Review
Advances of Nanotechnology in the Stem Cells Research and Development
Jiajia Ji, Jing Ruan, Daxiang Cui *
Department of Bio-Nano-Science and Engineering, National Key Laboratory of Nano/Micro Fabrication Technology, Key Laboratory for Thin Film and Microfabrication of Ministry of Education, Institute of Micro-Nano Science and Technology, Shanghai Jiao Tong University, Shanghai200240, China.
* Corresponding author. Email: dxcui@sjtu.edu.cn
Citation: J. Ji, et al. Advances of Nanotechnology in the Stem Cells Research and Development. Nano Biomed. Eng., 2010, 2(1): 67-89.
DOI: 10.5101/nbe.v2i1.p67-89
Abstract
In decades, stem cell nanotechnology has become a new promising field for stem cell research and development. So, stem cell nanotechnology has attracted lots of researchers’ attention and made great progress. The unique properties of nanomaterials and nanostructures which applied in the fundamental research of stem cell-based therapies have been recognized. Nanomaterials and nanotechnology have been highlighted as promising candidates for efficient control over proliferation and differentiation of stem cells, revolutionizing the treatment of neurodegenerative disorders, neuroprotection in traumatic brain injury, improving the osteospecific differentiation and function, tissue engineering scaffold, dental implant application, drug delivery, gene therapy and cell imaging or tracking. Here we summarize the main progress in this field, explore the application prospects in injuries, diseases, regenerative medicine, etc. and discuss the methods and challenges with the aim of improving application of nanotechnology in the stem cell research and development.
Keywords: Nanomaterials; Nanostructure; Stem cells; Imaging; Therapy
1. Introduction
Stem cell nanotechnology, referring to the application of nanotechnology in stem cell research and development, has emerged as a new promising field [1-3]. Stem cells are cells found in most, if not all, multi-cellular organisms. They are characterized by the ability to renew themselves through mitotic cell division and differentiating into a diverse range of specialized cell types. They exist mainly in the areas such as muscle, blood, bone marrow, skin and organs like the brain and liver, etc. [4-7]. Stem cells are divided into two types of embryonic stem cells (ESCs) and adult stem cells. ESCs are pluripotent stem cells derived from the inner cell mass of the blastocyst, an early-stage embryo. Adult stem cells are not pluripotent but multipotent, found throughout the body after embryonic development, that multiply by cell division to replenish dying cells and regenerate damaged tissues [8-9]. Since Evans, et al. [10] firstly reported to isolate embryonic stem cells in 1981, stem cell research have become a hot spot and provide a new application prospects for diseases, injuries and regenerative medicine [11]. Especially human induced pluripotent stem cells (ips) were successfully established in 2007; the heartening report attracted the world's eyes and brought new hope for stem cell-based therapy [12-15]. However, several obstacles must be overcome prior to their therapeutic application can be realized, which include to develop advanced technology to deeply understand and control microenvironmental signals and novel methods to track and guide transplanted stem cells to realize controllable proliferation and differentiation [16-18]. Up to date, the control over the proliferation and differentiation of stem cells is still a challenging task. The emergence of nanotechnology brings new opportunities to stem cells research and development. Nanotechnology is the study of the controlling of matter on an atomic and molecular scale. Generally nanotechnology deals with structures of the size 100 nanometers or smaller in at least one dimension, and involves developing materials or systems within that size [19-20]. Such materials and systems can be designed to exhibit uniquely physical, chemical and biological properties. As we all know that the nanomaterials own four basic effects including small size effects, surface effects, tunnel effects and quantum size effects. Ultimately these effects can bring new technological opportunities as well as new challenges [21-23]. For example, nanomaterials have been highlighted as promising candidates for improving traditional tissue engineering materials. Importantly, nanomaterials exhibit superior cytocompatible, mechanical, electrical, optical, catalytic and magnetic properties compared to conventional (or micron structured) materials, which provide exciting alternative materials to finally solve the numerous problems associated with traditional implants. The application of nanomaterials and nanotechnology in stem cell research and development exhibits attractive technological prospects, which takes a new chance to solve current problems that stem cell research and development meets. In particular, the effects of structure and properties of nanomaterials on the proliferation and differentiation of stem cells have become a new interdisciplinary frontier in regeneration medicine and material science [24-25], which exhibit attractive application prospects for tissue engineering and regenerative medicine to develop biological substitutes which are used to restore, maintain, or improve damaged tissue and organ functionality [26]. Here we summarize the main advances of stem cell nanotechnology over the past few years, explore the application prospects in injuries, diseases, regenerative medicine, etc. and discuss the issues, approaches and challenges with the aim of improving application of nanotechnology in the stem cell research and development.
2. Advance of Nanotechnology in Stem Cell Research and Development
In recent several years, with the development of nanotechnology, the application of nanotechnology in stem cell research and development has made considerable progress. For example, magnetic nanoparticles with the excellent magnetic properties have been used to isolate, sort and track stem cells [27], quantum dots as fluorescent probes have been used to label and track stem cells [28], nanomaterials such as carbon nanotubes(CNTs) [29], fluorescent CNTs [30] and dendrimers [31] have been used to deliver gene or drugs into stem cells. Nanomaterials with unique nanostructures can be designed to regulate proliferation and differentiation of stem cells [32], nanomaterial-based scaffolds for tissue engineering have been designed, fabricated and explored for application in the injuries [33]. All these advances accelerate the stem cell-related research and development.
2.1 Stem cell nanotechnology for cell isolation
Magnetic nanoparticles(MNPs), as shown in Figure.1, because of their superparamagnetic property, have broad application prospects in the terms of thermotherapy [33], magnetic resonance imaging(MRI) [34], tissue and organ repair [35], immunoassay [36], drug/gene delivery [37], cell separation and purification [38], etc. A single component of the stem cell is needed in stem cell therapy. So how to isolate the kind of stem cells we need from a mixture of a variety of cells in a low-cost, efficient and convenient mode is a big challenge. So far some studies have reported that magnetic nanoparticles can directly label and then isolate stem cells by magnetic force or flow cytometry. For example, Jing, et al. [39] reported that they successfully isolated and enriched peripheral blood progenitor cells (PBPCs) from human blood circulation with the use of magnetic nanoparticles combined with Cd34 antibody. They tested CD34+ cell by immunomagnetic labeling and isolated them using the continuous quadrupole magnetic flow sorter (QMS).Seven commercial progenitor cell labeling kits were assessed by measuring magnetophoretic mobility of KG-1a cell line, a high CD34 expression cell line, with the use of the cell tracking velocimeter (CTV). The commercial CD34 progenitor cell isolation kits from Miltenyi Biotec and Bergisch Gladbach were used to purify the progenitor cells from eleven fresh samples and eleven cryopreserved clinical leukapheresis samples derived from different donors. Results showed that the KG-1a cells were strongly labelled and isolated with a purity of 60-96%, an enrichment rate of 12-16% and a throughput of (1.7-9.3) x 104 cells/s. These data remain well within the clinical effective range. So the isolated CD34 progenitor cells can be effectively used in targeted patient therapy.
2.2 Stem cell nanotechnology for molecular imaging and tracing
The latest studies found that nanoparticles such as quantum dots, magnetic nanoparticles and gold nanorods can be used as markers for imaging and tracing of stem cells [40-43]. Quantum dots, because of their unique optical properities, have been increasingly improved in cellular imaging [44], immunoassays [45], DNA hybridization [46], and optical barcoding [47] and so on. With the advances in technology, quantum dots gradually provide a new technological platform for bioanalytical sciences and biomedical engineering. Ohyabu, et al. [48] reported that quantum dots could conjugate with an antibody to form composites, which can finally labeled MSCs cells by internalized. These kind of labeled MSCs underwent normal adipocyte, osteocyte, and chondrocyte differentiation in vitro and in vivo, which highly suggest that QDs can be applied to the long-term in vivo imaging diagnostics. Berry, et al. [49] reported that quantum dots functionalized with the HIV-1 tat peptide could be markedly uptake into intracellular and intranuclear positions of human bone marrow derived cell populations, and can be used to image and tract stem cells. As well as quantum dots, magnetic nanoparticles were also used for imaging and tracing of stem cells [31, 39]. As a representative of magnetic nanoparticles, superparamagnetic iron oxide nanoparticles (SPIO) have been wildly used for stem cell labeling and isolation, magnetic resonance imaging (MRI), tracing transplanted stem cells, etc. [50].Here we show another example, dextran-coated iron oxide nanoparticles which were covalent modification with fluorescent molecules could be used to monitor the engraftment process through labeling HSCs [51]. Fluorescent molecules existed and functioned for fluorescence-activated cell sorting and purification by eliminating spurious signals. Transplantation of purified primary human blood lineage-depleted and CD34+ cells into immunodeficient mice allowed detecting labeled human HSCs in the recipient bones. Flow cytometry endpoint analysis confirmed the presence of MNP-labeled human stem cells in the marrow [52]. On the other hand, the use of stem cell therapy for different disorders of the central nervous system (CNS) has been extensively reported and recognized. Endorem-labeled GFP MSCs were injected into rats in an experimental model of stroke [53]. Rats with grafting stem cells were examined weekly for a period of weeks post-transplantation. The lesion was visible on MR image as a hyperintense signal. One week after grafting, MRI showed that a hypointense signal was found in the lesion, which was intensified during the second and third weeks. Its intensity conformed to Prussian blue staining or GFP labelling. MSCs labeled with Endorem were injected intravenously into the femoral vein in modes of transversal spinal cord lesion [53-54]. MR images of longitudinal spinal cord sections from non-grafted animals showed the lesion cavity with a strong hyperintensive signal. But in the same situation, lesions of grafted animals were seen as dark hypointense areas. Compared to control rats, the lesions gathering by MSCs in grafted animals become considerably smaller, which suggested a positive effect of the MSCs on lesion repair [55].Moreover, several successful applications of MR tracking can be found in other organs, such as heart [56], liver [57], kidney [58] and pancreatic islets [59]. It is reported that bronchioalveolar stem cells (BASCs) were successfully isolated from the murine lung using magnetic nanoparticle-based surface-enhanced Raman spectroscopic dots (M-SERS Dots) [60]. We also observed that fluorescent magnetic nanoparticles(FMNPs) [61] could conjugate with brcaa1 antibody and formed FMNP-labeled brcaa1 probes. While the prepared probes incubated with CCE stem cells for 30 minutes, the probes can be internalized into CCE stem cells. Because of the internalized probes with the superparamagnetic properties, these stem cells with fluorescent signals can be isolated directly in vitro in magnetic fields. Another report supported that the QDs covered carbon nanotubes can be internalized into stem cells, and realized labelling stem cells [62]. Ruiz-Cabello, et al. [63] also investigated the stem cellular internalization of cationic and anionic perfluoro-15-crown-5-ether (PFCE) nanoparticles using cell culture plates with different surface coatings by F-19 MR. Results showed that the viability and proliferation of anionic and cationic PFCE-labeled neural stem cells (NSCs) did not differ from unlabeled cells. Cationic PFCE nanoparticles were superior to anionic particles for intracellular fluorination. After injecting PFCE-labeled NSCs into the striatum of mouse brain, cells were readily identified in vivo by MRI without changes in signal or viability over a 2-week period after grafting. These results highly suggest that neural stem cells can be efficiently fluorinated with cationic PFCE nanoparticles and visualized in vivo over prolonged periods with an MR sensitivity of approximately 140 pmol of PFCE/cell. Liu, et al. [64] reported that 100 nm carboxylated nanodiamond (ND) particles can be taken into stem cells by macropinocytosis and clathrin-mediated endocytosis pathways, and the cell growth ability was not altered by endocytic ND particles after long-term cell culture for 10 days in both A549 lung cancer cells and 3T3-L1 embryonic fibroblasts. Finally, the cell retained a single ND's cluster in cytoplasm after sub-cultured for several generations. Interestingly, ND's clusters were carried inside of cells but did not induce damages after long-term cell culture. These observations highly suggest that endocytic ND particles are non-cytotoxic in cell division and differentiation, which can be applied for the labeling and tracking of cancer and stem cells [65].

Figure1. A and B, HR-TEM images of MNPs before and after dendrimer modification (MNPs and G5.0 dMNPs, respectively) C, TGA curves of dMNPs. D, FT-IR spectra of MNPs and G5.0dMNPs E, Growth of PAMAM dendrimer on the surface of MNPs for nonviral gene transfection based on complexation with an asODN. Nine steps are shown in the process: APTS was added to form amineterminated MNPs (G0 dMNP), excessive methacrylate was added to get a ester-terminated MNPs, ethylenediamine was added to form amine-terminated G1.0 dMNP, methacrylate and ethylenediamine were added alternately to get dMNP with generation from 1.0 to 5.0, complexation between dMNP and asODN, adsorption of dMNP-asODN complexes onto cancer cells surface, dMNP-asODN complexes were endocytosed by cancer cells, endosome-containing dMNP-asODN complexes were located around the nucleus, and dMNP and asODN escaped from the endosome into cytoplasm [37].
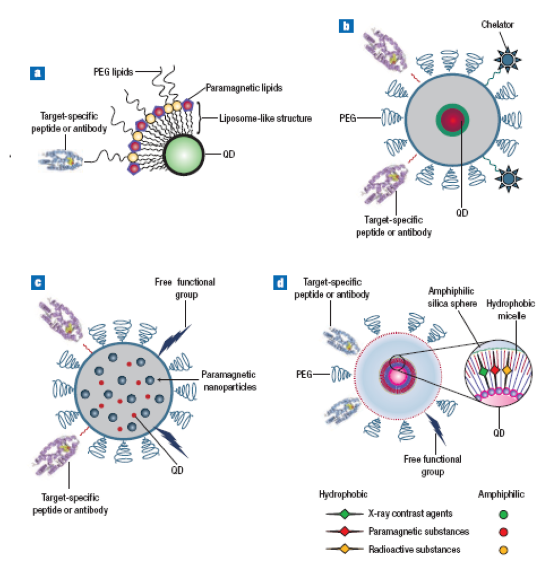
Figure 2. Various designs of multimodal QD probes. (a,b) Quantum dots having different molecules for target-specific interaction, and, attached to the surface, paramagnetic lipids, and chelators for nuclear-spin labeling. (c) The silica sphere has QDs and paramagnetic nanoparticles inside and target-specific groups attached to the outside. (d) The structure of a multimodal QD probe, based on silica-shelled single-QD micelles [49].
2.3 Stem cell nanotechnology for gene delivery
The rapid development of generating progenitor cells with in vivo reconstitution functions has accelerated biomedical applications of embryonic stem cells (ES) in the treatment of debilitating genetic, traumatic, and degenerative conditions [66]. Physical methods such as electroporation and nucleofection offer the advantage of high delivery efficiency but frequently cause severe damage to ES cells [67]. Viral vectors, including retro-, lenti-, and adenoviruses, can produce high-efficiency transfection, but their disadvantage of easy mutagenesis ignificantly decrease clinical applications [68]. Therefore, non-viral vectors such as polymeric nanoparticles and liposomes are currently recognized as the most promising nanotechnology and have the potential clinical applications [69, 70]. In our research, we report that No.5 generation of polyamidoamine dendrimer-functionalized fluorescent multi-walled carbon nanotubes(dMNTs) can enter into mouse embryonic stem cell line CCE efficiently [61]. While incubated dMNTs dose are more than 20 g/ml, they can cause CCE cells become smaller as the incubation time increases, and inhibit cell growth in dose- and time-dependent means. On the other hand, dMNTs less than 5 g/ml dose can improve CCE differentiation. Dendrimers is a novel special class of organic molecules: they can take different functional groups through a series of chemical modifications, and their interior cavities can serve as storage areas for a lot of genes or drugs [71]. Dendrimers may be a good nonviral delivery vector because it has the advantages of simplicity of use, and ease of mass production compared with viral vectors that are inherently risky for clinical use. Polyamidoamine (PAMAM) dendrimer-modified magnetic nanoparticles can markedly enhance the delivery efficiency for antisense oligonucleotides [72-74]. The prepared dMNTs may be a highly efficient gene delivery system for ES cells. So they have potential applications in ES research. As shown in Figure.4, nanoparticles such as magnetic nanoparticles [36, 48] and quantum dots can enter into human stem cells. As shown in Figure.4 D,I, J and K, we observed that SiO2 wrapped CdTe nanoparticles can enter into murine CCE stem cells and can be clearly observed that quantum dots existed in the induced-differentiated neurons, hematopoietic cells, and endothelia cells which did not exhibit strong cytotoxicity. More recently, a molecular delivery system by using atomic force microscopy (AFM) and nanoneedle has been developed to transfer gene into living cells [75-77]. Han SW et al described a low-invasive gene delivery method that uses an etched AFM tip or nanoneedle that can be inserted into a cell nucleus without causing cellular damage. The nanoneedle is 200 nm in diameter and 6 um in length and is operated using an AFM system. The probabilities of insertion of the nanoneedle into human MSCs and human embryonic kidney cells were higher than those of typical microinjection capillaries. A plasmid containing the green fluorescent protein (GFP) gene was adsorbed on a poly-l-lysine-modified nanoneedle surface, which was then inserted into primary cultured single human MSCs. A highly efficient gene delivery of over 70% was achieved in human MSCs, which compared very favorably with other major nonviral gene delivery methods (lipofection ~50%, microinjection ~10 %). Green, et al. [78] reported that they successfully prepared the small (similar to 200 nm), positively charged (similar to 10 mV) particles formed by the self assembly of cationic, hydrolytically degradable poly(beta-amino esters) and plasmid DNA. They also created an OCT4-driven GFP hES cell line to allow the rapid identification of nanoparticles that facilitate gene transfer while maintaining the hESC undifferentiated state. Using this cell system, they synthesized nanoparticles that have gene delivery efficacy that is up to 4 times higher than that of the leading commercially available transfection agent, Lipofectamine 2000.Importantly, these nanoparticles have minimal toxicity and do not adversely affect hESC colony morphology or cause nonspecific differentiation. Soenen, et al. [79] prepared the magnetoliposomes, and used magnetoliposomes to immobilize enzymes, both water-soluble and hydrophobic ones, were successfully investigated their potential applications including MRI, hyperthermia cancer treatment and drug delivery.
2.4 Effects of CNTs on proliferation and differentiation of stem cells
Carbon nanotubes, because of unique mechanical, physical and chemical properties, have great potential applications in various fields including molecular electronics, medical chemistry and biomedical engineering [80-85]. Carbon nanotubes can be functionalized to achieve improved properties and functions such as biocompatibility and biomolecular recognition capabilities [86-87]. Protein-conjugated carbon nanotubes can across the cellular membrane and enter into cytoplasm and cell nucleus [88-89]. Carbon nanotubes which can be filled with DNA or peptide molecules have high potential in gene or peptide storage and delivery system in molecular therapy of diseases [90-91]. In our previous work, we investigated the effects of single walled carbon nanotubes (SWCNTs) on human embryonic kidney cell line HEK293 cells [92]. We observed that SWCNTs can inhibit HEK293 cell proliferation and decrease cell adhesive ability in a dose- and time-dependent manner. HEK293 cells exhibit active responses to SWCNTs such as secretion of some 20–30 kd proteins to wrap SWCNTs, aggregation of cells attached by SWCNTs and formation of nodular structures. As shown in Figure.5, cell cycle analysis showed that 25 g/ml SWCNTs in medium induced G1 arrest and cell apoptosis in HEK293 cells. Biochip analysis showed that SWCNTs can induce up-regulation expression of cell cycle-associated genes such as p16, bax, p57, hrk, cdc42 and cdc37, down-regulation the expression of cell cycle genes such as cdk2, cdk4, cdk6 and cyclin D3, and down-regulation expression of signal transduction-associated genes such as mad2, jak1, ttk, pcdha9 and erk. Western blot analysis showed that SWCNTs can induce down-regulation expression of adhesion-associated proteins such as laminin, fibronectin, cadherin, FAK and collagen IV. SWCNTs inhibit HEK293 cells growth by inducing cell apoptosis and decreasing cellular adhesion ability. It is also observed that SWCNTs stimulate human osteoblast cells and human fibroblast cells to appear many protuberance on the surface compared with the control, which is one kind of active protective reaction of stimulating cells. Regarding the mechanism of nanoparticles such as CNTs entering into cells, receptor-mediated endocytosis may be responsible for the phenomena. A theory model is also suggested that the optimal size of particles entering into cells is between 25 nm and 700 nm or so, too small nanoparticles are very difficult to enter into cells because of cellular surface tension force and adhesion. The further mechanism of effects of CNTs on human ES cells is being investigated from the following four scales such as molecular, cellular, animals and environment levels. Barron and his collaborators investigated effects of a range of different types of CNTs, including single-walled nanotubes (SWCNTs), multi-walled nanotubes (MWCNTs) and functionalized CNTs on hMSCs, and revealed that at low concentrations of COOH-functionalized SWCNTs, the CNTs had no significant effect on cell viability or proliferation [93]. In addition, by fluorescently labeling the COOH functionalized SWCNTs; the CNTs were seen to migrate to a nuclear location within the cell after 24 h without adversely affecting the cellular ultrastructure. Moreover, the CNTs had no affect on adipogenesis, chondrogenesis or osteogenesis. So far CNTs have been considered to be one novel and emerging technology in gene or drug delivery, tissue engineering and regenerative medicine. At low concentrations, CNTs have minimal effect on MSCs viability and multipotency. Therefore, they have great potential to advance the field in a number of ways including: 1) Development of nanovehicles for delivering biomolecule-based cargos to MSCs; 2) Creation of novel biomedical applications for electroactive carbon nanotubes in combination with MSCs. Since carbon nanotubes are electrically conductive, there is a huge potential for the manipulation of MSCs differentiation pathways to create electroactive cells such as those found in the heart. In particular, specific applications could result in novel MSCs based cell therapies for electroactive tissue repair; novel biomolecule delivery vehicle for manipulation of MSCs differentiation pathways and electroactive CNTs scaffolds for damaged electroactive tissues.
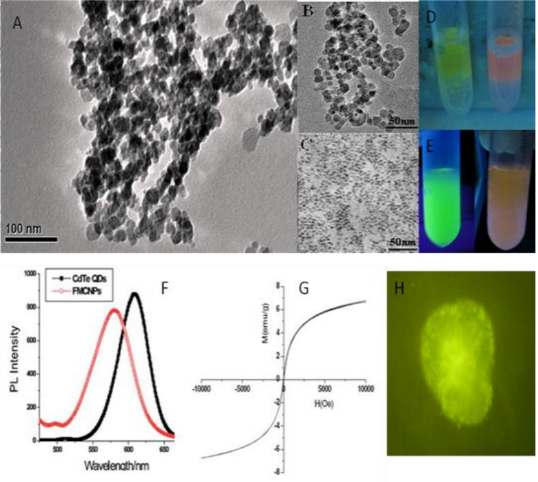
Figure 3. TEM images of (A) FMNPs (B) magnetic nanoparticles and (C) quantum dots (D) aggregated fluorescent magnetic nanoparticles under the magnetic field (E) prepared fluorescent magnetic nanoparticles with red or green color (F) PL spectra of CdTe QDs and FMNPs (G) Field-dependent magnetization curve of FMNPs at room temperature. (H) Fluorescent microscope image of FMNPs inside murine ECC stem cells [62]
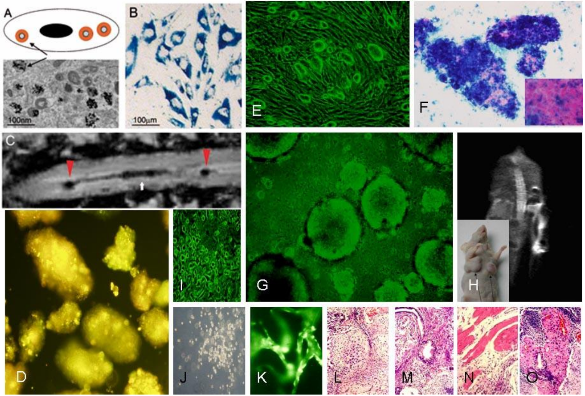
Figure 4. Drawing and transmission electron microscopy (TEM) image (A) and Prussian blue-positive cells (B) showing nanoparticles inside the cell (arrow). C: T2-weighted image of a rat spinal cord injected with nanoparticle-labeled MSCs. Arrowheads mark the injection sites, arrow the lesion populated with cells; implanted nanoparticles labeled mouse embryonic stem cells were labeled with superparamagnetic iron oxide nanoparticles (E-G). Cells were grafted intravenously. In vivo MRI was used to track their fate (H). Prussian blue staining confirmed the presence of iron oxide nanoparticles inside the cells (F). After 4 weeks post-implantation, grafted cells migrated to the lesion site and formed teratomas composed of tissue of all 3 germ layers (L-M); In vitro differentiation of quantum dots labeling of human embryonic stem cells (D) into neurons (I), hematopoietic cells (J) and endothelia cells (K) [48].

Figure 5. Apoptosis of HEK293 cells induced by SWCNTs. (A) morphological changes of HEK293 cells cultured with 25μg/ml SWCNTs for three days; (A0): showing cells become round and floating with apoptotic characterizatics; control: showing normal morphological cells; A1: showing nodular structure composed of SWCNTs and apoptotic cells; A2: showing apoptotic cells attached by SWCNTs. B1: DNA electrophoresis of cells cultured with 25 μg/ml SWCNTs for 1- 5 days, M: molecular Marker; no.1-5 denote the results of cells cultured for day 1- 5, respectively; B2: DNA electrophoresis results of control cells cultured for day 1-5; C: the cell cycle distribution of HEK293 cells cultured with 25 μg/ml SWCNTs for four days, the percentage of sub-G1 cells ( apoptosis cells) was 43.5%[92].
2.5 Application of 3D nanostructures in stem cell tissue engineering
The combination of stem cells with tissue engineering principles enables developing the stem cell-based therapeutic strategy for human diseases. Stem cell and progenitor cell directional differentiation is currently one hotspot, the differentiation of stem cells that conjugate 3D materials is considered as the most perspective tissue engineering. Up to date, various micro/nanofabrication technologies have been used to guide stem cells to develop into three-dimensional biodegradable scaffolds [94-95]. Nanostructured scaffolds are designed to trigger stem cells to become specific cell types compromising the tissues and organs in the body. Inside these scaffolds, cells deposit their own matrix and as the scaffold degrades, they form a 3D tissue structure that mimics the body’s natural tissues. For example, Gelain et al reported that they had developed a 3-D cell culture system using a designer peptide nanofiber scaffold with mouse adult neural stem cells [96]. They synthesized 18 different peptides which directly incorporate various functional motifs to promote cell adhesion, differentiation and bone marrow homing activities. These functionalized peptides self-assemble into nanofiber scaffolds where cells can be fully embedded by the scaffold in 3-D dimension. Without addition of soluble growth factors and neurotrophic factors, two of these scaffolds functionalized with bone marrow homing motifs not only significantly enhanced survival of the neural stem cells, but also promoted differentiation towards cells expressing neuronal and glial markers. As shown in Figure.6 and Figure.7, carbon nanotube patterns can be used to guide growth and alignment of mesenchymal stem cells (MSCs). The MSCs exhibited preferential growth on CNT patterns, and the cell culture results suggested that the CNT patterns did not have a harmful effect on the MSCs. The result clearly shows that CNT patterns have enormous potential as a new platform for basic research and applications using stem cells [97]. Stem cell differentiation is closely associated with their microenvironment. The regulation of stem cells depends largely on their interaction with a highly specialized microenvironment or “niches” [98]. Secreted factors, stem cell neighboring cell interactions, extracellular matrix (ECM) and mechanical properties collectively make up the stem cell microenvironment. The niche secretes appropriate chemicals to direct the differentiation and development of stem cells. For example, Scadden’s group has identified the elements of the microenvironment that control the behavior of mammalian stem cells [99]. Mineral components are important to stem cell localization; matrix components are important to constraint of stem cells; and bone-forming osteoblasts are also very important to the support and proliferation of stem cells, the calcium-sensing receptor, located on the surface of HSCs and other cells are critical for stem cells to find their niche. A key challenge in stem cell microenvironment research is how to develop in vitro system that accurately imitates the in vivo microenvironment [100]. Nanotechnology can be utilized to create in vivo-like stem cell microenvironment to determine mechanisms underlying the conversion of an undifferentiated cells into different cell types [101]. A better solution is currently under investigation: growing the stem cells on a so-called “lab-on-a-chip” [102]. This is a silicon chip with nano reservoirs. The chip surface contains about a thousand reservoir cavities, with each reservoir only about 500 nanometers across. A reservoir holds a small amount of liquid chemicals similar to what the stem cells would be exposed to in the niche. Each reservoir is sealed with a lipid bilayer equivalent to a cell membrane. These reservoir bilayers also contain the same voltage-gated channels found in cells. A small charge of electricity can then be applied to any individual reservoir to open the channels allow the chemicals to spill out, delivering them to any particular stem cell at any specified time of development. The nano reservoir chip technology also allows the possibility of growing cells layer by layer, making compound tissues, which are otherwise difficult to produce. Substrate topography influences a wide range of stem cell behaviors in a manner distinct from surface chemistry. One physical difference in the topography of divergent basement membranes is the size of pores and ridges. In vivo, cells never see flat surfaces: on the nanoscale, no basement membrane or extracellular matrix is flat. The great majority of features in the extracellular environment are in the submicron to nanoscale range, ensuring that an individual cell can be in contact with numerous topographic features [103-105]. For example, Castano, et al focused on the thickness of polypyrrole films and their potential as a biocompatible material for rat MSCs [106]. Others have investigated the potential of electrospun porous scaffolds of randomly oriented 500 nm to 900 nm diameter nanofibers for cartilage repair [107-108]. Nanofibrous structures can favorably modulate osteoblast, osteoclast, and fibroblast activities toward implant and/or scaffold materials [109]. Nanofibrous matrices are introduced as scaffolds that may have a better structural resemblance to target tissues than their bulk counterparts, because major components in tissues are nanoscale structures and cells appear to attach and proliferate better on nanoscale structures than on bulk materials. So far there is a rapidly growing interest in synthesis of natural polymer based nanofibers because of their proven biocompatibility and resorbable biodegradation products. Advantageous attributes of natural polymers include hydrophilicity, nontoxicity, less immune reaction, as well as enhanced cell adhesion and proliferation. However, fabrication of natural polymer nanofibers by electrospinning is challenging. Chitosan and alginate, two abundant natural polymers, have been widely used in tissue engineering, but none had been fabricated into nanostructured matrices until in recent two years. Zhang’s group reported that they successfully used Chitosan- and alginate- based nanofibrous matrices to mimic the ECM of articular cartilage that primarily consists of type II collagen and proteoglycans (glycosaminoglycan, GAG) [110]. A kind of nanopit template was etched with the special conglomeration surface and nanopits less than 100 nanometer in diameter. In the flat culture surface and nutrient medium of nanopit align ordered, the stem cell could not differentiate. But in the nutrient medium concurrent of ordered and unordered align naopit, the stem cell could grow to the calcify ossature cell. The stem cell could obtain the signal from the template. The surface of the transplanted tissue is the nanoengineering surface, it can induce the stem cell grow into the ossature. Obviously, surface character plays an important role in stem cell development and it is a relative simple way to control stem cell.
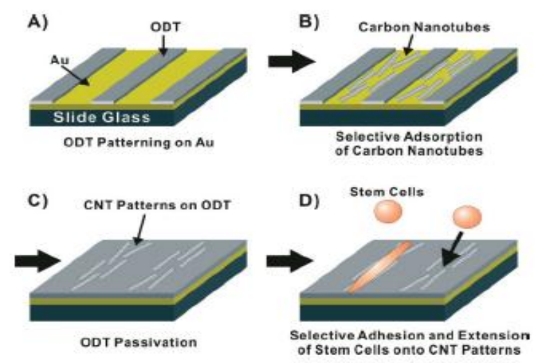
Figure 6. Schematic diagram depicting the directed growth of mesenchymal stem cells (MSCs) on large-scale carbon nanotube patterns. (A) Patterning of non-polar 1-octadecanethiol (ODT) SAM while leaving some bare Au area. (B) Selective adsorption and precision alignment of carbon nanotubes directly onto a bare Au surface. (C) Passivation of the exposed bare Au surface between the aligned carbon nanotubes with ODT. (D) Directed growth of MSCs onto the carbon nanotube patterns [97].

Figure 7. A) MSCs adhesion on various nanostructures and self-assembled monlayer (SAM) on Au or SiO2 surfaces. MSC spreading was characterized by measuring the cell area in actin filament fluorescence images. The surfaces studied are SWCNTs on Au (SWCNT/Au), SWCNTs on cystamine SAM on Au (SWCNT/Cys), SWCNTs on MHA SAM on Au (CNT/MHA), SWCNTs on APTES SAM on SiO2 (CNT/ APTES), ZnO nanowires on Au (ZnO/ Au), V2O5 nanowires on cysteamine SAM on Au (V2O5/ Cys), OTS SAM on SiO2 (OTS/ SiO2), and APTES SAM on SiO2 ( APTES/ SiO2). B) Fluorescence microscope image of actin filaments in MSCs adsorbed onto SWCNT patterns on Au surface. SWCNTs were adsorbed onto bare Au with ODT SAM as passivation layer. C) Optical microscope image of MSCs adhered onto mwCNTs/ ODT SAM patterns (50 μm wide mwCNT regions and 100 μm wide ODT regions) with ODT passivation after 24 h of cell culture. The mwCNT regions appear as dark areas around the MSCs. D) Elongation of MSCs on bulk swCNT substrates or swCNT line patterns as in B). E) Fluorescence microscope image of vinculins represaenting focal adhesions of MSC adsorbed onto swCNT patterns on Au. F) Immunofluorescence image of the fibronectins adsorbed on the swCNT patterns on Au substrate [97].
2.6 Stem cell nanotechnology for treatment of diseases in regenerative medicine
The human body is an intricate biochemical-mechanical system, with an exceedingly precise hierarchical organization in which all components work together in harmony across a wide range of dimensions. Many fundamental biological processes take place at surfaces and interfaces (e.g.cell-matrix interactions), and these occur on the nanoscale. For this reason, current health-related research is actively following a biomimetic approach in teaming how to create new biocompatible materials with nanostructured features. The ultimate aim is to reproduce and enhance the natural nanoscale elements present in the human body and to thereby develop new materials with improved biological activities. Stem cell nanotechnology has a potentially revolutionary impact on the basic understanding and therapeutic approaches for regenerative medicine. So far some great advances have made over several years.
2.6.1 Stem cell nanotechnology for the treatment of neurodegenerative disorders and brain injuries
Nanotechnology plays more and more important role in stem cell therapy. For example, Stupp, et al. [111-112] reported that paralyzed mouse which is lead by spinal cord injury recover walking function after injection the nanofibre which conjugating the laminin and nerve stem cells 6 weeks later. The neurite sprouting/guiding epitope combine the integrin which adjust cell differentiation could actuate signal and stimulate neuraxis extension. After 24 hours, nerve stem cells begin to differentiate on damage position and generate new neuron which inhibit colloid cell form cicatrix and help recovery nerve. The nanofibre was degraded after 8 weeks. The experimental mice suffer severe spinal cord injury similar to human extremely severe damage caused by traffic accident. The regenerate method has great potential application in disease therapy such as Parkinson disease, apoplexy, cardiopathy, diabetes and so on [113]. For the treatment of neurodegenerative disorders (NDs), conventional drug delivery systems do not provide adequate cyto-architecture restoration and connection patterns that are essential for functional recovery in NDs, due to limitations posed by the restrictive blood-brain barrier. Nanomaterials can get through the blood-brain barrier. Nanomaterials-based drug delivery systems, as shown in Figure.8 and Figure.9, have been actively explored for the treatment of NDs. Especially, nanotechnology employs engineered materials or devices that interact with biological systems at a molecular level and could revolutionize the treatment of neurodegenerative disorders (NDs) by stimulating, responding to and interacting with target sites to induce physiological responses while minimizing side-effects. Nanomaterials and nanotechnology have been actively explored the application in the therapy of NDs, in particular Alzheimer’s and Parkinson’s diseases, and developed some innovative therapeutic modalities for the treatment of NDs [114]. For the treatment of neurodegenerative disorders (NDs), a lot of neural stem cells are needed. However, how to obtain enough neural stem cells is a challengeable task. A major limitation in the translation of stem cells technology to clinical applications is the lack of efficient control over their proliferation and differentiation. Some research results fully demonstrate that biomaterials with nanoscale surface topography can influence cell ignalin like adhesion, proliferation and differentiation. Therefore, the identification of biomaterials that support appropriate ES cell attachment, proliferation and differentiation into neural cells is an attractive strategy for therapy of NDs. For example, as shown in Figure.10, thin films of gold with surface topography of varying roughness were designed and fabricated by using a combination of microfabrication techniques, which can be used to direct differentiation of ES cell-derived neural precursors. As shown in Fig.11, the ES-derived neural precursors best adhered on gold and underwent the highest differentiation on gold films with root mean square surface roughness (R-q) of 21 nm (72 +/- 6%) after five days of culture in the absence of traditional soluble neurotrophic factors. Moreover, when cells were seeded on a combination of micro-scale grooves with nanoscale surface roughness, axonal outgrowth orientation was observed to be influenced by the grating axis [115]. Ultimately, substrate patterning may hold special utility in the design of neural prostheses because repair of neurological injuries requires directional guidance. Stem cell nanotechnology has also been explored for the treatment of traumatic brain injury (TBI). As we know, traumatic brain injury is challenging and there is a need for neuroprotective therapies. One of the problems is to translation of promising animal experimental results into clinically successful therapies. The complexity of sequelae of TBI requires a multifaceted approach. In addition to the investigation of drugs for neuroprotective effect in TBI, new technologies based on cell/gene therapies, biomarkers and nanobiotechnology are being employed for the integration of neuroprotection with neuroregeneration and they have promising future [116]. For example, although transplantation of ES cells-derived neural progenitor cells has been demonstrated with success for either spinal cord injury repair in small animal model, but controlling of ES cell differentiation into complex, viable, higher ordered tissues are still challenging. Recent reports showed that the use of electrospun biodegradable polymers as scaffolds not only enhance the differentiation of mouse ES cells into neural lineages but also promote and guide the neurite outgrowth. A combination of electrospun fiber scaffolds and ES cells-derived neural progenitor cells could lead to the development of a better strategy for nerve injury repair [117]. Besides the data mentioned above, nanoscale biomaterials are also actively explored to apply to the treatment of nervous system disorders. For example, recently developed biomaterials can enable and augment the targeted delivery of drugs or therapeutic proteins to the brain, allowing cell or tissue transplants to be effectively delivered to the brain and help to rebuild damaged circuits. Similarly, biomaterials are being used to promote regeneration and to repair damaged neuronal pathways in combination with stem cell therapies. Many of these approaches are gaining momentum because nanotechnology allows greater control over material-cell interactions that induce specific developmental processes and cellular responses including differentiation, migration and outgrowth [118]. Nitric oxide (NO) has been shown to inhibit neointimal hyperplasia after arterial interventions in several animal models. NO-based therapies have great potential in clinical application. Combining nanofiber delivery vehicles with NO chemistry can create a novel, more potent NO-releasing therapy that can be used clinically. Primary experiment showed that the spontaneously self-assembling NO-releasing nanofiber gels can be used to prevent neointimal hyperplasia [119-120].
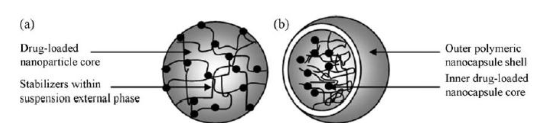
Figure 8. Illustrations of (a) functionalized and stable nanoparticles within a suspension and (b) the typical structure of a drug-loaded nanoparticle[114]
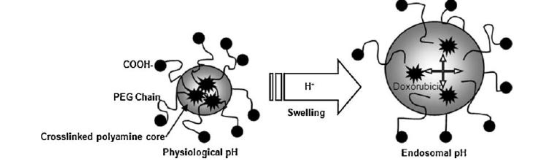
Figure 9. Schematic of endosomal release and intracellular delivery of doxorubicin using pH-sensitive PEGylated nanogels[114].
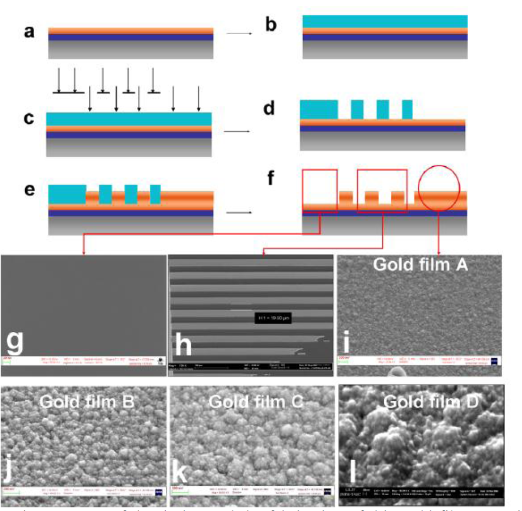
Figure 10. (a)-(f) Schematic summary of the design and the fabrication of thin gold films. (g)-(i) Representative SEM images of a gold film demonstrating three test surfaces realized on the chip; namely control (plarar gold),combined microgrooves with nano-roughness and nano-roughness respectively.(j) SEM images of gold films realized in this study qualitatively showing nano-agglomerations of increasing size.[115]
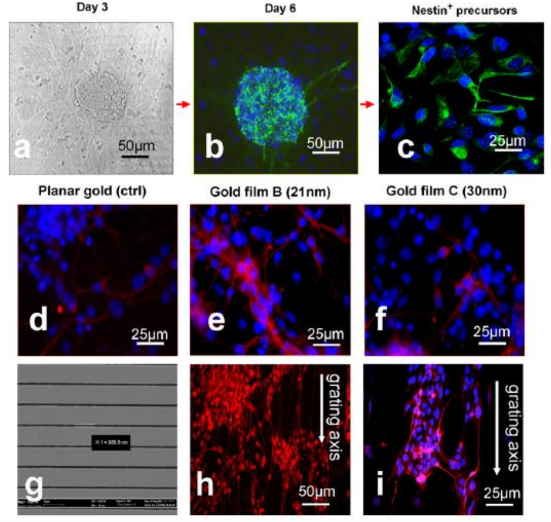
Figure 11. (a)-(c) Representative micrograph of the induction stages of the mES neural differentiation protocol [green nestin and blue DAPI stain for nuclear DNA]. (d)-(f), differentiation of neural precursor into post-mitotic TUJI-positive neurons(red) as a function of surface topography.(g) Representative SEM image of gold film with microscale grating on which axonal growth was orientated to the grating axis(h) and (i) [115]
2.6.2 Stem cell nanotechnology for cartilage and bone tissue engineering
Cartilage and bone tissue engineering has been widely investigated but is still hampered by cell differentiation and transplant integration issues within the constructs. Scaffolds represent the pivotal structure of the engineered tissue and establish an environment for neo-extracellular matrix synthesis. They can be associated to signals to modulate cell activity. The hydroxyapatite (HA) has been used for the cartilage repair, the membranes of electrospun fibers of poly-l-lactic acid (PLLA) loaded with nanoparticles of HA were designed and fabricated for the putative chondrogenic differentiation of human mesenchymal stem cells (hMSCs).Results showed that the hMSCs were seeded on PLLA/HA and bare PLLA membranes and cultured in basal medium, using chondrogenic differentiation medium as a positive control. After 14 days of culture, SOX-9 positive cells could be detected in the PLLA/HA group. Cartilage specific proteoglycan immunostain confirmed the presence of neo-extracellular-matrix production. Co-expression of CD29, a typical surface marker of MSCs and SOX-9, suggested different degrees in the differentiation process. We developed a hydroxyapatite functionalized scaffold with the aim to recapitulate the native histoarchitecture and the molecular signaling of osteochondral tissue to facilitate cell differentiation toward chondrocyte. PLLA/HA nanocomposites induced differentiation of hMSCs in a chondrocyte-like phenotype with generation of a proteoglycan based matrix. This nanocomposite could be an amenable alternative scaffold for cartilage tissue engineering using hMSCs [121]. Due to the fast progress being made in tissue regeneration therapy, biomaterials which used as scaffolds are expected to play an important role in future clinical application. Aoki, et al. reported a thin 3D carbon-fiber web as a scaffold for bone-tissue regeneration. The 3D web is consisted of high-purify carbon fibers in a nanoscale structure, as shown in Figure.12.When the thin carbon-fiber web (TCFW) and recombinant human bone morphogenetic protein 2(rhBMP-2) composite is implanted in the murine back muscle, new ectopic bone is formed, and the values of the bone mineral content and bone mineral density are significantly higher than those obtained with a collagen sheet, as shown in Fig.13, the rhBMP-2/TCFW composite repairs a critical-size bone defect within a short time period. These results suggest that the TCFW functions as an effective scaffold material and will play an important role in tissue regeneration in the near future [122]. Topographical modification of an orthopaedic implant may be a viable method to guide tissue integration and has been shown in vitro to dramatically influence osteogenesis, inhibit bone resorption and regulate integrin mediated cell adhesion. Integrins function as force dependant mechanotransducers, acting via the actin cytoskeleton to translate tension applied at the tissue level to change cellular function via intricate signaling pathways. In particular the ERK/MAPK signaling cascade is a known regulator of osteospecific differentiation and function. Biggs, et al. reported the effects of nanoscale pits and grooves on focal adhesion formation in human osteoblasts (HOBs) and the ERK/MAPK ignaling pathway in mesenchymal populations. Nanopit arrays disrupted adhesion formation and cellular spreading in HOBs and impaired osteospecific differentiation in skeletal stem cells. HOBs cultured on 10 nm wide groove/ridge arrays formed significantly less focal adhesions than cells cultured on planar substrates and displayed negligible differentiation along the osteospecific lineage, undergoing up-regulation in the expression of adipospecific genes. Conversely, osteospecific function was correlated to increased integrin mediated adhesion formation and cellular spreading as noted in HOBS cultured on 100 wide groove arrays. The osteospecific differentiation and function was linked to focal adhesion growth and FAK mediated activation of the ERK/MAPK signaling pathway in mesenchymal populations [123]. Topographical modification and surface micro-roughness of these devices regulate cellular adhesion, a process fundamental in the initiation of osteoinduction and osteogenesis. In particular, nanotedulology has allowed the development of nanoscale substrates for the investigation into cell-nanofeature interactions. Human osteoblasts (HOBS) were cultured on ordered nanoscale pits and random nano “craters” and “islands”. Nanotopographies affected the formation of adhesions on experimental substrates. Adhesion formation was prominent on planar control substrates and reduced on nanocrater and nanoisland topographies; nanopits, however, were shown to inhibit directly the formation of large adhesions. STRO-1+ progenitor cells cultured on experimental substrates revealed significant changes in genetic expression, which implicates nanotopographical modification can be used as a significant modulator of osteoblast adhesion and cellular function in mesenchymal populations [124]. The primary human osteoblasts (HOBs) were cultured on ordered nanoscale groove/ridge arrays fabricated by photolithography. Grooves were 330 nm deep and 10, 25 or 100 nm in width. Nanotopographies significantly affected the formation of focal complexes (FXs), focal adhesions (Fas) and supermature adhesions (SMAs). Planar control substrates induced widespread adhesion formation; 100 mm wide groove/ridge arrays did not significantly affect adhesion formation yet induced up-regulation of genes involved in skeletal development and increased osteospecific function; 25 nm wide groove/ridge arrays were associated with a reduction in SMA and an increase in FX formation; and 10 nm wide groove/ridge arrays significantly reduced osteoblast adhesion and induced an interplay of up- and down-regulation of gene expression, which highly indicates that groove/ridge topographies are important modulators of both cellular adhesion and osteospecific function and, critically, that groove/ridge width is important in determining cellular response [125]. Biggs, et al. reported that nanohybrid scaffolds mimicking extracellular matrix are promising experimental models to study stem cell behavior in terms of adhesion and proliferation. Ca-deficient hydroxyapatite nanocrystals (d-Hap) were synthesized by precipitation. Fibrous PCL/d-HAp nanohybrids were obtained by electrospinning, d-HAp content ranging between 2 and 55 wt % Electrospun mats showed a non-woven architecture, average fiber size was 1.5 +/- 0.5 nm, porosity 80-90%, and specific surface area was 16 m2/g. Up to 6.4 wt % d-HAp content, the nanohybrids displayed comparable microstructural, mechanical and dynamo-mechanical properties. Murine ES cells response to neat PCL and to nanohybrid PCL/d-HAp (6.4 wt %) mats was evaluated by analyzing morphological, metabolic and functional markers. Cells growing on either scaffold proliferated and maintained pluripotency markers at essentially the same rate as cells growing on standard tissue culture plates with no detectable signs of cytotoxicity, despite a lower cell adhesion at the beginning of culture. These results indicate that electrospun PCL scaffolds may provide adequate supports for murine ES cell proliferation in a pluripotent state, and that the presence of d-HAp within the mat does not interfere with their growth [126].
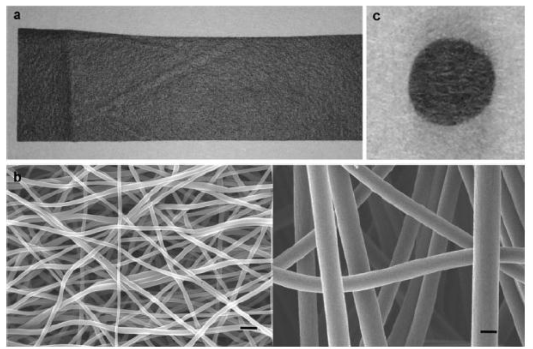
Figure 12. Images of the TCFW a) macroscopic view of the TCFW knitted into a soft black sheet. B) SEM images of TCFWs with diameters of 250nm (left) and 1000 nm (right). Fine fibers built up to a nano- to microscale pore structure. C) Macroscopic view of the 1000-nm- diameter TCFW clipped into circular implants (5mm in diameter). Scale bar in (b): 1 μm [122]

Figure 13. Repair of critical –sized bone defects in the rat ilia by the rhBMP-2/TCFW composite implants. A-c) Soft X-ray photographs of the regions of rat ileum bone defects at 4 weeks after the operation. Bone defects without implantation were not repaired and were of a critical size with a diameter of 4mm (a). Very slight bones were formed in the defect containing the TCFW without rhBMP-2 (b). The bone defects were completely restored by the TCFW with 5μg of rhBMP-2(arrow) (c. d) Histology of the regions of rat ileum bone defects obtained 4 weeks after implantation in the TCFW with 5 μg of rhBMP-2 group. A section of the host-defect interface (arrow heads) shows that new bone (NB) with hematopoietic marrow and bony trabeculae was formed adjacent to the host bone (HB). Scale bar: 50μm [122]
2.6.3 Stem cell nanotechnology for tissue engineering Scaffolds
Embryonic stem cells represent a potentially unlimited cell source for tissue engineering applications. However, in order to be used for such applications, embryonic stem cells differentiation must be controlled to the desired lineages. Smith, et al. reported the effects of nanofibrous architecture and biochemical cues on the osteogenic differentiation of embryonic stem cells compared to the more traditional architecture without the nanofibrous features in two dimensions (thin matrix or flat films) and three dimensions (scaffolds) in vitro. After three weeks of culture the nanofibrous thin matrices were capable of supporting mRNA expression of osteogenic differentiation markers in embryonic stem cells without osteogenic supplements, while solid films required osteogenic supplements and growth factors to achieve mRNA expression of osteogenic differentiation markers. Nanofibrous scaffolds substantially enhanced mRNA expression of osteogenic differentiation markers compared to solid-walled scaffolds, nanofibrous thin matrices or solid films. After 4 weeks of culture, nanofibrous scaffolds were found to contain 3 times more calcium and stronger osteocalcin stain throughout the scaffolds than the solid-walled scaffolds. Overall, the nanofibrous architecture enhances the osteogenic differentiation and mineralization of embryonic stem cells compared to the solid-walled architecture in both two and three-dimensional cultures [127]. The human body is a complicated biochemical-mechanical system, with an exceedingly precise hierarchical organization which all components work together in harmony across a wide range of dimensions. Many fundamental biological processes take place at surfaces and interfaces, and these often occur on the nanoscale. So far the major techniques have been adopted to yield novel nanostructured versions of familiar biomaterials, focusing particularly on metals, which can beneficially guide biological processes, exerting influence on cellular behavior [128]. For example, Tambralli, et al. reported the development of a hybrid, nanostructured, extracellular matrix (ECM) mimicking scaffold by a combination of nanofibrous electrospun poly (epsiv-caprolactone) (ePCL) nanofibers and self-assembled peptide amphiphile (PA) nanofibers. The PAs have ECM mimicking characteristics including a cell adhesive ligand (RGDS) and matrix metalloproteinase-2(MMP-2) mediated degradable sites. PA self-assembly into nanofibers (diameters of 8-10 nm) using a solvent evaporation method. This evaporation method was then used to successfully coat PAs onto ePCL nanofibers (diameters of 300-400 nm), to develop hybrid, bioactive scaffolds. The PA coatings did not interfere with the porous ePCL nanofiber network. Human mesenchymal stem cells (hMSCs) were seeded onto the hybrid scaffolds to evaluate their bioactivity. Significantly greater attachment and spreading of hMSCs were observed on ePCL nanofibers coated with PA-RGDS as compared to ePCL nanofibers coated with PA-S (no cell adhesive ligand) and uncoated ePCL nanofibers. Overall, this novel strategy presents a new solution to overcome the current bioactivity challenges of electrospun scaffolds and combines the unique characteristics of ePCL nanofibers and self-assembled PA nanofibers to provide an ECM mimicking environment [129]. A fast electrochemical anodization treatment, applying different anodic currents, was used to produce a nano/submicron-scale network oxide layer on Ti metal surface for biomedical implant application. Results showed that a nano/submicron-scale TiO2 network layer with a lateral pore size of 20-160 nm could be rapidly produced on Ti surface through electrochemical anodization treatment. Increasing the applied anodic current led to an increase in pore size of TiO2 network. The nano/submicron-scale TiO2 network layer significantly enhanced the whole blood coagulation and hBMSCs adhesion on Ti surface [130]. Silver nanoparticles were prepared by the polyol process, i.e. by the reduction of silver nitrate with ethylene glycol in the presence of polyvinylpyrrolidone PVP. Thereby, the silver nanoparticles were colloidally stabilized by the polymer. The synthesis of nanoparticles of different size and shape (cubes, rods and spheres) was possible by changing the reaction conditions such as reagent ratio and temperature. The biological activity of spherical PVP-coated silver nanoparticles (about 100nm diameter) was tested on human mesenchymal stem cells (hMSC) in comparison with equivalent amounts of silver ions (silver acetate). hMSC were treated with silver concentrations in the range of 50 ng/mL to 50 ng/mL for 7 days under cell culture conditions. Cytotoxic cell reactions occurred at >= 2.5 ng/mL for nanoparticles and >= 1 ng/mL for silver acetate, indicating a critical role of the silver ions for toxic reactions [131]. A computer-controllable scaffold structures were made by a layer manufacturing process (LMP) with addition of nano- or micro-sized particles. By using the LMP, a new nano-sized hydroxyapatite/poly(epsilon-caprolactone) composite (n-HPC) scaffold and a micro-sized hydroxyapatite/poly(epsilon-caprolactone) composite (m-HPC) scaffold were made for bone tissue engineering applications. The scaffold macropores were well interconnected, with a porosity of 73% and a pore size of 500 mm. The compressive modulus of the n-HPC and m-HPC scaffolds was 6.76 and 3.18 MPa respectively. Both n-HPC and m-HPC exhibited good in vitro biocompatibility. Attachment and proliferation of mesenchymal stem cells were better on the n-HPC than on the m-HPC scaffold. Moreover, significantly higher alkaline phosphatase activity and calcium content were observed on the n-HPC than on the m-HPC scaffold. In an animal study, the LMP scaffolds enhanced bone formation, owing to their well-interconnected pores. Radiological and histological examinations confirmed that the new bony tissue had grown easily into the entire n-HPC scaffold fabricated by LMP. We suggest that the well-interconnected pores in the LMP scaffolds might encourage cell attachment, proliferation, and migration to stimulate cell functions, thus enhancing bone formation in the LMP scaffolds [132].
3. Bio-safety of Stem Cell Nanotechnology During Embryonic Development
With the rapid development of nanomaterials and nanotechnology, bio-safety of nanomaterials and nanotechnology has caused many governments’ concern. Up to date, the effects of nanomaterials on environment and healthcare have been being actively investigated. However, few reports are closely associated with the area of developmental toxicity. How to clarify the mechanism of effects of nanomaterials and nanotechnology on the embryonic development is a big challengeable problem. For example, Park, et al. reported that when mouse embryonic stem cells were exposed to silica nanoparticles at concentrations ranging from 1 to 100 ng/ml, the embryonic stem cells test showed a concentration dependent inhibition of differentiation of stem cells into contracting cardiomyocytes by two silica nanoparticles of primary size 10 (TEM 11) and 30 (TEM 34) nm while two other particles of primary size 80 (TEM 34) and 400 (TEM 248) nm had no effect up to the highest concentration tested. Inhibition of differentiation of stem cells occurred below cytotoxic concentrations, indicating a specific effect of the particles on the differentiation of the embryonic stem cells. The impaired differentiation of stem cells by such widely used particles warrants further investigation into the potential of these nanoparticles to migrate into the uterus, placenta and embryo and their possible effects on embryogenesis [133]. Deng, et al. reported the neurotoxicity of different sized zinc oxide (ZnO) nanoparticles in mouse neural stem cells (NSCs). A cell viability assay indicatedthat ZnO nanoparticles manifested dose-dependent, but no size-dependent toxic effects on NSCs. Further test showed that the ZnO nanoparticle toxicity come from the dissolved Zn 2+ in the culture medium or inside cells, which highlight the need for caution during the use and disposal of ZnO manufactured nanomaterials to prevent the unintended environmental and health impacts [134]. In recent years, the genotoxicology (the study of genetic aberrations following exposure to test agents) of nanomaterials is reported. For example, DNA damage may initiate and promote carcinogenesis, or impact fertility. The metal nanoparticles such as metal-oxide nanoparticles, quantum dots, fullerenes, and fibrous nanomaterials, can damage or interact with DNA, for example, to cause genotoxic responses, such as chromosomal fragmentation, DNA strand breakages, point mutations, oxidative DNA adducts and alterations in gene expression profiles. However, there are clear inconsistencies in the literature and it is difficult to draw conclusions on the physico-chemical features of nanomaterials that promote genotoxicity [135]. Conversely, some reports clearly showed that the surface functionalized nanomaterials own good biocompatibility and advantageous bioactivity. For example, superparamagnetic iron oxide (SPIO) nanoparticles are very useful in cell imaging. However, biosafety concerns associated with their use, especially on therapeutic stem cells, have arisen. Huang, et al. reported that Ferucarbotran, an ionic SPIO, was not toxic to human mesenchymal stem cells (hMSCs) under the conditions of experiments but instead increased cell growth. Ferucarbotran-promoted cell growth is due to its ability to diminish intracellular H2O2 through intrinsic peroxidase-like activity. Also, Ferucarbotran can accelerate cell cycle progression, which may be mediated by the free iron (Fe) released from lysosomal degradation and involves the alteration of Fe on the expression of the protein regulators of the cell cycle [136]. Vaijayanthimala, et al. reported the biocompatibility and endocytosis mechanism of fluorescent nanodiamonds (FNDs) in cancer cells (HeLa) and pre-adipocytes (3T3-L1). Results showed that the mechanism of the FND uptake in both cells is by energy-dependent clathrin-mediated endocytosis. In addition, the surface charge of FND influences its cellular uptake, as the uptake of poly-L-lysine-coated FNDs is better than that of oxidative-acid-purified FNDs at the same concentration in regular medium with or without serum. The proliferative potential of FND-treated and untreated cells does not exhibit any significant differences when measured at bulk cultures, and more stringently at clonal cell density. Further biocompatibility studies indicate that the in vitro differentiation of 3T3-L1 pre-adipocytes and 489-2 osteoprogenitors is not affected by the FND treatment, which highly suggest that FNDs are biocompatible and ideal candidates for potential applications in human stem cell research [137]. Low, et al. reported the biocompatibility of thermally-oxidised, aminosilanised porous silicon membranes and their potential to support human ocular cells in vitro and in vivo in the rat eyes. The membranes with pore sizes of 40-60 nm slowly dissolved, but the material could be maintained in tissue culture medium in vitro for at least two weeks without visible degradation. When implanted under the rat conjunctiva, the material did not erode the underlying or overlying tissue. The implant underwent slow dissolution, but remained visible at the operating microscope for over 8 weeks. End-stage histology indicated the presence of a thin fibrous capsule surrounding the implant, but little evidence of any local accumulation of acute inflammatory cells or vascularization. Human lens epithelial cells and primary human corneal explants adhered to the porous silicon membranes, where they remained viable and underwent division. Primary corneal epithelial cells supported on membranes were labelled with a cell tracker dye and implanted under the rat conjunctiva. Seven days later, labelled cells had moved from the membrane into the ocular tissue spaces. A porous silicon membrane may have value as a biomaterial that can support the delivery of cells to the ocular surface and improve existing therapeutic options in patients with corneal epithelial stem cell dysfunction and ocular surface disease [138-139]. Up to date, the effect of nanomaterials on embryonic development is not fully clarified, further researches should focus on investigating their biocompatibility, genotoxicology and advantageous bioactivity with potential application prospects.
4. Challenges and Prospects
In recent years, application of nanotechnology in stem cells has made great advances, and it is becoming an emerging interdisciplinary field. Stem cell nanotechnology is developing towards imaging, active tracing and controllable regulation of proliferation and differentiation of stem cells. However, like any emerging field, stem cell nanotechnology also faces many challenges. The mechanism of interaction between nanomaterials and stem cells is still not clarified well [140].How nanomaterials and nanostructures to affect the nanomaterials inside stem cells to be metabolized are great challenges. How to use current knowledge and principles to fabricate novel multifunctional or homogenous nanostructures, the processing, characterization, interface problems, high quality nanomaterials availability, nanomaterials tailoring, and the mechanisms governing the behavior of these nanoscale composites on the surface of stem cells are also great challenge for present existing techniques. The ips cells were prepared by using HIV virus-based gene delivery system. Using nanomaterial-based gene delivery system to replace virus-based gene delivery system will be a great challenge. However, stem cell nanotechnology shows great attractive prospects and stem cells are developing towards application of generative medicine. We believe that stem cell nanotechnology will be broadly applied in treatment of injuries and degenerative diseases in the near future.
5. Conclusions
Stem cell nanotechnology provides a novel chance for stem cells research and development, speeding up the exploration of application of stem cells in generative medicine. Nanomaterials such as quantum dots, fluorescent CNTs and fluorescent magnetic nanoparticles have been used for imaging and tracing, gene or drug delivery, scaffolds for tissue engineering, designed nanostructures have been used to regulate the proliferation and differentiation of stem cells, which will speed up the understanding and controlling the microenvironmental signals, helping to solve the current bottleneck problems of stem cells-based therapy. Although stem cell nanotechnology faces many challenges, marriage of stem cells and nanotechnology have exhibited attractive technological prospects, and will dramatically advance our ability to understand and control stem cell-fate decisions and develop novel stem cell technologies, which will eventually lead to stem cell-based therapeutics for human diseases.
6. Acknowledgements
This work is supported by China National 973 Project (No.2010CB933901), National 863 Key Project(No.2007AA022004),National Natural Scientific Fund (No.30672147), and the science and technology foundation of shanghai (No.072112006).
References
Received 8 January, 2010; accepted 10 February, 2010; published online 5 March, 2010.
Copyright: (c) 2010 J. Ji et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.