Article
Dextran-Based Nanocarriers as Efficient Media Delivery Vehicles to Cell Production Bioreactors
Edmund Anton 1, 2, 3, Kambalapally Swetha 1,2, Wilson Thomas 2, R.J. Nicolosi 2, 3
1 Biomedical Engineering and Biotechnology PhD Program.
2Center for Health and Disease Research, Division of Nanomedicine; 3University of Massachusetts Lowel Solomont way, Lowell, MA 01854.
* Corresponding authors. E-mail: anton.edmund@genzyme.com; Anton_edmund@student.uml.edu
Citation: A. Edmund et al., Dextran-based Nanocarriers as Efficient Media Delivery Vehicles to Cell Production Bioreactors. Nano Biomed Eng. 2010, 2(2): 126-132.
DOI: 10.5101/nbe.v2i2.p126-132
Abstract
Decreasing the volume of media required to maintain cell viability not only reduces contamination of bioreactors from the upstream process, but may contribute to cost-containment measures in the biopharmaceutical industry.Based on our recent finding that dextran containing nanocarriers increased CHO cell density up to 20 fold compared to its cellulose-containing microcarrier counterpart (manuscript submitted), we then investigated thepossibility of reducing media volume tomaintain cell viability, by utilizing the same dextran-based nanocarrier prepared from a self assembling nanoemulsion (SANE) method, and an adherent Chinese hamster ovary (CHO) cell culture line to evaluate media volume requirements. At the same 60 mL volume of media, cell viability after day 3 was 6 fold greater in CHO cells exposed to dextran-containing nanocarriers compared to cellulose-based microcarriers. When CHO cells were exposed to 60 mL of media containing dextran-based nanocarriers compared to 100 mL of media for cellulose microcarriers, at day 6, cell density was up to 7 fold greater. Similarly, cell lysate protein concentrations at day 6 was nearly 3 fold greater for CHO cells exposed to dextran containing nanocarriers compared to the cellulose-based microcarriers. Furthermore, nanocarriers had 59% greater glucose concentration, used as a measure of the polymer dextran and cellulose content levels in the nanocarriers and microcarriers, respectively. In conclusion, nanocarriers with increased numbers of dextran molecules, developed in these studies may be useful to further optimize media volume requirements for maximum culture growth.
Keywords:Controldelivery; Dextran; Nanocarrier; Microcarrier; Media optimization; Adherent cells
1. Introduction
In biopharmaceuticals applications, production bioreactors have generated proteins such as enzymes, anti-bodies successfully for the last decade (1). Growth of the cell culture in the bioreactor mainly depends on the volume and composition of media and its upstream delivery as it provides necessary nutrients to the cell culture (2). Although media volumes for biologics production purposes have remained relatively unchanged, the excess requirement for more efficient media delivery, as greater demands on the process are made, isone of the root causes of unwanted contamination (3). On the other hand, optimizing media usage is an ongoing challenge to improve bio production while maintaining cost of production at a minimum (4). In addition, optimizing media usage for production reactors is a” kaizen” or “lean manufacturing” goal of modern business thinking (5).Using in vitro studies, nanocarriers with nanoemulsion-producing techniques have been reported to be an efficient delivery system across various indications (6-9). We are also proposing that our dextran-containing nanocarriers can also be an efficient way of delivering mediato the bioreactor, a possibility receiving some sort from the recent report that dextran nanobubbles enhance oxygen delivery (10).By utilizing dextran-containing nanocarriers, we are hypothesizing that a new process can be developed for biopharmaceutical application which would alter mediarequirements for the production reactor. The reducedvolume of media required to maintain optimum cell growth can also be cost-saving.Recently, we have developed a self assembly nanoemulsion (SANE) process using a modified phase inversion temperature (PIT) method to formulate dextran based nanocarriers (manuscript submitted). In the present study this same dextran-based self-assembling nanoemulsion (SANE) was applied to determine whether the volume of media required to maintain cell growth of the adherent CHO cells could be reduced.
2. Methods and Materials
2.1 Nanocarrier preparation
Microcarriers (Cytopore 2®) were purchased from GE Healthcare Biosciences (Pittsburgh, PA). The components of the nanoemulsions produced for comparative evaluation of both nano and microcarriers were a vegetable oil which, for this application was rice bran oil (RBO) (Tsuno, Japan), the surfactant Solutol HS 15 (BASF, Ludwigshafen, Germany) and distilled / deionized water (Millie Q, Bedford, MA). The RBO (0.5 g) was added to a 50 mL autoclaved beaker along with 2.5 g of the surfactant Solutol and 75 mg Dextran (MolWt Mr~1500 from Sigma Cat. 31394, Sigma, and Saint Louis, MO) and combined with up to 25 mL of deionized water with gentle mixing. A modified phase inversion temperature (PIT) used to form the SANE process (patent-pending) (also referred to as HLB (Hydrophile–Lipophile temperature) varies depending upon the HLB number (Hydrophile–Lipophile balance) of the surfactant. PIT increases with increases in HLB number. The mixture was heated and stirred for 5 min @ 50-60 °C until the three components form a homogeneous mixture. An additional 23 mL of distilled water was added while the mixture was stirring at 60 °C for 5 min. At this stage it formed an O/W emulsion. During heating, when the PIT (or HLB temperature) of the system was reached (65-70 °C, phase inversion zone) for 2-3 minutes, the surfactant was in equilibrium with the oil and water phases. Heating and stirring was continued up to 2 minutes beyond the PIT up to 80 °C. At this temperature the system inverts to a W/O emulsion. The emulsion was subsequently cooled to room temperature by placing it in cool water for 30 to 60 minutes to obtain an O/W emulsion. The particle sizes of the nanoemulsion were determined by dynamic laser light scattering using the Malvern Zetasizer-S (Malvern Instruments Inc., Southborough, MA) at 25 ºC. The range of particle sizes whichcan be measured by the Zetasizer is from 0.6 to 6000nm. The measurement of zeta (ξ) potential is based on the following principle: colloidal particles of nanocarriers dispersed in the RBO and Solutol are electrically charged due to their ionic characteristics and dipolar attributes. Each particle dispersed in the mixture is measured using the Malvern Zetasizer Nano series Zen3600 (Malvern Instruments Ltd., Enigma Business Park, Grovewood Road, Malvern, WorcestershireWR14 1XZ, U.K.)
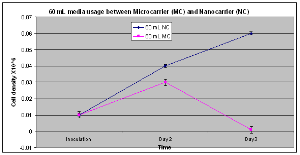
Figure 1. With microcarriers, cell density at day 2 was 7 times greater than day 1 (p<0.05) whereas the nanocarrier increased cell density 9 times higher during that same timed period (p< 0.01). On day 2, cell density of nanocarrier-containing cultures was 2 fold higher than microcarrier contained medium on 2nd day (P<0.04). However, at the third day of culture, microcarrier-containing cultures were unable to maintain little or no cell growth compared to the nanocarrier for which cell density continued to rise.
2.2 Cell culture and preparation for inoculation
Chinese hamster ovarian (CHO) cells were obtainedfrom American Type Cell Culture Collection (ATCC) (Manassas, VA), and they were maintained in a disposable Petri Dish with 25 mL (minimum essential medium) MEM culture medium supplemented with glucose (2.0 g/L) and 10% fetal bovine serum in a 5% CO2:95% O2 maintained-incubator at 37 ºC. The cell passage was carried out at 80–90% confluency at a 1:5 ratio with 0.15% trypsin in CaCl2, MgCl2-free PBS. The cells were counted using a hemacytometerbefore inoculation. Two sterilized 250 mL spinner flasks, which served as bioreactors were prepared for cultivation and each were filled with 100 mL MEM plus 25 mL of freshly prepared nanocarriers, or microcarriers (230 µM particlesize of Cytopore 2®), respectively. Microcarriers were washed with PBS, and a hydration step was performedto remove any air trapped from the microcarrier beforebeing added to the spinner flask. Each flask was placed on a stir plate and inoculated with 2 mL CHO cells and maintained in a 5% CO2:95% O2 atmosphere at 37 ºC. Stirring was set to minimum speed for agitation. The duration of the growth studies were up toseven days.The first series of experiments were performed to determine the minimum volume in the crude bioreactorsystem (250 ml spinner flask) necessary to maintain optimum cell growth and proper agitation. Samples were taken at 24 hour intervals up to seven days from each spinner flask for microscopic examination and growth determination. The cells were counted using a Hemacytometer before inoculation.
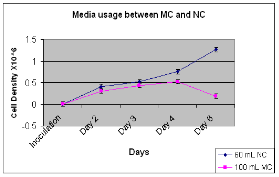
Figure 2. Nanocarriers had 50% and 55%, increased cell density (p < 0.05) from the 1st and 3rd day after 2nd and 4th day of culture, respectively. However, at day 6, nanocarriers despite having only 60 ml media compared to 100 mL of microcarrier, showed a cell density that was nearly 7 fold greater (p<0.004).
2.3 Cell density and cellular protein measurements
Numbers of viable cells were counted under a light microscope using a hemacytometer for determining culture cell density. Cell lysate protein, as a gross estimate of biologics production was determined using, the Bradford assay (11). Briefly, two mL of culture media were taken from the spinner flask, centrifuged at 1000 rpm for 10 min, washed 2 times with ice cold PBS, and centrifuged between washes. Lysate buffer was added to cells and the cell suspension transferred into a centrifuge tube and allowed to incubate for 15 minutes to completely lyse cells in the cold room. The lysate was centrifuged at 1000 rpm for 15 minutes and the supernatant immediately transferred to a fresh centrifuge tube. The pellet was discarded and the supernatant [cell lysate] was diluted up to 10x for measurement of the cell lysate protein concentration.
2.4 Colony formation assay
Colony formation assay is a convenient tool for visually determining viable cells or colony formation. The use of cell colony formation assays for research and clinical applications to assess the functional integrity of cells after in vitro manipulations is extensive (12). In this assay, viable colonies are stained with crystal violet for visualization. Confluent cells grown in tissue culture flasks were maintained in MEM media. Thesecells were then seeded into appropriate 100x20 mmculture plates and allowed to attach with microcarrier or nanocarriers for up to 72 hours. The media was subsequently removed after 72 hours incubation and the cells were washed with PBS, followed by the addition of 40 µL formaldehyde for 20 minutes. Crystal violet (0.5 mL) was added to each plate for an additional 20 minutes. Color intensity is directly proportional to the cell density which was measured after the third day of cell culture.
2.5 Concentration of glucose in dextran-nanocarrierand cellulose-microcarrier
Sigma GAGO-20 glucose kits (Saint Louis, MO)were used to determine the total content of these glucosecontaining polymers of dextran for the nanocarrier and cellulose for the microcarrier.
2.6 Statistical analysis
Data was analyzed using Sigma stat software (JandelScientific, San Rafael, CA, USA) (13). One way ANOVA analysis was done on these triplicate groups followed by the Students “t” test. All values were expressed as mean + SEM and statistical significance wasset at the minimum p < 0.05.
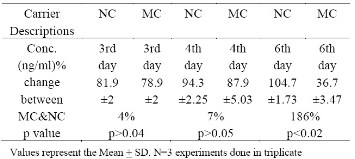
Table1. Total protein content of cell lysates from CHO cells exposed to 100 mL microcarrier (MC) and 60 mL nanocarrier (NC)
3. Results
3.1 Celldensities between cultures exposed to micro and nanocarriers
Earlier studies (data not shown) indicated that a volume of 100 ml of media was appropriate for optimal cell growth in 250 ml spinner flask with proper agitation and therefore was utilized as our control media volume. Whennanocarrier or microcarrier-containing cultures were exposed to 60 ml of media (Fig. 1), microcarrier-containing cultures had 7 times higher cell density at day 2 vs. day 1 (p<0.04). In contrast, nanocarrier-containing cultures had 9 times higher cell density at day 2 vs. day 1 (p<0.04). Comparisons between the microcarrier and nanocarriers at day 2 revealed that nanocarrier containing-media supported a higher cell density than microcarrier-containing media (p<0.02).Furthermore, after the 3rd day of culture, CHO cells exposed to nanocarrier containing-media had 6 fold greater cell densities compared to microcarrier containing media.
3.2 Celldensities between cultures exposed to microcarrier and nanocarriers
Using 60 ml media for the nanocarrier and 100 mL media for the microcarrier experiments (Fig 2), at day 3, nanocarrier-containing cultures had 50% higher cell density (p<0.004) than 2nd day while the microcarrier-containing culture had 40% higher cell density than 3rd day (p<0.03). At day 6, CHO cells exposed to nanocarriers compared to microcarriers had 7 fold greater cell densities.
Particle size andZeta potential Measurements
As shown in Figure3a, particlesize of dextran-containing nanocarrier was 25.15 nm and the polydisperse index (PDI) was 0.312. As shown in Figure3b, particlesize of the nanoblank was 20.12 nm and the polydisperse index (PDI), was reduced to 0.065 as the nanoblank lacked the dextran polymer. Microcarrier particle sizes and PDI [not shown] were beyond the limits of determination by the Malvern Zetasizer indicating that it was greater that6000 nm.As shown in Figure 3c, the zeta potential for the dextran-nanocarrier was-3.62 mV compared to -0.00365 mV zeta potential for the microcarrier.
Colony formation assay
The colony formation assay after visual inspection at the end 72 hrs showed that the CHO cells exposed to nanocarrier-containing media had higher color intensity than the microcarrier (Fig 4), indicative of higher greater cell density.
3.3 CHO cell lysate protein measurements between nano and microcarrier-containing cultures
At a 60 ml media volume, the cultures exposed to microcarriers failed to support cell growth and detecta-ble cell lysate protein, and, therefore cell lysate protein concentration was determined on or after 3rd day. In Table 1, CHO cell lysates exposed to 60 mL nanocarrier-containing cultures and 100 mL microcarrier-containing cultures and analyzed for protein content gave the following results: at day 3, cell lysate protein concentration after exposure to nanocarrier-containing medium was 4% higher than cell lysates (81.9 +2 ng/ mL) prepared from cells exposed to microcarrier containing medium (78.9 +2 ng/mL) but not statistically significant. Similarly, at day 4, although the differences were not statistically significant, cell lysate protein concentration prepared from cells exposed to nanocar-rier-containing media was 94.3+2.25 ng/mL compared to cell lysates prepared from cells exposed to microcarriers (87.9+5.03 ng/mL). However, at day 6, cell lysates prepared from cells exposed to nanocarrier-containing media contained 104.7+1.73 ng/mL and 36.7 + 3.47 ng/mL, for the nanocarrier and microcarrier-containing media, respectively (p < 0.02).
3.4 Micro and nanocarrier glucose concentrations
Glucose measurement as an indicator of polymer concentration of the micro and nanocarrier indicated that dextran-containing nanocarriers had 59 % higher glucose concentration (719 + 139 mg/L) than cellulose-containing microcarrier (411 + 22 mg/L) (p< 0.05).
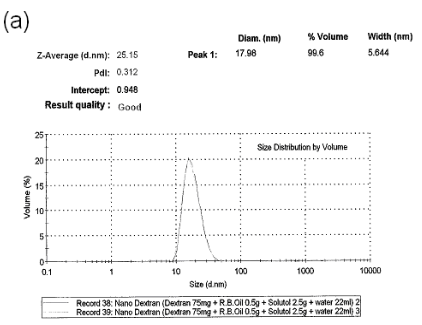
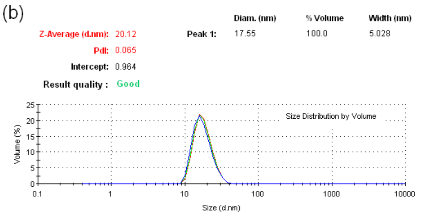
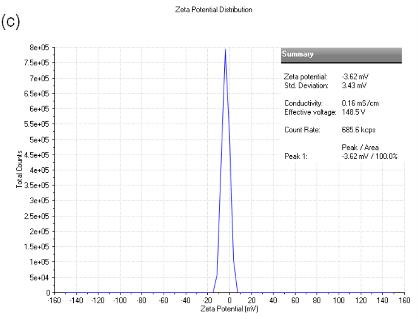
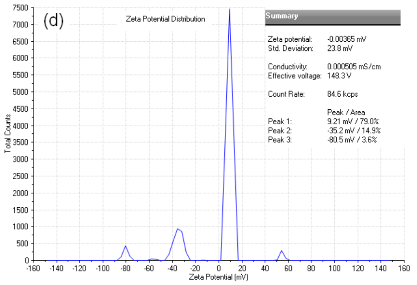
Figure 3. (a) particle size analysis of dextran-containing nanocarriers showing the Z-average size distribution of the particle of 25-30 nm. And a PDI =0.312. (b) particle size analysis of nanoblank was 20 nm with a PDI of 0.065 without added dextran particles. (c) Zeta potential analysis of nanocarrier. As shown, particles have the mean of -3.62 mV zeta potential withthe zeta deviation of 3.43 mV. (d) Zeta potentialanalysis of micro and nanocarrier revealed that the nanocarrier had a zeta potential with a mean of - 3.62 compared to the micro carrier which was -00365 mV demonstrating a reduced zeta potential for the nanocarrier vs. microcarrier.
Additional observations
Visibly, nanocarrier-containing cultures had less foaming than microcarrier containing medium(Fig. 5).
4. Discussion
Nanocarriers utilized in nanoemulsion techniques have proven to be efficient delivery systems (6-9). Optimizing media usage for production reactors is a ”kaizen” or “lean manufacturing” term used in modern business thinking (5). Taken together, we hypothesized that optimization and utilization of dextran-based nanocarriers would reduce the volume of media necessary to maintain the growth of adherent CHO cells. Microcarriers have been widely employed for many years to increase cell density in bioreactor systems (14). Structural modifications such as increases in porosity have provided the microcarrier an extended surface area in the interior and additional attachment space (15) which can support higher cell concentration with equivalent beads. The more recent porous containing-microcarriers which were made from cellulose (16) have an average pore diameter of about 30 µM (17). Thus, the cells in the interior of the macro porous microcarrier are shielded from fluid flow and are hence not susceptible to the detrimental effects of high shear (18). This raises the possibility that potential gradients of nutrients, oxygen, and metabolites along the radius of the microcarrier may cause growth limitation (17). However, in dextran-containing nanocarrier systems described in this report, the adherent membranes of cells are attached to the dextran component of the nanocarrier, instead of being entrapped in the porous cellulose-containing microcarriers. Our results indicatethat cell viability can be maintained at lower media volume in CHO cells exposed to nanocarriers compared to microcarriers with the latter also demonstrating a higher cell lysate protein concentration in nano-carrier containing culture (Table 1). These results indicate that dextran-containing nanocarriers enhance media utilization more effectively.Foam formation and the subsequent cell damage/losses in the foam layer have been reported to be associated with major problems affecting cell growth and monoclonal antibody (MAb) production in stirred and sparged bioreactors ( sprinkle techniques to provide oxygen to the reactor) for both serum supplemented and serum-free media (19). The suspended cells and protein aceous compounds (including recombinant protein product) tend to be entrapped in the foam layer. The cells entrapped in the foam layer are subjected to nutrient and oxygen deficiency, resulting in the reduction of suspended biomass and productivity. These cells may also release proteases and secondary metabolites, and can generate a thick layer that adheres to the reactor wall (wall growth), impeller shaft and the sensors, hindering and disturbing the flow pattern of culture fluid (20). Under extreme foaming conditions, the foam layer could migrate up the gas outlet port and clog the air venting filters, restricting gas flow and making the culture susceptible to contamination (21). Approaches to decrease the foaming include reducing agitation speed and aeration rate without significantly impacting mixing intensity and mass transfer rates to serve as a mechanical foam breaker (20). On the other hand, in our self assembled micelle structure, dextran particles are located in and outside of the structure. These dextran particles may serve as a bridge in between cell membranes and the nanocarrier which floats in oxygen and nutrients contained in the media. This may be the partial explanation for the nanocarrier causing less foaming than the microcarrier containing medium (Fig 2). Having less foaming can also be associated with enhanced oxygen and nutrient delivery to the cells and optimize the nutrients media concentration.Furthermore, dextran used for treating hypovolemiamay also help in volume expansion with improved blood flow (22). Along with the above dextran characteristics, our SANE formulation techniques promoted more efficient media delivery to the culture. In addition, microcarriers made from cellulose (16) coupled with our glucose measurements as an indicator of polymer concentration, showed reduced concentration of polymer in the microcarrier compared to the nanocarrier.In the colony formation assay (Fig 3), color intensity visualization showed that there were greater numbers of cells with the reduced volume of medium for thenanocarrier vs. the microcarrier-containing media. Thismay be explained by, the increased number of dextran particles in our nanocarrier, increasing attachment with the CHO cell membrane more effectively, and at reduced volume of medium. The increased attachment of the cells to the dextran polymer is also likely enhanced because of the dramatic reduction in the zeta potential as a result of the formation of nanoemulsion, as we have reported previously (23,24)From these studies, we conclude that dextran based nanocarriers produced by the SANE method, can improve media utilization. Accordingly, as nanocarriers enhance medium volume requirements for maximum cell growth, these formulation techniques can be used by the biopharmaceutical application for optimizing the cell growth with reduced volume of media.
Figure 4. Colony formation assay at 72 hrs between nanocarrier and microcarrier containing cultures: In-creasing color intensity indicates that plate A with the media containing nanocarrier has a higher cell density than plate B having the microcarrier - containing medium.

Figure 5. Foaming picture between nanocarrier and microcarrier containing culture: nanocarrier containing culture didn’t have any foaming during agitation while microcarrier containing culture showed foaming after 3rd day.
References
Received 12 June, 2010; accepted 25 June, 2010; published online 27 June, 2010.
Copyright: (C) 2010 A. Edmund et al. This is an openaccess article distributed under the terms of the Creative Commons Attribution License,which permits unrestricted use, distribution, andreproduction in anycredited.