Article
In Vitro Evaluation of Antiproliferative Effects of Self-Assembling Nanoemulsion of Paclitaxel on Various Cancer Cell Lines
Mukta Bagul 1, 2, Srikanth Kakumanu 1, 2, Thomas Wilson 1,2, Robert Nicolosi 1,2*
1 Center for Health and Disease Research-Division of NanoMedicine, Department of Clinical Laboratory and Nutritional Sciences; and 2 The Biomedical Engineering/Biotechnology Ph.D. Program, University of Massachusetts Lowell, Lowell, MA 01854.
*Corresponding author. E-mail: Nicolosi.Robert@yahoo.com
Citation: M. Bagul, et al., In Vitro Evaluation of Antiproliferative Effects of Self-Assembling Nanoemulsion of Paclitaxel on Various Cancer Cell Lines. Nano Biomed. Eng., 2010, 2(2): 100-108.
DOI: 10.5101/nbe.v2i2.p100-108
Abstract
Paclitaxel is routinely used in cancer chemotherapy to treat patients with ovarian, breast, lung, head and neck cancers. However, because of its low aqueous solubility, it’s been administered as a Cremophor EL and ethanol solution, which is associated with increased toxicity and high therapeutic dose requirements. The goal of the present study was to formulate paclitaxel into a self assembling nanoemulsion (SANE) and demonstrate the effectsof paclitaxel SANE formulation on the inhibition of cell proliferation in breast (80 %), colon (60 %), and pancreaticcell lines (60 %) compared to blank nanoemulsion. In addition, nanoemulsions of paclitaxel with a mean particle size of 20 nm dramatically reduced zeta potential and showed up to 12 fold greater apoptosis in the PL-45 pancreatic cancer cell line compared to a blank nanoemulsion. In conclusion we have developed a SANE formulation of paclitaxel having a particle size of 20 nm which significantly inhibited cell proliferation, dramatically reduced zeta potential and increased apoptosis by 12-fold when compared to a blank and nanoemulsion, thus indicating the therapeutic potential for SANE as an anti-cancer drug delivery system.
Keywords: Self assembling nanoemulsions; Paclitaxel; Pancreatic; Breast and colon cancer cell lines; Cell proliferation
1. Introduction
Current statistics suggest that cancer still remains one of the main causes of death in North America [1-4]. While chemotherapy is widely used for the treatment of various cancers, often times, the higher mortality rates are more associated with the adverse side effects of the intervention rather than the cancer itself [5-7]. These high mortality rates and adverse side effects are usually the result of (a) most of the anti-cancer drugs (> 70%) being lipid soluble and (b) therapeutic doses are often very high. Nanoemulsion-based drug delivery systems can be used to enhance the chemotherapeutic properties of a drug by reducing the particle size of the typical anti-cancer drug formulation up to 100 fold (microns to20 nm) thereby increasing the drug’s bioavailability (absorption into the blood stream) and efficacy (inhibition of uncontrolled cell proliferation) [8-11]. One such drug used in breast cancer chemotherapy is paclitaxel (Taxol®) which has very strong antitumor activity, but has low aqueous solubility thus requiring a high therapeutic dose for effectiveness [12-15]. Paclitaxel is an anti-microtubule agent extracted from the needles and bark of the Pacific yew tree taxus brevifolia [12]. It is used in the treatment of advanced ovarian and breast cancer, lung cancer, and head and neck cancers [16, 17]. Instead of causing disassembly of microtubules, paclitaxel forms extremely stable and nonfunctional microtubules, which causes inhibition of many cell functions and the interruption of the cell cycle [18]. Paclitaxel is a potent anticancer drug, but its use is limited because it is a Pglycoprotein substrate associated with drug resistance [19] and its aqueous solubility is poor [12]. Over the years, various formulations of paclitaxel have been made to improve the efficacy of the drug [20]. Efforts to overcome its poor aqueous solubility have included the use of Cremophor EL, which is associated with several adverse side effects [21, 22]. As a result, there have been several attempts to develop a Cremophor EL free-formulation including but not limited to the use of Peglyated PGLA-based formulations [23]. The nanoparticulate formulation Abraxane containing albumin is the most recent commercially available preparation of a Cremophor EL-free paclitaxel [24]. Thus nanoemulsion formulations of paclitaxel of differing compositions to optimize the efficacy of paclitaxel, and subsequently reduce adverse side effects seemed plausible.The toxicity associated with paclitaxel treatment can be non-specifically distributed in the body where it affects both cancerous as well as normal cells, therebylimiting the dose achievable within the tumor and alsoresulting in suboptimal treatment due to excessive toxicities [22]. Use of drug-loaded nanoparticles or nanoemulsions can enhance the intracellular concentration of the drug as well as bind to specific receptors and then enter the cell bypassing the recognition of P-glycoprotein, one of the main drug resistance mechanisms [19]. However, limitations such as poor oral bio-availability, instability in circulation, inadequate tissue distribution and toxicity still exist [25].There are various types of nanoformulations used in drug delivery. These include drug delivery systems that are submicron sized particles (92 – 200 nm), that canbe made using a variety of materials and configurationssuch as but not limited to micelles, dendrimers, nanoemulsions, liposomes, viruses and organometallic compounds [9, 10, 24, 26, 27].Our laboratory has developed a self assembling nanoemulsion (SANE) delivery system [patent pending] for drug delivery. Nanoemulsions are a class of stable emulsions formed by monolayers of phospholipids composed of surfactant and oil suspended in water. The nanoemulsions are often referred to as “Approaching Thermodynamic Stability” [25]. Compared to typical suspension preparations which can be thousands of nanometers in size, nanoemulsion delivery systems utilized and reported from our lab [28-32] as well as those of others with particle sizes approximately 100 nm or less have been shown to increase bioavailability and efficacy of a number of compounds [25, 33, 34]. The concept of zeta potential and how its reduction can be associated with increased efficacy of drug deliverysystems has been raised by reports from our lab [30, 33] and others [35-37]. The objective of this study was to develop and evaluate a Cremophor EL-free nanoemulsion formulation paclitaxel that would increase its efficacy and specificity thereby decreasing the adverse side effects commonly associated with this drug treatment, utilizing in vitro studies of breast, pancreatic and colon cancer cell lines.
2. Materials and Methods
2.1 Cell culture line, growth conditions and gents
The human breast cancer cell line MCF-7, pancreatic cell lines PL-45 and P10.05, and the colon cancer cell line CCL- 221 were purchased from the American Type Culture Collection (ATCC, Manassas, VA). These cultures of human tumor cell lines were propagated at 37 ºC in 5% CO2 / 95% O2 after the appropriate number of subcultures as indicated by the manufacturer using ATCC-formulated RPMI-1640 Medium and 10% (v/v) fetal bovine serum (FBS).
2.2 Nanoemulsion preparation
Paclitaxel was purchased from (Sigma Aldrich, St Louis MO). Rice bran oil (Tsuno, Japan) and the surfactant Solutol HS-15 were purchased from (BASF, Germany) respectively. Four milligrams of rice bran oil were weighed in a beaker. The mixture of rice bran oil and Solutol HS-15 was heated and stirred for 5 min using a magnetic stirrer, until the paclitaxel visually appeared to have dissolved in oil @ 50 -60 °C. After an equal amount of Solutol HS was added, the mixture was heated and stirred again for 5 min at 60 °C until the three components form a homogeneous mixture. Distilled water was added to a final volume of 50 mL while the mixture was stirred at 60 °C. At this stage it forms an O/W emulsion. During heating when the PIT (or HLB temperature) of the system is reached (65-70 °C, phase inversion zone), the surfactant is in equilibrium with the oil and water phases. Heating and stir-ring was continued beyond the PIT up to 80 °C. At this temperature the system inverts to a W/O emulsion. The emulsion was cooled to room temperature to obtain an O/W emulsion.
2.3 Encapsulation efficiency of nanoemulsion
Paclitaxel was analyzed by HPLC (Agilent 1100 series, Santa Clara, California, USA). A reverse phase Zorbax C18 column (250 x 4 mm, 5 um) at room temperature was used. The detector wavelength was set at 227 nm and injection volume was 20 μL. The mobile phase was a mixture of acetonitrile and water (50:50 v/v) at a flow rate of 1 mL/min. The nanoemulsion was diluted in 1 mL of acetonitrile and then further dilutions were made using the mobile phase. A 100 μL aliquot was injected into the HPLC for measurement.
2.4 In vitro cytoxicity assay
Cell number and viability were assessed using the MTS reagent (Promega Corporation, Madison, Wisconsin USA), according to the manufacturer’s instructions. Cultured cells were plated at a density of 5000 cells per well into 96-well, flat-bottomed tissue culture plates. MCF-7 cells were incubated with 0.25 and 0.125 μM concentrations of nanoemulsion containing paclitaxel while the PL-45, P10.05 and CCl-221 cells were treated with 3 μM, 1 μM, 0.3 μM, 0.1 μM, 0.030μM, 0.010 μM, 0.003 μM, 0.001 μM and 0.0003 μM concentrations of paclitaxel. Untreated cells and na-noemulsions containing no drug (blank nanoemulsion) were used as controls. After 72 hr incubation, MTS reagent was added and incubated for an additional 2 hr and then the cell plates were measured at a wavelength of 490 nm using Spectra MAX (Molecular Devices, Sunnyvale, California). Cell inhibition was calculated using the following equation: Cell Inhibition (%) = (1- (Abss/Absctrl))x100
where Abss is the absorbance of the cells tested with the paclitaxel and nanoblank formulations and Absctrl is the absorbance of untreated cells.
2.5 Apoptosis assayusingthe pancreatic cancer
PL-45 cells were seeded in a tissue culture-treateddish containing a slide with 50,000 cells per plate and cultured at 37 °C for 24 hr. Cells were then incubated for an additional 72 hr with 300 nM, 30 nM and 15 nM of the nanoemulsion formulations of paclitaxel or blank nanoemulsions. Untreated cells were used as the control. Samples were then fixed by adding 4% paraformaldehyde made up in PBS for 10 min to the cell plates containing the slides. Ten µg/mL of Hoechst dye (Invitrogen, Carlsbad, CA) was added to the fixing step. The samples were then washed twice in PBS. Cover slips were mounted on glass slides which were examined using a fluorescent microscope (Olympus America Inc, Center Valley Pa). Random fields of cells were selected to obtain unbiased distributions.
2.6 Colony formation assay
Colony formation properties of tumor cells were assayed using the clonogenic survival assay. PL-45 cellswere seeded in 10 cm tissue culture-treated plates at a suitable density for 24 hr. The cells were then treatedwith 0.03µM and 0.1 µM of paclitaxel as a nanoemulsion formulation or blank nanoemulsion. Untreated cells were used as control. After 48 hr, the treated cells were trypsinized, counted and plated in 6-well dishes at suitable concentrations. The cells were cultured for 10 days. After 10 days of incubation at 37°C under 5% CO2 / 95% O2, the cells were fixed and stained with1 % crystal violet (Sigma Aldrich, St Louis MO).
2.7 Statistical methods
SigmaStat software was used for all statistical evaluations (Jandel Scientific, San Rafael, CA, USA) [38]. A repeated measures one-way analysis of variance (RM ANOVA) was used to analyze the data. When statistical significance was found by ANOVA, the Student - Newman - Keuls separation of means was used to determine differences. All values are expressed as mean ± SD and all measured parameters underwent statistical analyses using the student’s “t” test. Statistical significance was set at the minimum p < 0.05.
3. Results
3.1 Nanoemulsionch characterization and encapsulation efficiency
The initial particle size Z-Average of the paclitaxel suspension contained peaks greater than 2000 nm which were reduced to 20 nm after SANE preparation (Fig.1). The polydisperity index (PDI) which is defined by the average molecular weight (Mw) divided by the average number of molecular weight particles (Mn) was 0.1 for the paclitaxel nanoemulsion formulation and was greater than 1 for the paclitaxel suspensions. Relative to the increased zeta potential of the paclitaxel suspended in DMSO suspensions (-15mV) the paclitaxel nanoemulsion formulations demonstrated a reduced zeta potential (1mV), i.e., decreased negative charge [less anionic and more cationic]. The blank na-noemulsion showed neutral or slightly negative (-0.mV) zeta potential. The particle size, polydispersity index and zeta potential of the paclitaxel nanoemulsion formulation remained unchanged (stable) compared to the paclitaxel suspension after one month when stored at 4 °C (data not shown). The HPLC analysis used to de-termine encapsulant efficiency indicated that 79 % of paclitaxel was entrapped in the nanoemulsion. The encapsulation efficiency of less than 100 % maybe to either disruption of the primary W/O phase or due to leaching out of material from the inner aqueous phase.
3.2 Effect of nanoemulsion of paclitaxel on cell proliferation activity
MCF-7 breast cancer, CCL-221 colon cancer and P10.05 pancreatic cancer cell lines were treated with various concentrations of the drug encapsulated in the nanoemulsion and the cells were incubated at 37°C under 5%CO2/95%O2 for 72 hrs. Although, CCL-221, PL-45 and P10.05 showed 80% cell growth inhibition when treated with the blank nanoemulsion and nanoemulsions encapsulating paclitaxel at the high concentration of 3000nM and 1000nM, nevertheless, paclitaxel-containing nanoemulsions effectively inhibited cell growth at the much lower concentrations of 300 nM, 100 nM, 30 nM and 10 nM compared to the blank nanoemulsion in CCL-221, PL-45, and P10.05, suggesting a very significant drug effect over carrier cytotoxicity. As shown in Figure 2, the MCF-7 breast cancer cell line exhibited 80 % growth inhibition at 0.25 μM and 0.125 μM when treated with nanoemulsion con-taining paclitaxel. The blank nanoemulsion showed minimal to no anti-cell proliferative activity. Nanoemulsions containing paclitaxel effectively inhibited cell growth of CCL-221 by 60 % (p<0.05) over blank nanoemulsion when treated at300 nM, 100nM, 30 nM and 10 nM. (Fig. 3a). As shown in Fig. 3b PL-45 cells were significantly inhi-bited by 50% (p<0.05) at concentrations as low as 30 and 10 nM when treated with nanoemulsion containingpaclitaxel. A sustained decrease of 60 % (p<0.05) at 300 nM, 100 nM, 30 nM, 10 nM, 3 nM in the cell growth was observed when P10.05 cells were treated with nanoemulsions containing paclitaxel. (Fig. 3c).
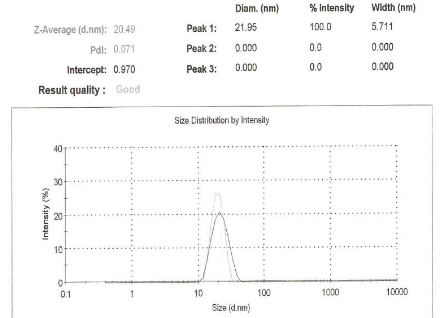
Figure 1. Z-average size distribution of the nanoemulsion of paclitaxel using dynamic laser lightscatteringparticle size analysis showing that the particle size of the nanoemulsion containing paclitaxel is reduced to 20nm.
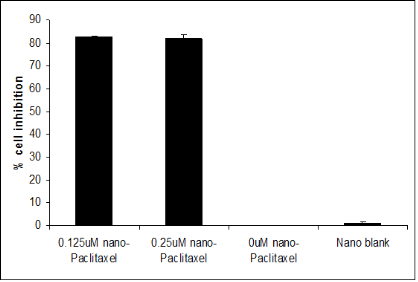
Figure 2. Determination of in vitro cellcytotoxicity on MCF-7 cells. MCF-7 exhibited 80% decrease in cell proliferation after treatment with 0.125 µM and 0.25 µM of nanoemulsion containing paclitaxel when compared to blank nanoemulsion and untreated cells.

Figure 3. Antiproliferative effects of (a) 60 % on CCL-21 when treated with 300nM, 100nM, 30nM and 10nM of paclitaxel encapsulated in nanoemulsion (b) 50% on PL-45 when treated with 300nM, 100nM, 30nM and 10nM of paclitaxel encapsulated in nanoemulsion (c) 60% when treated with 300nM, 100nM, 30nM and 10nM of paclitaxel encapsulated in nanoemulsion on P10.05 was observed when cells were treated for 72 hours. Untreated cells were used as control. Error bars represent the standard deviation of the mean for two sets of independent experiments done in riplicates. Statistical analysis by one way ANOVA and student t-test: * p<0.05 as compared to blank nanoemulsion.
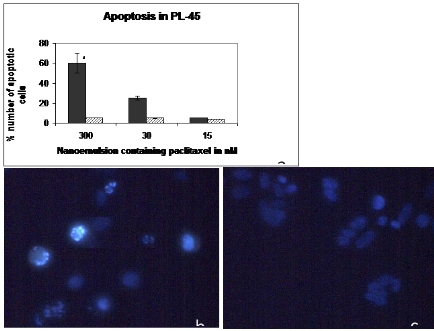
Figure 4. Apoptosis assay (a) Percent number of apoptotic cells staining of PL-45 cells after 48 hours of incubation with nanoemulsions containing 300nM, 30nM and 15nM of paclitaxel and blank nanoemulsion. * p< 0.05 as compared to blank nanoemulsion (b) Represents the visualization of apoptosis using Hoechst staining on PL-45 cells treated when treated with 300nM of nanoemulsion containing paclitaxel and (c) blank nanoemulsion. A total number of 100 cells were counted and the results were based on two random fields picked on the slide in two different independent experiments. The apoptotic bodies were counted from random fields on the slides. Blank nanoemulsion was used as a control. The percent apoptotic cells were based on the number of cells counted by visualization of the apoptotic cells. * p<0.05 as compared to nano blank.
3.3 Apoptosis assay
To determine whether paclitaxel-containing nanoemulsion had apoptotic activity, PL-45 cells were treated with different concentrations of paclitaxel; the blank nanoemulsion was used as a control in order to reduce or eliminate any effects caused by the blank nanoemulsion itself. The results revealed that nanoemulsion of paclitaxel-treated cells showed 60 % (P<0.05) apoptotic activity demonstrating a 12-fold increase in apoptotic activity as compared to 3% apoptotic activity of the blank nanoemulsion control itself (Figure 3a) when treated with 300nM of paclitaxel encapsulated in the nanoemulsion. The nuclei of untreated cells showed homogenous fluorescence with no evidence of segmentation and fragmentation after Hoechst staining. Interestingly, exposure of cells to nanoemulsions containing paclitaxel led to segregationof cell nuclei into segments, which indicates break- down in chromatin followed by DNA condensation, the classical signs of apoptosis. A special note should be made of the fact that the blank nanoemulsion demonstrated minimal apoptotic effects.
3.4 Colony formationassay
Colonies formed after 10 days were counted manually. A rapid growth in forming larger colonies was observed in the wells treated with blank nanoemulsions. In contrast, no larger colony formation was observed in cells treated with nanoemulsions containing paclitaxel as shown in Fig.5, where the numbers of colonies formed in wells treated with drug free nanoemulsion and untreated control were significantly more (75%) than the wells treated with Nan emulsion of paclitaxel.
4. Discussion
Over the past decade, various delivery methods anddrug formulations for cancer drugs have been used to minimize drug toxicity, improve therapeutic effects and broaden the therapeutic window of the drug [39, 40]. Many anticancer chemotherapeutic drugs are water insoluble and highly toxic limiting their clinical efficacy [19, 41]. Accordingly, organic solvents are used for drug delivery of cancer drugs that overcome the insolubility associated with these hydrophobic drugs [42]. But additional toxicities are associated with these organic solvents. Therefore, there is a need for novel formulations, which (a) convert lipid soluble drugs to stable water dispersion and reduce the side effects caused by the drug itself and also the vehicles and (b) increase the therapeutic efficacy of the drugs [8, 43]. Novel methods such as nanodeliverysystems would also improve biodistribution of cancer drugs and the pharmacokinetic profile [25].Paclitaxel is one such anti-neoplastic drug that is used in cancer therapy known for its aqueous insolubility [16, 16], which, as a result, is associated with increased binding to blood protein limiting its ability in treatment of solid tumors [10, 26]. Currently, paclitaxel is administered in two forms, one as Taxol® that is formulated by dissolving the mixture of dehydrated ethanol and Cremophor EL and Abraxane, which is an albumin, based nanoparticle formulation [23, 44]. Studies have indicated that most of the toxicity associated with Taxol is from the surfactant Cremophor-EL [20,45, 46].In this study, we have developed a nanoemulsion formulation for delivery of paclitaxel, which is Cremophor EL free. The 20 nm particle size of this nanoemulsion of paclitaxel is up to 50 fold less than the conventional drug suspensions, which is one of the optimal characteristics of a carrier-mediated drug delivery. Nanoemulsions containing paclitaxel exhibited excellent stability over a month (data not shown), which is an important parameter in a drug delivery system. In agreement with paclitaxel being one of the fundamental anti tumor agent used in treatment of early or advanced breast cancer therapy [13, 15, 47] we have demonstrated consistent 80% inhibition achieved in a breast cancer cell line when treated with 25 µM and 125 µM of paclitaxel encapsulated in nanoemulsions (Fig 2), but at significantly less concentrations than traditionally utilized (40%) (data not shown). Moreover, a sustained inhibition of 50-60% was achieved at 300 nM, 100nM,30 nM and 10 nM in colon and pancreatic cancer cell lines when treated with paclitaxel encapsulated in thenanoemulsion. Most interestingly, this unique nanoemulsion formulation of paclitaxel showed enhanced anti-cell proliferative capacity and effective apoptotic properties on pancreatic cancer cells compared to the blank nanoemulsion which revealed only minimal anti-proliferative and apoptotic effects. Taken together, the cell proliferation inhibitory abilities of nanoemulsions containing paclitaxel and colony formation clearly indicate the in vitro efficacy of the nanoemulsion containing paclitaxel when compared to blank nanoemulsion. Accordingly, this novel formulation can provide a distinct framework for safe and efficient drug delivery, especially for poorly soluble drugs. These findings will require additional studies involving the evaluation of this SANE formulation of paclitaxel in animal models such as the immune-compromised xenograft nude mouse.
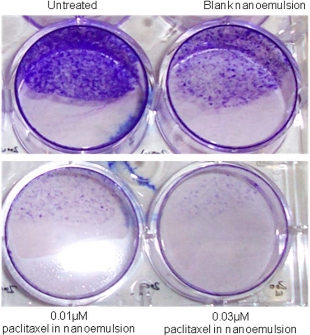
Figure 5. Colony formation assay: The effect of0.01µM and0.03 µM nanoemulsion encapsulating paclitaxel, blank nanoemulsion and no treatment on colony formation properties of PL-45 cells after 48hr treatment ofthe drug. Compared to nanoblank and untreated controls, a 75% decrease in colony formation was observed.
Acknowledgements
The authors would like to thank the graduate studentsand staff at the Center of Health and Disease Research at UMass Lowell for their support and assistance in conducting the study.
References
Received 16 May, 2010; accepted 20 June, 2010; published online 26 June, 2010.
Copyright: (C) 2010 M. Bagul et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.