detection
PICEs on the different nanofibers were cultured for 7 d then trypsinized, and total RNA was extracted from the cells using QIAamp RNA mini kits (QIAGEN, USA). 5 μL of RNA was reverse transcribed with MMLV reverse transcriptase (Promega, Southampton, United Kingdom) and an oligo (dT) 15 primer into cDNA which was amplified using the design primers. Heat activation, 95 ℃ for 4 min, duplex amplification was performed using a thermocycler for 30 cycles ac- cording to the following program: 95 ℃ for 55 sec, 59 ℃annealing for 55 sec, and 72 ℃ extension for 50 sec. Followed by a final extension of 72 ℃ for 10 min. PCR fragments of PCNA bands were visualized in a 1.5 % agarose gel and at 100V electrophoresis with β-actin (318 bp) as an internal standard. The relative intensity was then compared (Photo Image Analysis Software 7.0, SONY, Japan).
2.6 Statistical analysis
Statistical analysis was performed using SPSS 10.0 software for windows. All assays were repeated with a minimum of n=3. Statistical comparisons were based on one-way analysis of variance (ANOVA). In all evaluations, P<0.05 was considered to be statistically significant.
3 Results and Discussion
3.1 Surface hydrophilicity
Figure 1 shows the CA measurement of electros pun SS, collagen and PU nanofiber scaffolds. Collagen nanofiber scaffolds exhibited a strong hydrophilic behavior; with the angle values obtained around 60° as summarized in Table 1. The CA value of SS scaffolds was 114.8°, and PU nanofiber scaffolds had the highest CA value at 130.4°. Generally, natural polymers which produced in aqueous environments have suitable surface for water contact. The results showed that the SS nanofiber scaffolds have less hydrophilic than collagen nanofiber scaffolds. It can be explained by the fact that SS has the unique hydrophobic aminoacid compositions and sequence, showing a predominance of glycine, alanine, and glutamine; there are also substantial amounts of leucine and tyrosine, which are among the larger ami no acids [24].
3.2 Water Stability
Table 2 shows the results of stability of nanofiber scaffolds in culture medium after 7 d. SS nanofiber scaffolds lost 5.55 % of their weight, and collagen scaffolds lost 16.20 % of their weight, respectively. PU nanofiber scaffolds showed the best stability and almost had not any weight loss after 7 d. It is known that collagen is polypeptide-based materials has strong hydrophilicity and low stability [25]. The results showed that SS nanofiber scaffolds in culture medium had higher stability than collagen nanofiber scaffolds. The basis for this lies in part in the amino acid sequence of silk protein, which imparts block copolymer like behavior to the protein: hard segments form a crystalline structure that is embedded in an elastic matrix [26]. It suggests that SS nanofibers are more stable as scaffolds.
3.3 Fiber diameter and porosity
Nanofiber diameters were calculated from average diameter of 100 segments of fibers which were measured from SEM images as shown in Figure 2. It was found that the average diameter of SS nanofibers was 173 nm, and the value was increase to 202 nm for collagen nanofibers. The average diameter of PU nanofibers (372 nm) was much greater than that of above two kinds of nanofibers as shown in Figure. 3. The highest porosity was found in the SS nanofibers which reached 65.62 %, however, the porosity for collagen and PU scaffolds were about 40 %. The change of fiber diameter and porosity among SS, collagen and PU nanofiber scaffolds could be explained by the conductivity in crease of electrospun solution. The addition charged ions to polymer solutions have been found to affect fiber diameter and lead fibers substantially decrease in diameter [27].
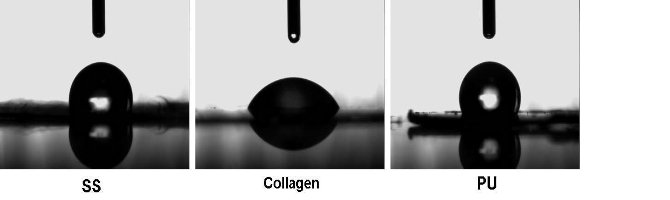
Figure 1. Photographs of CA measurement of nanofiber scaffolds after distilled water dripping on them 30s later.SS and collagen have higher polarity compared to PU, which results in the formation of salts between solvent molecules and the amino groups, the increased charges Therefore leads to a greater deposition area of electros pun fibers. In addition, the more strong hydrophobic amino acids groups in SS lead to the higher porosity.
3.4 Cell adhesion, migration and proliferation
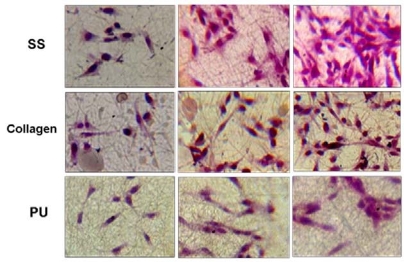
The results of HE staining showed that the PIECs attached and kept normal morphology on the surface of different nanofiber scaffolds after 6h of culture. After cultured for 2d, the number of cells on SS and collagen were higher than that on PU nanofiber scaffolds, and the number of cells on SS nanofibers was significantly higher than that on collagen and PU after 5 d as shown in Figure 4 suggesting that the PIECs have a rapid growth on SS nanofiber scaffolds. MTT assay was used to quantify the adhesion and proliferation of PIECs on different fiber scaffolds, as shown in Figure 5. The results showed that the adhesion of cells on fiber scaffolds have no significantly difference compared with TCP for 6h incubation. The MTT value for collagen nanofibers was a little higher than that for SS nanofibers after the first 2 d, but the highest MTT value can be observed for SS nanofibers after cultured for 5 d, which is about 20 % higher than that for collagen fibers, the difference between two groups is statistically significant (p<0.05). That could be explained by the different water stability between SS and collagen scaffolds. Due to the strong hydrophilicity, the structure of collagen nanofibers can be destroyed gradually in the medium after cultured for several days. Compared to collagen nanofibers, the PIECs on SS nanofibers showed higher cell proliferation. Figure 6 shows the SEM morphology and migration of PIECs on nanofiber scaffolds after 5d of culture. It can be observed that cells migrated into the SS and collagen nanofibers and adopted a spreading polygonal shape, indicating that these materials have good cytocompatibility, whereas for PU nanofiber scaffolds PIECs were shown to attach to fiber scaffolds but difficult to migrate into it, the cells adopted a fusiform shape instead of spreading.
Table 2. Stability of nanofiber scaffolds in cell culture medium
|
Nanofibers |
Before W0(×10-2g) |
After W1(×10-2g) |
Lost weight W (%) |
Stability |
|
SS |
1.08±0.01 |
1.02±0.01 |
5.55% |
+ |
|
Collagen |
1.15±0.02 |
0.60±0.02 |
47.82% |
―― |
|
Collagen* |
1.11±0.01 |
0.93±0.01 |
16.20% |
― |
|
PU |
1.02±0.01 |
1.00±0.01 |
1.96% |
+ + |
Note: Data were representatives of three independent experiments and all data were given as Means±SD (n=3); “*” means collagen nanofibers scaffolds were crosslinked by steam of 25 %(v/v) glutaraldehyde. “+” means sta- ble; “++”means quite stable; “―”means unstable; “――”means quite unstable.
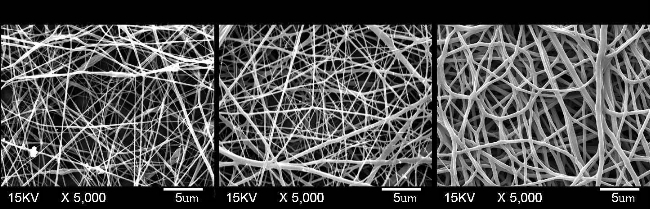
Figure 2. SEM images of electrospun SS, collagen and PU nanofiber scaffolds.

Figure 3. Average fiber diameter and porosity of SS, collagen and PU nanofiber scaffolds.
6h 2d 5d
Figure 4. Hematoxylin and eosin (HE) staining images of PIECs after cultured for 6 h , 2 days, and 5 d . The cells were seeded equally on various nanofiber scaffolds before culture, and making sure vision of each photo was typical.

Figure 5. Adhesion and proliferation of PIECs on nanofiber scaffolds. (: SS vs Collagen and TCP, : p<0.05; : MTT adsorption value of SS scaffolds at 6 h, 2 d and 5 d, p<0.01.)
3.5 Expression of PCNA gene of PIECs
The detection of expression of PCNA gene on molecular level was chosen to analyze the proliferation of PIECs cultured on different nanofiber scaffolds (Figure 7). The intensity relative absorption of PCNA gene, comparing with β-actin as an internal standard was shown in Figure 8. It was found the number of cells on SS nanofibers was approximately double more than that on collagen and TCP, and even four times more than that on PU after 7 d culture. That indicated the PIECs on SS nanofibers favored better proliferation than those on other scaffolds, and the results were in accordance with the results of HE staining and MTT assay In vitro biocompatibility of scaffolds in vascular tissue engineering is determined by various factors. It has been reported that the polymer properties, such as chemical composition, surface hydrophobicity, surface morphology and surface energy play important roles in regulating cell growth [28]. The SS and collagen nanofibers have aminoacid side chains on their surface which greatly influences the interaction between cells and nanofibers. In addition to chemical composition, the hydrophilicty of nanofibers is another important factor influencing their biocompatibility. It was demonstrated that higher protein adsorption and protein conformational change always occurred on hydrophobic surface because proteins are difficult to deabsorb from hydrophobic surface [29]. Furthermore, the porous scaffolds with interconnected pore network are important to provide sufficient space for cell growth and tissue regeneration.
4 Conclusions
Three kinds of nanofiber scaffolds including SS, collagen, and PU were fabricated by electrospinning technique. These scaffolds were then evaluated and compared with each other based on vascular tissue engineering application. The results showed that SS nanofiber scaffolds possess strong surface hydrophobility, good water stability and suitable surface morphology. Furthermore, the SS nanofiber scaffolds can promote better PIECs attachment, proliferation and phenotypic morphology than collagen and PU nanofiber scaffolds. The results strongly suggest the potential application of ss nanofibers as vascular engineering scaffolds.

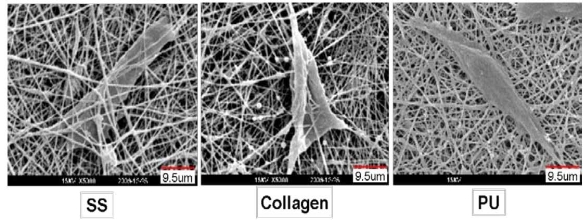
Figure 6. SEM images of interaction between PIECs and nanofiber scaffolds.
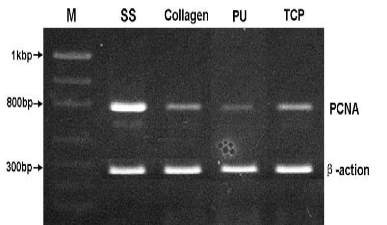
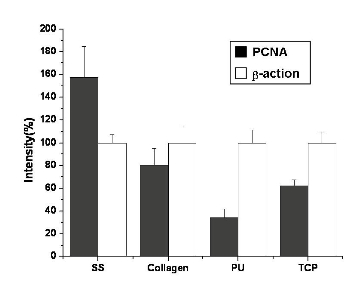
Figure 7. Expression of PCNA from PIECs cultured on nanofiber scaffolds for 7 days, and β-actin as an internal standard. The PCR primers designed as sense PCNA (5'-CTC CTT CCC GCC TGC CTG TA-3'), antisense PCNA (5'-AAT GCC TAA GAT CCT TCT TCA TCC-3'), sense β-actin (5′-ATC ATG TTT GAG ACC TTC AAC A-3′) and antisense β-actin (5′-CAT CTC TTG CTC GAA GTC CA-3′). Histograms showed fluorescence intensity of bands expressed as a percentage of the intensity of β-actin.
Acknowledgements
This work was supported by the National High Technology Research and Development Program of China (2008AA03Z305), Natural Science Foundation of Shanghai (07ZR14001), Doctoral Fund of Ministry of Education of China (200802551014), Open Foundation of State Key Laboratory for Modification of Chemical Fibers and Polymer Materials, and the Program of Introducing Talents of Discipline to Universities (B07024).
References
Received 10 November, 2009; accepted 6 December, 2009; published online 9 December, 2009.
Copyright: (c) 2009 C. He et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.