Article
Electrochemical Property and Cell Toxicity of Gold Electrode Modified by Monolayer PAMAM Encapsulated Gold Nanorods
Xueqing Zhang, Bifeng Pan, Kan Wang, Jing Ruan, Chenchen Bao, Hao Yang, Rong He, Daxiang Cui *
Department of Bio-Nano-Science and Engineering, National Key Laboratory of Nano/Micro Fabrication Technology, Key Laboratory for Thin Film and Microfabrication of Ministry of Education, Institute of Micro-Nano Science and Technology, Shanghai Jiao Tong University, Shanghai 200240, China.
Note: Xueqing Zhang and Bifeng Pan are equally contributed to this work
* Corresponding author. E-mail: dxcui@sjtu.edu.cn
Citation: X. Zhang et al. Electrochemical Property and Cell Toxicity of Gold Electrode Modified by Monolayer PAMAM Encapsulated Gold Nanorods. Nano Biomed Eng. 2010, 2(3), 182-188.
DOI: 10.5101/nbe.v2i3.p182-188.
Abstract
Herein we exploit the molecular engineering capability to immobilize monolayer of polyamidoamine dendrimer on gold electrode, which exhibit enhanced charge transfer and biocompatibility. Polyamidoamine (PAMAM generation 5.0) dendrimers were used as template/stabilizers for gold nanoparticle growth, with Au@PAMAM nanoparticles serving as surface modifier to produce monolayer film. TEM, UV-vis spectroscopy, and AFM were used to characterize the formation of monolayer Au@PAMAM on gold surface. The cyclic voltammetry (CV) and Impedance measurements of Au@PAMAM modified gold electrodes demonstrate electrochemistry properties of modified electrode. Furthermore, Au@PAMAM coating can greatly increased the biocompatibility of gold electrode as determined by cell growth curves
Keywords: Monolayer; Gold electrode; Cyclic voltammetry; Biocompatibility
Dendrimers are a special class of organic molecules that can undergo a series of chemical modifications through surface chemistry while their interior cavities serve as templates for nanoparticle growth [1-3]. Po- lyamidoamine (PAMAM) dendrimers, in particular, have been recently reported as very effective nanopar- ticle stabilizers, allowing their use as nanoreactors [4,5]. Dendrimer molecules possess three basic architectural components [6,7]: an initiator core (e.g., ethylenedia- mine), interior layers often called “generations”, which comprise repeating units attached to the initiator core, and the shell which generally consists of functionalized groups attached to the outermost interior layer. It is well-known that dendrimers of lower generations tend to exist in relatively open forms [6,7], while high generation dendrimers take on a spherical three- dimensional structure. The latter structure, however, is very different from conventional linear polymers, which adopt random-coil structures. Thus, many inter- esting reactions using dendrimers have been reported because they can provide reaction sites in the interior or on the periphery of the dendrimers and are very use- ful as model systems [8,9]. Because of the unique physical and chemical characteristics, nanosized mate- rials and nanoscale technologies are changing many basic scientific concepts [10-13]. One of the most widely studied and used nanomaterials is gold nanopar- ticles [14,15]. For example, gold nanoparticles have been proposed for many potential applications such as superstructured material [16], optical devices [17], mo- lecular switch (memory switch) [18], high-temperature superconductive material [19], quantum computer [20],and biomedical use [21]. It has been predicted that tens or even hundreds of tons of gold nanoparticles will be produced worldwide every year. Monolayers of PAMAM dendrimers have been pre- pared by electrostatic self-assembly from aqueous solu- tions [22]. Bliznyuk et al. [23] proposed a model of molecular ordering, which assumes highly deformed (compressed parallel to the surface) ellipsoidal shapes for macromolecules in condensed monolayers. The molecular dimension (thickness) versus molecular mass dependence is described by a scaling law. Cheng and Cox [24] employed the layer-by-layer (LbL) depo- sition technique to fabricate multilayer films consisting of polyoxometalates (POMs) and G4-PAMAM. They demonstrated the fabrication of uniform and well- defined multilayered supramolecular structures consist- ing of inorganic POMs and organic PAMAM for appli- cation in catalysis. Hybrid nanoparticles of carboxyl- terminated PAMAM dendrimers containing encapsu- lated Pt nanocrystals were prepared [25], and reports on the fabrication of Au [26], Cu [27], and Pd [28] na- noparticles within PAMAM dendrimers have also been made. Highly monodispersed, 1-2 nm diameter, Au nanoparticles were prepared by Kim et al. [29] using PAMAM dendrimers as templates (Au@PAMAM). The synthesis was carried out in water and took less than 30 min, requiring no subsequent purification. He et al. [30] reported the electrostatic LbL assembly of a Au-dendrimer nanocomposite using poly(sodium 4- styrenesulfonate) (PSS) as the oppositely charged po- lyelectrolyte leading to nanoscale uniform multilayers of Au-dendrimer nanoclusters. An advantage of PAMAM nanoreactors is the small nanoparticle diame- ter. Metal nanoparticles (less than 4 nm in diameter) are interesting because of their inherent size-dependent optical, electrical, catalytic, and magnetic properties. These materials have been integrated into new kinds of biosensors, have shown effects of particle size on hete- rogeneous catalytic reactions, and have been used for the fabrication of nanometerscale electronic devices, supercapacitors, and data storage devices. It was found that gold electrode was highly toxic to cells [31]. As enlightened by these studies, we con- ducted the biocompatibility study of the gold electrode with and without dendrimer coating. Dendrimer coat- ing provide an opportunity to reduce the comparative toxicity particularly originated by gold electrode. In this work, we report the fabrication of nanostructured films comprising gold nanoparticle-containing amine- terminated G4 PAMAM dendrimer. Nanosized Au na- noparticles were grown inside PAMAM molecules us- ing formic acid as the reduction agent. The objectives of this study were to reduce the cytotoxicity to cells caused by the above mentioned gold electrode.

Figure 1. (Step 1) Preparation of thoil-terminated PAMAM dendrimer, (step 2) preparation of gold nanoparticles by using polyamidoamine dendrimer as template.
2.1 Materials
Poly(amidoamine) (PAMAM) dendrimers with sur- face amine groups (generation 5.0) were prepared according to the literature [1-7]. Methyl mercaptoacetate (98%) was purchased from Sigma Co. (U.S.A.). Tetrachloroauric acid, sodium borate (NaBH4), and N, N’- dimethylfomamide (DMF) were obtained from Shang- hai Chemical Reagent Corporation (Shanghai, China).All materials were of analytical grade and used as re- ceived. Gold electrode, platinum electrode, and calo- mel electrode are purchased from Tianjin Aida Tech Corporation (Tianjin, China). Human breast cancer cell line (MCF-7) was obtained from ATCC Company.
Figure 2. Monolayer Au@PAMAM Nanocomposite on gold electrode surface
2.2 Preparation of thoil-modified PAMAM dendrimer
A proper amount of methyl mercaptoacetate in water was added to 10 mg freshly prepared amine-terminated PAMAM dendrimer (G 5.0) dissolved in 20 ml water. The mixture was reacted with stirring at 50˚C for 9 h to complete the formation of the thiol-terminated dendri- mer. The solvent and residual methyl mercaptoacetate were removed under vacuum at 70 ˚C to get a thick honey-colored oil. The chemical structure of thiol- modified PAMAM dendrimer is shown in Figure 1 (step 1).
2.3 Preparation of gold nanoparticles by using po- lyamidoamine dendrimer as template (Figure 1, step 2)
Tetrachloroauric acid (0.5 M, HAuCl4) and PAMAM dendrimer solutions (0.5 M) in DMF solvent were prepared. To 10 ml of DMF, 40 μl of 0.5M HAuCl4 solu- tion was added with stirring. The resulting HAuCl4 solution has a concentration of 2×10−3 M. To 10 ml of DMF, 160μl of PAMAM dendrimer (0.5 M) in DMF was added resulting in a concentration of 8×10−3 M. This solution (10 ml) was added to the HAuCl4 solution. The mixture of HAuCl4 and PAMAM dendrimer was stirred for 60 min, and then NaBH4 was added. The resulting grey mixture turned slowly to a light red colour. After stirring for 15 min, the gold nanoparticles prepared by this procedure were wine red. Such par- ticles are called Au@PAMAM nanocomposite, and they are well known to exhibit an extinction band located at approximately 520 nm.
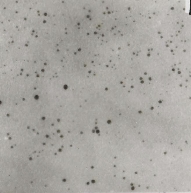
Figure 3. HR-TEM image of Au@PAMAM nanocomposites
2.4 Formation of monolayer Au@PAMAM on gold electrode surface (Figure 2)
Gold electrode were dipped into above 1 wt% Au@PAMAM DMF solution and incubated for 24 h at room temperature to form stable Au@PAMAM mono- layer on gold electrode surface. A control experiment was also conducted by using PAMAM dendrimer only.
2.5 Characterization:TEM, FT-IR, UV-vis, AFM
High-resolution transmission electron microscopy (HR-TEM, Hitachi H-700H) was used to confirm gold nanoparticle size. Fourier transform infrared (FT-IR) spectroscopy was conducted with an FTS135 infrared spectrometer (BIO-RAD, USA). Atomic force micro- scopy (AFM) imaging was performed by Nanoscope III (Digital Instruments/Veeco Metrology Group, USA). UV–vis spectra were measured at 20 ˚C with a UNICAM UV 300 spectrometer (Thermo Spectronic, U.S.A.) equipped with a 10mm quartz cell. The 300– 800 nm wavelength region was scanned as it includes the absorbance of the gold nanoparticles.
2.6 Electrochemical Property of gold electrode before and after Au@PAMAM modification
All electrochemical characterizations were carried out using a three-electrode setup, with modified gold as the working electrode, platinum foil as the counter electrode, and saturated calomel as the reference elec- trode. Cyclic voltammetry measurements were carried out (CHI 660A TX, USA) at a scan rate of 0.05 V/s in the potential window, −0.2 to 0.5 V in the presence of 0.1 M NaF as the supporting electrolyte under inert atmosphere. Accessibility studies were carried out us- ing 3 mM potassium ferrocyanide/potassium ferricyanide in 0.1 M NaF. Impedance spectra were collected in the frequency range of 100 kHz to 100 MHz (5210, EG&G PARC, USA) with 5 mV rms AC perturbation at 0.2 V DC bias.
2.7 Cell biocompatibility of gold electrode: mea- surement of cell growth curve
Human breast cancer MCF-7 cells were cultured for 24 h in RPMI 1640 containing 1×105 mU/mL of peni- cillin, and 0.1 mg/mL of streptomycin supplemented with 10% (v/v) FCS at 3˚7C in a humidified 5% CO 2 incubator. In order to investigate the biocompatibility of gold electrode, we grew MCF-7 cell on the Au@PAMAM modified gold plate surface. Gold plate without Au@PAMAM modification was used as con- trol experiment. Inverted Microscope was used to ob- served cell morphology and growth status. Cell count- ing was conducted at 1, 2, 3, and 4 days culture to draw cell growth curves.
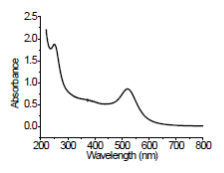
Figure 4. UV–vis spectrum of Au@PAMAM nanocomposite in DMF solvent at 20˚C
The HRTEM photos indicating the particle size and shape are shown in Figure 3. Every gold nanoparticle has a spherical form, and the average diameter is 1-2 nm. No gold nanoparticles were seen as aggregates by HR-TEM, indicating gold nanoparticles were stabilized and separated by PAMAM dendrimers. It is not easy to directly observe the PAMAM dendrimer from TEM micrograph.The formation of Au@PAMAM nanocomposites was further demonstrated by the characteristic peak at ~520 nm as shown in Figure 4.

Figure 5. (a) Gold electrode without Au@PAMAM modifica- tion, (b) gold electrode with Au@PAMAM modification, indi- cating monolayer Au@PAMAM on gold electrode surface.
3.1 AFM observation of gold electrode before and after Au@PAMAM modification
It can be easily seen from AFM images that there are Au@PAMAM monolayer on the gold electrode surface. Before Au@PAMAM modification, gold electrode is seen as a smooth plane shown in Figure 5a. After Au@PAMAM modification, gold electrode is coated by a layer of nanocomposite as clearly shown in Figure 5b. voltammetry and impedance measurements. The electron transfer rate between a diffusion species and an electrode surface is a function of the distance between them in addition to other parameters as given by the Marcus theory. Well-ordered and tightly packed mono- layers act as a barrier and block the Faradaic process between a diffusing species and the electrode surface. The defects can be quantitatively assessed from the current response in the voltammetry by calculating the heterogeneous rate constant associated with the Fara- daic process. Figure 6 shows the cyclic voltammo- grams of the bare Au electrode in 0.1 M H2SO4 solu- tion. Figure 7 shows the cyclic voltammograms of bare gold electrode and monolayer Au@PAMAM -modified gold electrodes in 3 mM [Fe(CN)6]4−/3− in the presence of 1.0 M KCl supporting electrolyte. A reversible redox response for the bare gold electrode is clearly observed. The voltammograms for the Au@PAMAM monolayer modified electrodes show an excellent blocking beha- vior as revealed by the observed low currents flowing in the cell. It is clearly observed that the monolayers prepared from the neat compound are highly blocking in nature.
3.2 Electrochemical characterization
The Au@PAMAM nanocomposites were assembled onto gold electrode for electrochemical characterization. The aim was to obtain enhanced charge transport, which may be important for a number of applications,especially in biosensors requiring redox mediators. To further exploit the enhanced transport, in subsequent electrochemical experiments we show that a new system based on Au@PAMAM can be easily detected using cyclic voltammetry (CV). The integrity and packing of the monolayers under electrochemical conditions were followed using cyclic voltammetry and impedance measurements. The elec-tron transfer rate between a diffusion species and an electrode surface is a function of the distance between them in addition to other parameters as given by the Marcus theory. Well-ordered and tightly packed mono-layers act as a barrier and block the Faradaic process between a diffusing species and the electrode surface. The defects can be quantitatively assessed from the current response in the voltammetry by calculating the heterogeneous rate constant associated with the Fara-daic process. Figure 6 shows the cyclic voltammo-grams of the bare Au electrode in 0.1 M H2SO4 solu-tion. Figure 7 shows the cyclic voltammograms of bare gold electrode and monolayer Au@PAMAM -modified gold electrodes in 3 mM [Fe(CN)6]4−/3− in the presence of 1.0 M KCl supporting electrolyte. A reversible redox response for the bare gold electrode is clearly observed. The voltammograms for the Au@PAMAM monolayer modified electrodes show an excellent blocking beha-vior as revealed by the observed low currents flowing in the cell. It is clearly observed that the monolayers prepared from the neat compound are highly blocking in nature. Impedance measurements were carried out to follow the charge transfer resistance for the reaction involving the redox couple [Fe(CN)6]4−/3− present in the electro- lyte. Figure 8 shows the Nyquist plots for the bare Au electrode and Au@PAMAM monolayer modified gold electrode in 1 mM [Fe(CN)6]4−/3− and 1.0 M KCl as the electrolyte at 0.2-V DC bias. The plot for the bare elec- trode shows a small semicircle in the high-frequency region followed by a straight line in the low-frequency region oriented at 45˚, showing that the process is diffusion controlled. The Au@PAMAM-modified electrode shows a large semicircle in the entire frequency region (Figure 8). This is similar to the one proposed by Fawcett and co-workers for alkaneselenol-based SAMs on gold. The results are analyzed by fitting with a standard Randle equivalent circuit comprising a parallel combination of constant phase element represented by Q and a Fara-daic impedance, Zf, in series with the uncompensated solution resistance, Ru. The Zf is a combination of charge transfer resistance, Rct, and the Warburg imped-ance, W, in series. This model is used to fit the data on a bare gold electrode and Au@PAMAM on gold elec-trode (straight-line behavior in the low-frequency re-gion). In the case of Au@PAMAM-modified elec-trodes with good order, the Zf consists only of charge transfer resistance, Rct. The Rct for the Au@PAMAM monolayers prepared from the neat compound and the ethanolic solution is 560 and 297 Ωcm2 and the rate constant values have been determined to be 3.2 × 10−4 and 8.4 × 10−4 cms−1, respectively. The impedance data for the Au@PAMAM monolayers follow a similar trend. The Rct values for Au@PAMAM monolayers prepared from neat and ethanolic solution are 2938 and 2055 Ωcm2 and the rate constants have been deter-mined to be 3.1×10−4 and 9.4×10−4 cms−1, respectively.
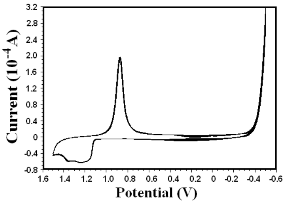
Figure 6. Cyclic voltammograms of bare gold electrode in 0.1 M H2SO4 solution.
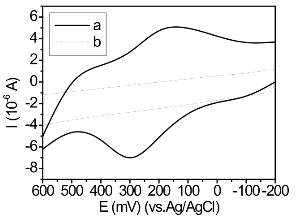
Figure 7. Cyclic voltammograms of 3 mM potassium ferrocya- nide/potassium ferricyanide in 1.0 M KCl supporting electrolyte on (a) Au@PAMAM modified electrode where the monolayer is formed from the neat compound, (b) bare gold surface.

Figure 8. (A) Impedance (Nyquist) plots of (■) bare gold, (▼) Au@PAMAM modified gold electrode at pH 7.0, and (●)Au@PAMAM modified gold electrode at pH 4.0.


Figure 9. (left) Incubation of MCF-7 cells on bare gold plate surface, (right) Incubation of MCF-7 cells on Au@PAMAM modified gold plate.
3.3 Cell biocompatibility of gold electrode: mea-surement of cell growth curve.
The cell number was counted by using the Trypan blue dye exclusion method and the percentage of cell growth was calculated as a ratio of numbers of PAMAM-asODN treated cells and control cells with 0.5% DMSO vehicle. From Inverted Microscope images (Figure 9) and Cell growth curves (Figure 10) show that the Au@PAMAM modified gold substrate exhibited lower cytotoxicity than the bare gold substrate, indicating Au@PAMAM modification can be used to increase biocompatibility of gold electrode. As shown in Figure 10, the inhibition rate of MCF-7 cells is ~10% for Au@PAMAM modified gold electrode, on the other hand, in the case of bare gold substrate, ~60% of MCF-7 cells were inhibited.
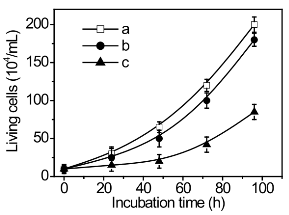
Figure 10. Effect of gold substrate on proliferation of MCF-7 cancer cells. (a) Control, (b) Au@PAMAM modified gold sub-strate, (c) gold substrate.
Gold nanoparticles with diameter of 1-2 nm were synthesized in DMF solvent by using G5.0 PAMAM dendrimer as template. Au@PAMAM nanocomposite with thoil surface was used to modify gold electrode to form monolayer Au@PAMAM on gold surface. Elec- trochemical properties of Au@PAMAM modified gold electrode were determined by cyclic voltammetry (CV) and Impedance measurements. Importantly, we found that biocompatibility has been increased greatly after Au@PAMAM modification.
Acknowledgement
This work is supported by the National Key Basic Re- search Program ( 973 Project ) (2005CB724300G and 2010CB933901), National 863 Hi-tech Project (2007AA022004), Important National Science & Technology Specific Projects (2009ZX10004-311), National Natural Scientific Fund (No.20771075 and No.20803040), Special project for nano-technology from Shanghai (No.1052nm04100), New Century Ex- cellent Talent of Ministry of Education of China (NCET-08-0350) and Shanghai Science and Technolo- gy Fund (10XD1406100).
References
Received 16 Jul, 2010; accepted 22 Sep, 2010; published online 4 Oct, 2010.
Copyright: (C) 2010 X. Zhang et al. This is an open access article distributed under the terms of the Crea- tive Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.