Article
The Study on Electrochemical Behaviors of the Interactions between Daunorubicin Hydrochloride and BSA
Jie Liu *, Bo Zhang
Taiyuan City Centre Hospital, Taiyuan, 030009.
* Corresponding authors. E-mail: tyzxyyyjk@163.com
Citation: J. Liu, et al., The study on Electrochemical Behaviors of the Interactions between Daunorubicin Hydrochloride and BSA. Nano Biomed. Eng. 2010, 2(2): 138-142.
DOI: 10.5101/nbe.v2i2.p138-142
Abstract
The Electrochemical behaviors of Daunorubicin hydrochloride, and the interactions between Daunorubicin hydrochloride and BSA, were studied in this paper, with Linear sweep voltammetry and Cyclic voltammety methods. The results showed that, there was a reductive peak at E=-0.66V in the Linear sweep voltammetry of Daunorubicin hydrochloride, on condition of 0.1 mol∙L-1 Na2SO4, pH 8.5B-R buffer solution. After BSA was introduced, the system peak current reduced. There was a maximal system peak current Δip″, when the concentration of Daunorubicin hydrochloride was 5.0×10-5 mol∙L-1. At the range of 5.0×10-8 mol∙L-1-1.0×10-4 mol∙L-1 of the concentration of BSA, it existed a good linear relation between the reduced valve of the peak current of Daunorubicin hydrochloride and the concentration of BSA. This method can be used in the detection of the concentration of BSA.
Keywords: Daunorubicin hydrochloride; Bovine serum albumin; electrochemical behaviors; Linear sweep voltammetry; Cyclic voltammety
1. Introduction
Daunorubicin (DNR) is one of the anthracycline anti-tumor agents widely used in the treatment of acute myeloid leukemia, mammary cancer, etc [1, 2]. The molecular structure of Daunorubicin has a anthracene nucleus plane [3] (Figure 1), can embed itself into the DNA base pair, then close combined with DNA, which can block DNA’s spatial structure, thus inhibits the composition of nucleic acid [4,5]. It is shown that the aglucon of Daunorubicin can be embedded into the DNA base pairs, as the interaction between the positive charge on glycosyl group and negative charge on DNA phosphate group, the complexes on DNR and DNA is stable, so inhibits the DNA replication [6]. Also, Daunorubicin can act on DNA free radicals, causing the rupture of DNA chain and ultimately cell death. The study on the action mechanism of Daunorubicincan help to clarity the agent’s storage and transport process in the body, which is an active research subject. In recent years, people has studied the selective binding molecular mechanism on DNR and DNA base sequence, indicated that DNR could identify special DNA sequence as its best binding sites, then the best base sequence between DNR and DNA was forecasted [7]. To present, the analytical methods of DNR include HPLC, Capillary Electrophoresis, ESI mass spectrometry etc [8-10]. It is a marginal subject between chemistry and biology that the study on the interactions of the agent molecular and Bovine serum albumin (BSA) [11, 12]. from the point view of chemistry, it mainly includes binding sites, binding constant, force type, the effect of coexist substance, the distribution of drug in blood etc. BSA is a abundant carrier albumen in plasma, and can interact with many endogenous substances. In the elements which involved in many in many important life processes. So the study on the interactions of drugs and BSA has great significance. In this paper, electrochemical determination as a novel method for Daunorubicin hydrochloride was built. With Linear sweep voltammetry and Cyclic voltammety methods, the interactions between Daunorubicin hydrochloride and BSA were studied. This method is an ideal analysis method for drugs, which has the advantage in simpleness, speedness, high sensitivity.

Figure 1. The molecular structure of daunorubicin
2. Materials and Methods
2.1 Materials and instruments
LK 2005-type Electrochemical workstation (Lanlike chemical Electronics high-tech Co., Ltd., Tianjin). Three-electrode system: reference electrode-Saturated calomel electrode (SCE), supporting electrode-platinum electrode, working electrode-Silver disk electrode (Φ: 50 µm). Type PHS-3C pH meter: (Ronghua Instrument Manufacturing Co., Ltd., Jintan). Type KQ-500B Ultrasonic cleaner (Ultrasonic Instrument Co., Ltd. Kunming). Daunorubicin hydrochloride standard stock solution (1.0×10-3 mol·L-1): Accurately weighed 0.0564 g Daunorubicin hydrochloride (mass fraction > 99.5 %), Dissolved in secondary distilled water and set the volume to l00 mL volumetric flask. Placed in brown reagent bottle, saved in cold and dark. Dilute to the desired concentration ready for use. Bovine serum albumin (BSA, Sigma Co., molecular weight: 68000) pH8.5 Britton-Robinson buffer solution, 0.1 mol L-1 Na2SO4 solution.
2.2 Exprimental section
(1) Electrode pretreatment: Use small metallographic sandpaper polish the silver disk electrode smoothly first, and use Al2O3 powder polished into a mirror on the thick cloth, then wash thoroughly with water and ethanol in sequence in the ultrasonic bath.
(2) The Electrochemical behaviors of Daunorubicin hydrochloride with Linear sweep voltammetry: In 10 mL biosome there are also many trace elements and micro mL comparison tube, mix 0.1 mol·L-1 pH8.5 Britton-Robinson buffer solution 1 mL, 0.1 mol·L-1 Na2SO4 1 mL, corresponding Daunorubicin hydrochloride standard solution(or sample solution) in sequence, and diluted to the desired concentration using secondary distilled water. Then transfer to the electrolytic cell for linear sweep voltammetry scanning on electrochemical workstation. Set initial potential as -0.4 V, record the value of reductive peak current at -0.66 V, the concentration of Daunorubicin hydrochloride was calculated by calibration curve method.
(3) The interactions between Daunorubicin hydrochloride and BSA with Linear sweep voltammetry and Cyclic voltammety methods: In 10mL comparison tube, mix 0.1 mol·L-1 pH 8.5 Britton-Robinson buffer solution 1 mL, 0.1 mol·L-1 Na2SO4 1 mL, 1.0×10-3 mol·L-1 Daunorubicin hydrochloride 0.5mL and different concentrations of BSA in sequence, diluted to the desired scale. After fully mixed, stand for 10 minutes at ambient temperature, with solution without BSA as control.
3. Results and Discussion
3.1 Voltammetric response of daunorubicin hydrochloride on Silver disk electrode
(1) Effects of supported electrolyte
HC1, HAc-NaAc, KH2PO4-Na2HPO4, Britton-Robinson buffer solution, Na2SO4, KC1, NaCl, NH3-NH4Cl and NaOH, all of which were 0.1 mol·L-1, were selected respectively as supported electrolyte to study the impact on the peak current. The results showed that, Daunorubicin hydrochloride had a reductive peak in all of these mediums, while the best peak shape and largest peak current appeared in Na2SO4 solution, so Na2SO4 was selected as supported electrolyte.
(2) Effects of base solution pH
Use Na2SO4 as supported electrolyte, measured at different concentrations with Linear sweep voltammetry method. As shown in Figure 2, the best peak shape and largest peak current appeared at the concentration of 0.1 mol L-1. While less than 0.1 mol·L-1, peak shape is poor; higher than 0.1 mol·L-1, the peak current decreased. So the concentration of Na2SO4 was selected 0.1 mol·L-1. The peak current was measured by changing the pH value of solution with Britton-Robinson buffer solution, at the condition of 0.1 mol·L-1 Na2SO4 as base solution, the concentration of Daunorubicin hydrochloride 5.0×10-5 mol·L-1. The results showed that, the peak current reached maximum at pH 8.5, and gradually became smaller with the increase of pH value. So thebase solution acidity was selected pH8.5. (Figure 3).
(3) Linear sweep voltammograms of Daunorubicin hydrochloride on silver disk electrode.
According to the optimal condition, 0.1 mol·L-1 Na2SO4 solution and pH8.5 Britton-Robinson buffer solution, different concentrations of Daunorubicin hydrochloride on silver disk electrode was analyzed with Linear sweep voltammetry, potential scanning range from -0.4V to -1.2V (Figure 4). The results showed there was a reductive peak at E=-0.66V in the Linear sweep voltammetry of the Daunorubicin hydrochloride, but no oxidation peak existed, which showed that the reaction was irreversible.

Figure 2. Election of Na2SO4 concentration
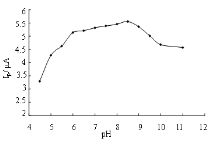
Figure 3. Effects between the pH and peak current

Figure 4. Linear sweep voltammetry curve of Daunorubicin hydrochloride: a. 0; b. 1.0×10-5 mol·L-1; c. 3.0×10-5 mol·L-1; d. 5.0×10-5 mol·L-1; e. 8.0×10-5 mol·L-1; f. 1.0× 10-4 mol·L-1 (scanning rate: 100 mV·s-1)
3.2 Electrochemical Behaviors of the interactions between Daunorubicin hydrochloride and BSA
(1) Voltammetry of the interactions between Daunorubicin hydrochloride and BSA
In pH 8.5 Britton-Robinson buffer solution, there is a reductive peak (Vp= -0.63V) of Daunorubicin hydrochloride on silver disk electrode, The peak current (Ip) is proportional to the scanning speed (υ), and the linear regression equation is E=-0.2086υ-0.6463 (Ep: V, υ: mV·s-1) with correlation coefficent r=0.998, showed that Daunorubicin hydrochloride in silver disk electrode reduction process is controlled by adsorption. After BSA was introduced, no extra peak existed in scanning range, peak potential were essentially same, (Figure 5) The more BSA added, the greater the peak current reduced. It is indicated that the BSA- Daunorubicin hydrochloride complexes have no electrochemical activity. Since BSA-Daunorubicin hydrochloride complexes formed, the concentration of dissociative Daunorubicin hydrochloride decreased, hence the corresponding peak current reduced.
(2) Effects of Daunorubicin hydrochloride dosage on the reaction system
In pH 8.5 Britton-Robinson buffer solution, BSA concentration 1.0×10-5 mol·L-1, the effects of Daunorubicin hydrochloride dosage were analysed with Cyclic voltammety method. The results showed that, the peak current Δip″ had a maximum when the concentration of Daunorubicin hydrochloride was 5.0×10-5 mol·L-1. So 5.0×10-5 mol·L-1 was selected as optimum concentration.
(3) The linear relation between the reduced valve of the peak current of Daunorubicin hydrochloride and the concentration of BSA.
Under optimum conditions, it existed a good linear relation between the reduced valve of the peak current of Daunorubicin hydrochloride and the concentration of BSA, at the range of 5.0×10-8 mol·L-1-1.0×10-4 mol·L-1 of the concentration of BSA. The linear regression equation is Ip (µA) =0.201c (×10-6 mol·L-1) -0.0056, with correlation coefficient r = 0.997 and minimum detectable concentration 5.0×10-9 mol·L-1. 10 times comparative experiment were measured further, with relative standard deviation 1.64%. It proves that this method can be used in the concentration detection of BSA.

Figure 5. Cyclic voltammetry curves and Linear sweep voltammogram curve of reaction system of BSA-Daunorubicin hydrochloride. 1. 5.0×10-5 mol·L-1 Daunorubicin hydrochloride; 2. 1+BSA 1.0×10-5 mol·L-1 3. 1+BSA 1.5×10-5 mol·L-1; 4. 1+BSA 2.0×10-5 mol·L-1. (a) Cyclic voltammetry curves (b) Linear sweep voltammogram curves.
4. Conclusions
(1) The Electrochemical behaviors of Daunorubicinhydrochloride were studied first, with Linear sweep voltammetry method. The results show that, there is a maximal reductive peak at E=-0.66V in the Linear sweep voltammetry of the Daunorubicin hydrochloride, with the conditions of 0.1 mol·L-1 Na2SO4, pH 8.5B-R buffer solution. There is no oxidation peak existed, which showed that the electrode reaction was an irreversible process.
(2) The Electrochemical behaviors of the interactions between Daunorubicin hydrochloride and BSA, were studied then, with Linear sweep voltammetry and Cyclic voltammety methods. After BSA was introduced, the system peak current reduced, which indicated that the Daunorubicin hydrochloride- BSA complexes have no electrochemical activity. There was a maximal system peak current Δip″, when the concentration of Daunorubicin hydrochloride was 5.0×10-5 mol·L-1. At the range of 5.0×10-8 mol·L-1-1.0×10-4 mol·L-1 of the concentration of BSA, it existed a good linear relation between the reduced valve of the peak current of Daunorubicin hydrochloride and the concentration of BSA. This method can be used in the detection of the concentration of BSA.
Acknowledgements
We thank Professor Yong Zhang for providing expert help with electrochemical observations. This work was supported by Taiyuan City Centre Hospital’s funding.
References
Received 10 June, 2010; accepted 6 July, 2010; published online 9 July, 2010.
Copyright: (c) 2010 J. Liu et al. This is an open access article distributed under the terms of the Creative unrestricted use, Commons Attribution License, Which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.