![]()
Article
Inter-prismatic matrix structure characterization of mollusk shell and its effect on crystal formation
Dapeng Yang1,2*, Peng Huang1, Bifeng Pan3, Yang Mo1*
1Department of Bio-Nano-Science and Engineering, National Key Laboratory of Nano/Micro Fabrication Technology, Key Laboratory for Thin Film and Microfabrication of Ministry of Education, Institute of
Micro-Nano Science and Technology, Shanghai JiaoTong University, Shanghai, 200240, China
2Department of Health Technology and Informatics, The Hong Kong Polytechnic University, Kowloon, Hong Kong, China
3Departments of Otolaryngology-Head and Neck Surgery, Stanford University School of Medicine, Stanford, CA 94305, USA.
*Corresponding authors. Email: Mo.Yang@inet.polyu.edu.hk (M. Yang); boyydp@sjtu.edu.cn (D.P. Yang).
Abstract
Mollusk biomineralization is an elaborate process in which cells, organic macromolecules, and calcium carbonate crystals are actively involved. Macromolecules (mainly are proteins and polysaccharide) act as a key role in regulating and limiting the size, orientation, polymorph and texture of inorganic phase. In this work, we focused on the inter-prismatic matrix of mollusk shell combining scanning electron microscopy (SEM), X-ray diffraction (XRD) and transmission electron microscopy (TEM) analytical techniques with CaCO3 recrystallization experiment to characterize its structure and effects on crystal formation. Our results show that the inter-prismatic matrix is not a sort of pure polymer, calcite nano-crystals are also located inside the inter-prismatic matrix. Interestingly, it seems that these nanocrystals have a preferred orientation, which means the inter-prismatic matrix do impose effect on the crystal formation. In vitro re-crystallization experiment using partially dissolved prismatic fragment as template indicates that the (104) faces of CaCO3 micro-crystals are closely associated with the walls of inter-prismatic matrix. Furthermore, a possible growth mechanism of mollusk shell prismatic layer was proposed.
Keywords: Biomineralization; Mollusk shell; Inter-prismatic matrix; Calcite, Dissolution;
Citation: D. Yang, et al. Inter-prismatic matrix structure characterization of mollusk shell and its effect on crystal formation.Nano Biomed. Eng.2010, 2(4), 218-224. DOI: 10.5101/nbe.v2i4.p218-224.
1. Introduction
Billions of years’ evolution endows living organism with fascinating shapes, structures and corresponding multi-function features which are attracting more and more scientists to throw themselves into this field called biomineralization [1,2]. Of all the minerals formed by organisms, calcium carbonate (CaCO3 ) is well known not only because it is widely distributed in nature, but also because it is a kind of typical mineral to reveal the biomineralization mechanism which is mystical to the curious people. On the other hand, understanding the design strategy behind the mineral will hold great promise for the future development of biological, chemical and materials sciences [3-9]. Mollusk is a kind of classic model organism for the research of biomineralization [4]. Most mollusk secretes CaCO3 crystals in the form of aragonite and/or calcite which are well studied using modern materials analysis and characterization techniques, such as X-ray diffraction (XRD) [10], scanning electron microscope (SEM) [11,12], transmission electron microscopy (TEM) [13], and atomic force microscopy (AFM) [14], etc. Based on these advanced analytical techniques, some hypotheses [15-23] are also put forward in attempt to reveal the formation mechanism of molluk shell. However, the shell is a special composite which includes two parts: inorganic phase and organic phase. Deduced alone from the perspective of inorganic phase, the authentic scenario is less far revealed. To fully understand the mollusk shell mineralization process, an organic phase view is also equally required. The extracellular matrix (organic phase) is made up of multifunctional macromolecules (mainly polysaccharides and proteins) [2] which play a key role in regulating and controlling CaCO3 crystals growth and orientation, determining the shape, size and polymorph [24-29], maintaining the mechanical performances [18], even displaying enzymatic functions [30] and involving in cell signalling [31]. Currently, there is a great deal of work relating with the purification and characterization of proteins from mollusk shell [32-38]. However, less concern is paid attention to the inter-prismatic proteins (insoluble in water) as a whole from a structural perspective. In this work, we focus on the insoluble inter-prismatic matrix extracted from the prismatic layer in Atrina pectinata, characterising its structures and certain habits through SEM, XRD, TME and CaCO3 re-crystallization experiment in vitro. In the end, we discussed the possible effects of inter-prismatic matrix on mollusk shell formation.
2. Materials and Methods
2.1 Chemicals
Sodium hydroxide (A.C.S. reagent, Aldrich), hydrochloric acid (A.C.S. reagent, Aldrich), Ethanol (anhydrous, Aldrich), Calcium chloride (A.C.S. reagent, Sigma-Aldrich), ammonium carbonate (A.C.S. reagent, Aldrich) and Ethylene Diamine Tetraacetic Acid (A.C.S. reagent, Aldrich) were used without further purification. De-ionized water was used throughout all the experiments.
2.2 Mollusc shell collection and Treatment
The studied shell of Mediterranean fan mussel (Atrina pectinata) was bought alive from local seafood market (Tongchuan Road, Shanghai). Nacre and prism layers were separated mechanically. In our studies, only the prismatic layer was used for the following experiments. The surface of prismatic layer was slightly etched with 1 molL-1 NaOH solution (to remove debris), carefully cleaned using DI water, and then cut into several small fragments. These fragments were subsequently separated into different parts for crystal dissolution and growth experiments.
2.3 Decalcified with HCl and FAM
The partially- and whole-decalcified prismatic samples were prepared as follows:two pieces (1 cm x1 cm) of previously weighed prismatic fragments (3.237 and 3.265 g) were simultaneously submerged into 10 ml 1 molL-1 HCl aqueous solutions, respectively. After vigorous reactions for 1 hour, one of them was taken out from the solution for drying. The remaining one was proceeded to react until 24 hours, took out from HCl solution and dried at room temperature. In contrast, another prism sample (3.243 g) was decalcified in 5% EDTA aqueous solution for 3 days. Prior to the observation of scanning electron microscopy, all the samples were sputtered with gold (2 nm).
2.4 CaCO3 Crystal Growth by inter- prismatic matrix as template
A piece of partially-dissolved prismatic fragment 1 molL-1 HCl, 30 min) was put flatly in a glass Petri dish and immersed in aqueous solutions of calcium chloride ([Ca2+] =10 mmolL-1). The Petri dish was then placed into a sealed desicator containing a 10 ml vial of ammonium carbonate powder. CaCO3 crystallization was induced by the slow diffusion of ammonium carbonate into calcium chloride solutions at room temperature for 6 hours. The resulting product was taken out followed by thorough washing with DI water and ethanol sequentially, and dried at room temperature before characterization.
2.5 Instruments for characterization
SEM/EDX observation:The morphology observation and qualitative energy dispersive X-ray (EDX) tests were performed using scanning electron microscopy (SEM/ EDX, JEOL JSM-6700). Gold-sputtered, the qualitative EDX spectra of original prismatic shell and interprismatic matrix were recorded using an Edax (Leo 1540 XB) EDX detector mounted on the scanning electron microscope. EDX measurements were performed at 10 kV, with a probe current of 20 μA. X-ray diffraction patterns of prism samples (powder, prismatic fragment and dissolved obtained polymer) were recorded by using a Bruker AXS GADDS X-ray diffractometer. TEM (HRTEM) imaging was done with a JEOL 2010- F TEM. The TEM foil was obtained using focused ion beam (FIB, Hitachi FE-2100) milling technique by cut perpendicularly to the inter-prismatic matrix. Before FIB cutting, the prismatic fragment was sputtered with gold.
3. Results and discussion
Morphology observation is performed with different method treated samples. Figure 1a. shows the SEM image of the top-surface of original prism shell. The topology is made up of many irregular polygonal unite cells which are separated by inter-prismatic matrix. The average diameter of individual unite cell is about 30 μm, meanwhile the thickness of the wall of inter-prismatic matrix is about 2 μm. After 30 minutes etching with HCl aqueous solution, some well-like structures are shown in Figure 1b. The depth of the well depends on the etching time. After complete dissolution, a honeycomb-like structure is shown in Figure 1c. One could see the layer by layer structures from the lateral walls, which indicate the periodic rhythmic deposition process of organic matrix [39]. Figure 1d is the higher magnification of Figure 1c, where some porous structures appear on the inter-prismatic wall. It is noted that there are no porous structures on original inter-prismatic matrix without any treatment. The same case also occurred through the use of 5% EDTA aqueous solution in figure 1e and 1f. Interestingly, both the inorganic phase and organic phase show the porous structures, which indicates that they might be calcium-containing materials (Note: EDTA is a strong Ca ions chelator). Figure 1f is the magnified picture of inter-prismatic matrix in figure 1e. The size of porous structure ranges from 50 to 100 nm. Representative EDX spectra for organic phase, inorganic phase of the original prism and dissolution obtained polymer are shown in Figure 1g-i. They exhibited strong Ca peaks as well as C and O peaks, and can be distinguished by the relative intensity.The inorganic phase shows the most intense Ca signals, which attribute to a great number of calcite crystals. It is interesting to note that the inter-prismatic matrix also presents Ca signals, which reduces in the HCl-attacked obtained polymer (The relative value of Ca is h>g>i). Based on these, we speculated that CaCO3 polymorph (crystalline or amorphous) might be included in the inter-prismatic matrix. Although Mg as an important element is often involved in the formation of biogenic CaCO3 [40] , we only observe the neglectable amount of Mg signal in our species. Additionally, all Au signal present in the spectra is the result of sputtering. X-ray diffraction patterns were then measured from the ground shell powder, original prism outer shell and completely dissolved prism surface. The powder X-ray diffraction pattern (Figure 2a) indicated that the mineral phase is predominantly calcite with rhombohedral crystallographic unit cell with parameters a=b=4.990 and c=17.061 Å. The signal corresponding to {104} plane is the most intense. In contrast, the number of peaks of original prism shell (Figure 2b) is much less than the powder XRD, indicating the shell has a preferred orientation, which has been confirmed by others. Dissolution obtained polymer also showed three broad peaks (Figure 2c) with d-space values of 1.568 nm (2θ=5.633˚), 0.3483 nm (2θ=25.551˚) and 0.2128 nm (2θ=42.449˚), implying these insoluble proteins are crystalline and might be ordered. To prepare the sample for TEM observation, a piece of prismatic fragment was cut perpendicularly to organic matrix using FIB instrument. FIB is an ideal tool for TEM sample preparation with little artefact and is being widely employed in dealing with geomaterials and biominerals [41]. The cutting thin slice (Figure 3a) includes three different parts: two inorganic phases (on both sides) which come from two neighbouring prisms and an organic phase (middle part) which derives from the inter-prismatic matrix. The width of cutting thin slice is 15 μm, while the thickness is less than 100 nm.The higher magnification of one of regions (circles) is shown in Figure 3b. One can observe that there are many black spots with average size of 3 nm inside the polymer, indicating the occurance of different materials. Selected area electron diffraction (SAED) shows not only a ringed but also a spot pattern (Inset, Figure 3b), which indicates a preferred orientation for these nanocrystals. HRTEM image shows that the inter-atomic distances of these spots are 0.218 and 0.217 nm (Figure 3c), which correspond to {202} planes of the calcite crystal structure. Similarly, Velázquez-Castillo et al. [42] observed that nano-metric aragonite crystals embedded on the proteinous material of both matrix and the bridges in the Nautilus pompilius, which means that two species might adapt to same design strategies to complete mineralization. Earlier studies showed that inter-crystalline macromolecules extracted from mollusc shell (both prismatic layer and nacreous layer) can affect the crystal polymorph and shape [24]. In order to study the effects of the insoluble intra-prismatic matrix to crystal nucleation, growth and orientation, we carried out CaCO3 crystallization experiments in vitro using HCl-partially dissolution obtained prism as template. After 6 hours mineralization time, some crystallites with the typical rhombohedral shapes are observed in Figure 4a. X-ray diffraction experiments (data not shown) confirm the presence of calcite phase of CaCO3 , which is consistent with the results of soluble proteins as additives from the same layer. Interestingly, some crystals adhered to the walls of organic matrix with certain {104} faces (figure 4b), while some regular calcite crystallites randomly oriented at the bottom. The preferential nucleation of {104} face indicates that macromolecules lateral walls contain appropriate chemical groups (-COOH, -OH) that interact with Ca ions in structural arrangements that resemble the ordering of the same ions in calcite particular face. In this way, the matrix acts as an organic template for epitaxial nucleation of CaCO3 through interfacial molecular recognition [43]. Since the lateral walls of inter-prismatic matrix could affect the crystallographic orientation in vitro experiment, we infer that it might act as the same role during mollusk shell formation. In fact, through the TEM observation on a single prism, we found that there are two differently oriented crystal arrangements which distribute in outside and inside, respectively (related data will publish elsewhere). This could be the results exerted together by inter- and intra-prismatic matrixes. The fabrication of highly ordered mollusk shell is a rather complex process in which cells, macromolecules and ions are actively involved [38,44]. Epithelial cells in mollusk secrete organic fluids which self-organize into polygonal cavities by interfacial tension [18]. It is believed that the polygonal cavities are pre-formed before filling the CaCO3 inorganic phase. The inter-prismatic matrix is composed mainly of glycine-rich proteins [4,20]. According to Addadi et al.’s proposal [20], the growth mechanism of the prismatic layer in Atrina rigida basically adopted the way of layer by layer superposition with alternating layers of Asprich-stabilized amorphous calcium carbonate (ACC) and chitin (Figure 5, Strategy 1). Especially, the successful introduction of ACC could effectively resolve a list of paradoxes encountered in mollusk biomineralization [45]. However, based on their model, it is difficult for us to imagine how the prismatic layer which is closely adherent to the nacreous layer becomes thicker and thicker with the increments of age, as the mantle cells cannot make contact with this layer. On the base of our results, one alternative assumption is that there might exist a channel in the inter-prismatic matrix so that a part of calcium ions or Asprich stabilized ACC could pass through it to reach mineralization sites (Figure 5, Strategy 2). Meanwhile, these Asprichstabilized ACC would fuse and crystallize inside the inter-prismatic matrix and on chitin latticework, respectively, due to the structural control. In the end, a monocrystalline was formed with likely occluded macromolecues. We are inclined to think that Strategy 1 collaborates with Strategy 2 during the whole process. Additionally, the inter-prismatic matrix not only plays the function as framework but also affords some charged or polar resides as template for calcite oriented nucleation. In fact, Marin et al. accurately mapped the localization of casparin (a kind of acidic protein, from prismatic layer) by immunogold and discussed its different functions [17]. They pointed out that caspartin may be involed in maintaining the crystallographic orientation of the whole prism. Our in vitro recrystallization experiment unambiguously elucidates this point.
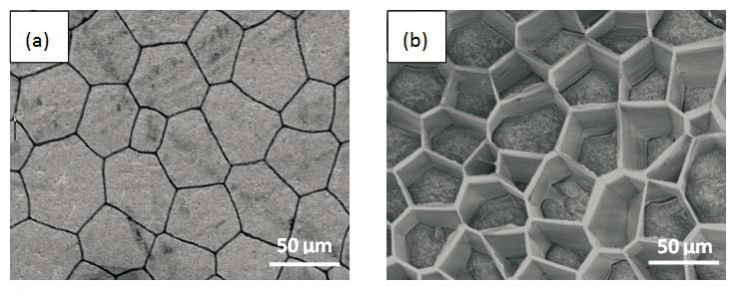
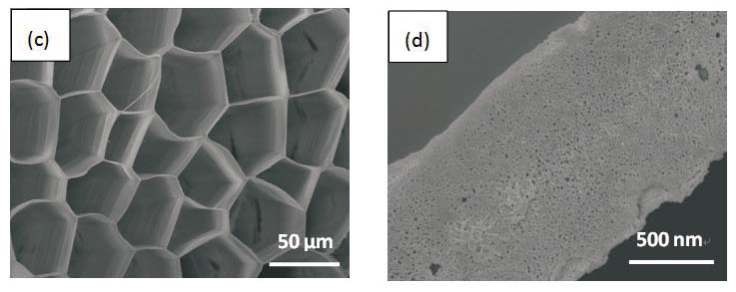
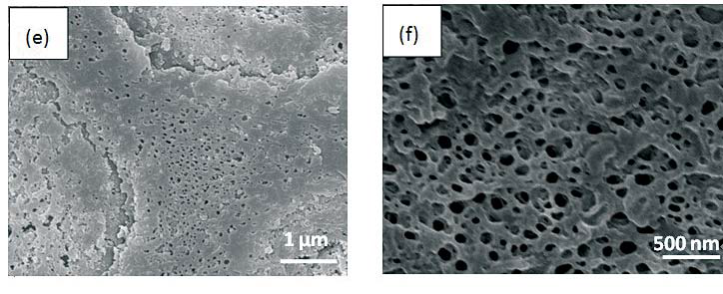
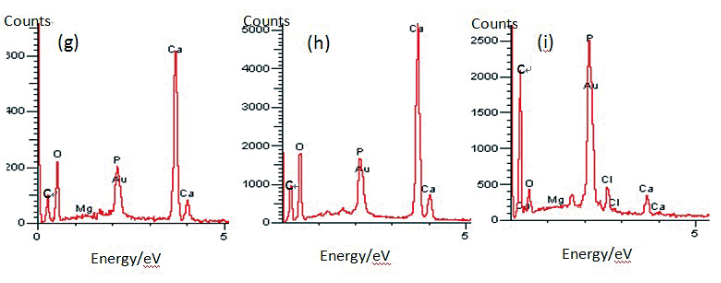
Figure 1. SEM images of (a) original prism top surface showing a polygonal structure, the width between the boundary of two neighbouring prismlike crystals are about 2 μm. (b) partially dissolved prism surface by 1molL-1 HCl acqueous solution with the arrays of well-like shape. The layered structure is clearly seen from the side walls of the wells. The depth of the well could be controlled by time. (c) the completely-dissolved honeycomblike inter-prismatic matrix. (d) a higher amplificaition of the top surface of inter-prismatic matrix wall revealing the porous structures which are formed by acidic dissolution. (e) partially dissolved prism surface using 5% EDTA acquous solution. The nano-porous structures are observed on both the inorganic and organic phase. (f) a higher magnification of top surface of inter-prismatic matrix in Figure. (e). EDX results of (f) inter-prismatic matrix of original prism surface; (g) the inorganic phase of original prism surface; (h) dissolution-obtained inter-prismatic matrix. Ca signals are present in the inter-prismatic matrix. The relative intensity of Ca signals is h>g>i.
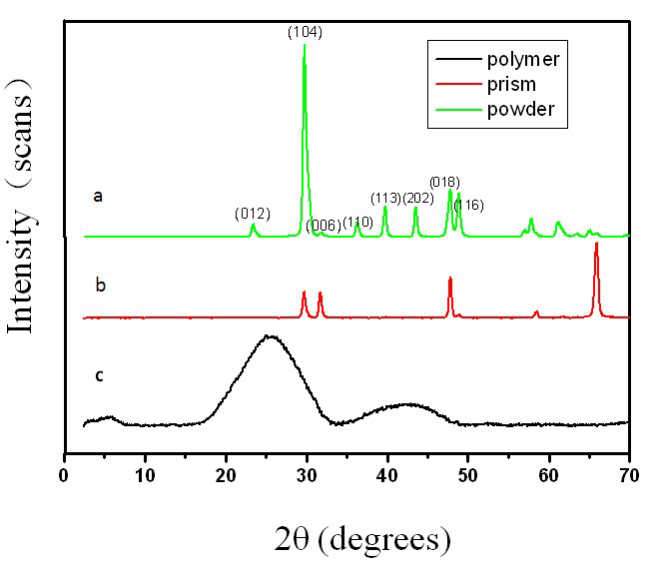
Figure 2. Comparative X-ray diffraction patterns of (a) ground prism powder; (b) original prism outer surface; (c) polymer obtained by completel dissolution of inorganic phase.
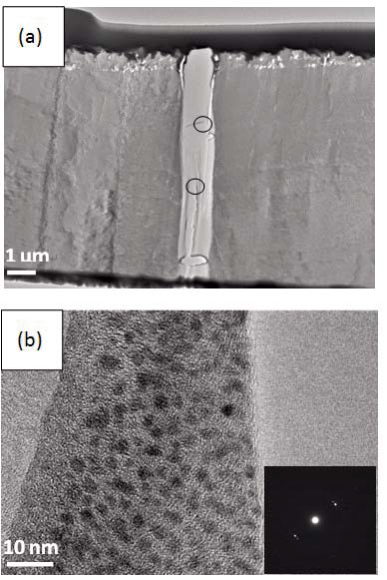
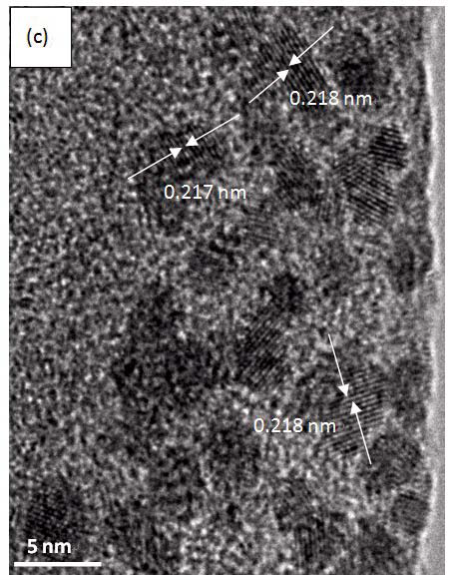
Figure 3. (a) TEM image of a piece of foil cut perpendicularly to inter-prismatic matix by FIB. The middle part is inter-prismatic matrix, which could be easily identified with both sides (inorganic phase) due to contrast. (b) magnified image of one of areas (circles) observed in (a). Inset is corresponding SAED pattern. (c) HRTEM image of one of areas in (b).
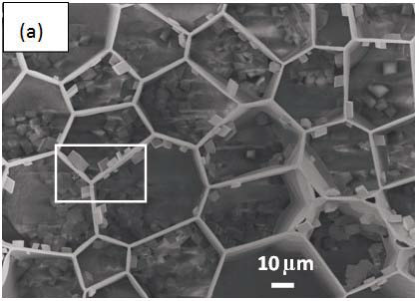
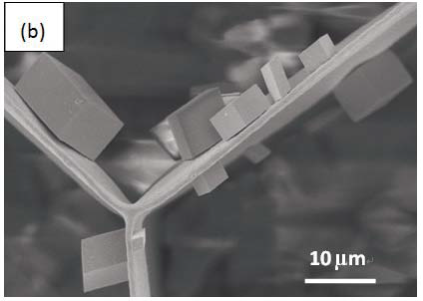
Figure 4. SEM images of insoluble polymer as template directing the crystal growth. (a) the distribution of CaCO3 crystals on/in polymer. Some crystals with {104} faces are closely adhered to walls of inter-prismatic matrix, others randomly arranged at the bottom. (b) a higher amplication of rectangular area in figure 4(a). One could clearly see that the {104} faces of rhombohedral CaCO3 micro-crystallites are oriented parallel to the interprismatic walls.
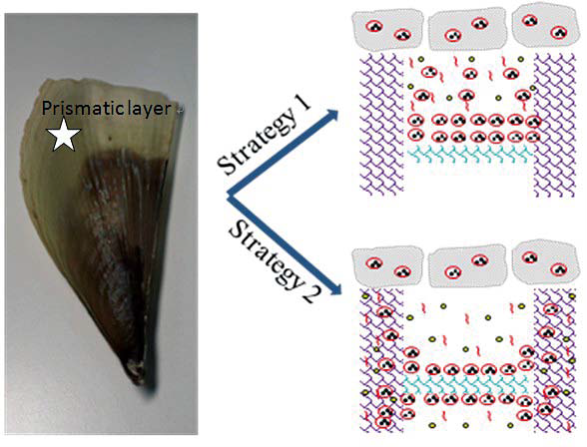

Figure 5. Schematic representation of growth mechanism from prismatic layer. We briefly think that the complex process is a combination of two pathways. Strategy 1: layer by layer additions with alternating ACC and chitin fibers to achieve mineralization process. Strategy 2: ions, molecules and/or other forms of minerals through the channel of inter-prismatic matrix to complete mineralization process. See the text for detailed illustrations.
4. Conclusion
In summary, we used dissolution and in situ recrystallization methods combining with modern materials analysis and characterization techniques to fully unveil the structure information of inter-prismatic matrix in mollusk shell. A basic fact is that the Ca is included in the inter-prismatic matrix of formed shell. There could be a channel in the inter-prismatic matrix for the transport of ions, macromolecules and/or other forms of minerals. Through dissolution, porous and layer by layer structures are visible on inter-prismatic matrix. The walls of interprismatic matrix influenced the orientation of CaCO3 crystals in vitro. Our finding and “channel hypotheses” would enrich people’s understanding to mollusk biomineralization process. Further insights into the interprismatic matrix will be required to test our hypotheses.
Acknowledgements
The authors thank Professor Han Ming-Yong (Institute of Materials Research Engineering, Singapore) for the use of his facilities and helpful discussions. This work is supported by the National Key Basic Research Program (973 Project) (2010CB933901), National 863 Hi-tech Project (2007AA022004, Important National Science & Technology Specific Projects (2009ZX10004-311), National Natural Scientific Fund (No. 20771075 and No.20803040), Special project for nano-technology from Shanghai (No.1052nm04100), New Century Excellent Talent of Ministry of Education of China (NCET-08-0350) , Shanghai Science and Technology Fund (10XD1406100) and Doctoral Program of Higher Education Research Fund(20070248050, 20070248107).
References
1. Mann S. Biomineralization: Principles and Concepts in Bioinorganic Materials Chemistry, Oxford: Oxford University Press; 2001.
2. Lowenstam HA, Weiner, S. On Biomineralization, New York: Oxford University Press;1989.
3. Sommerdijk NAJM, With Gd. Biomimetic CaCO3 mineralization using designer molecules and interfaces. Chem. Rev. 2008; 108:4499-4550. doi:10.1021/cr078259o
4. Spencer Evans J. “Tuning in” to mollusk shell nacre- and prismatic-associated protein terminal sequences. Implications for biomineralization and the construction of high performance inorganic-organic composites. Chem. Rev. 2008;108:4455- 4462.doi:10.1021/cr078251e
5. Meldrum F C, Cölfen H. Controlling mineral morphologies and structures in biological and synthetic systems. Chem. Rev. 2008;108:4332-4432.doi:10.1021/cr8002856
6. Oaki Y, Imai H. The hierarchical architecture of nacre and its mimetic material. Angew. Chem., Int. Ed. 2005;44:6571-6575. doi:10.1002/anie.200500338
7. Yu S H. Bio-inspired crystal growth by synthetic templates.Top. Curr. Chem. 2007;271:79-118. doi:10.1007/128_070
8. Fricke M, Volkmer D. Crystallization of calcium carbonate beneath insolube monolayers: suitable models of mineralmatrix interactions in biomineralization? Top. Curr. Chem.2007;270: 1-41. doi:10.1007/128_063
9. Meldrum FC. Calcium carbonate in biomineralisation and biomimetic chemistry. Int. Mater. Rev. 2003;48:187-224.doi:10. 1179/095066003225005836
10. Pokroy B, Zolotoyabko E. Microstructure of natural plywoodlike ceramics: a study by high-resolution electron microscopy and energy-variable X-ray diffraction. J. Mater. Chem. 2003; 13:682-688. doi:10.1039/b300167a
11. Xie L, Wang XX, Li J. The SEM and TEM study on the laminated structure of individual aragonitic nacre tablet in freshwater bivalve H.cumingii Lea shell. J. Struct. Biol. 2010; 169:89-94. doi:10.1016/j.jsb.2009.09.002
12. Nudelman F, Shimoni E, Klein E, Rousseau M, Bourrat X, Lopez E, et al. Forming nacreous layer of the shells of the bivalves Atrina rigida and Pinctada margarit i f e r a : An environmental- and cryo-scanning electron microscopy study. J. Struct. Biol. 2008;162:290-300. doi:10.1016/j.jsb.2008.01. 008
13. Kudo M, Kameda J, Saruwatari K, Ozaki N, Okano K, Nagasawa H, et al. Microtexture of larval shell of oyster, Crassostrea nippona: a FIB-TEM study. J. Struct. Biol.2010; 169:1-5. doi:10.1016/j.jsb.2009.07.014
14. Li XD, Chang WC, Chao YJ, Wang R, Chang M. Nanoscale Structural and Mechanical Characterization of a Natural Nanocomposite Material: The Shell of Red Abalone. Nano Lett. 2004;4:613-617. doi:10.1021/nl049962k
15. Addadi L, Joester D, Nudelman F, Weiner S. Mollusk shell formation: a source of new concepts for understanding biomineralization processes. Chem.-Eur. J. 2006;12:980-987. doi:10.1002/chem.200500980617.
16. Addadi L, Weiner S. A pavement of pearl. Nature.1997, 389, 912-5. doi:10.1038/40010
17. Marin F, Pokroy B, Luquet G, Layrolle P, Groot KD. Protein mapping of calcium carbonate biominerals by immunogold. Biomaterials. 2007;28:2368-2377.doi:10.1016/j.biomaterials. 2007.01.029
18. Checa AG, Rodríguez-Navarro AB, Esteban Delgado FJ. The nature and formation of calcitic columnar prismatic shell layers in periomorphian bivalves. Biomaterials. 2005;26:6404-6414. doi:10.1016/j.biomaterials.2005.04.016
19. Schäffer T E, Ionescu-Zanetti C, Proksch R, Fritz M, Walters D A, Almqvist N, et al. Does abalone nacer form by heteroepitaxial nucleation or by growth through mineral bridges? Chem. Mater. 1997;9:1731-1740. doi:10.1021/cm960429i
20. Nudelman F, Chen HH, Goldberg HA, Weiner S, Addadi L. Lessons from biomineralizaiton: comparing the growth strategies of mollusc shell prismatic and nacreous layers in Atrina rigida. Faraday. Discussions.2007;136:9-25.doi:10.1039/ b704418f
21. Checa AG, Rodríguez-Navarro AB. Self-organisation of nacre in the shells of Pterioida (Bivalvia: Mollusca).Biomaterials. 2005;26:1071-1079. doi:10.1016/j.biomaterials.2004.04.007
22. Rousseau M, Meibom A, Gèze M, Bourrat X, Angellier M, Lopez E. Dynamics of sheet nacre formation in bivalves. J. Struct. Biol.2009;165:190-195. doi:10.1016/j.jsb.2008.11.011
23. Yu-Min Lin A, Chen PY, Meyers MA. The growth of nacre in the abalone shell. Acta. Biomaterialia. 2008;4:131-138.doi:10. 1016/j.actbio.2007.05.005
24. Falini G, Albeck, S, Weiner S, Addadi L. Control of aragonite or calcite polymorphism by mollusk shel macromolecules. Science .1996;271:67-69. doi:10.1126/science.271.5245.67
25. Metzler R A, Evans J S, Killian C E, Zhou D, Churchill T H, Appathurai N P, et al. Nacre Protein Fragment Templates Lamellar Aragonite Growth. J. Am. Chem. Soc. 2010;132:6329- 6334.doi:10.1021/ja909735y
26. Albeck S, Aizenberg J, Addadi L, Weiner S. Interactions of various skeletal intracrystalline components with calcite crystals. J. Am. Chem. Soc.1993;115:11691-11697. doi:10.1021/ja000 78a005
27. Belcher A M, Wu X H, Christensen R J, Hansma P K, Stucky G D, Morse D E. Control of crystal phase switching and orientation by soluble mollusc-shell proteins. Nature. 1996; 381:56-58. doi:10.1038/381056a0
28. Takeuchi T, Sarashina I, Iijima M, Endo K. In vitro regulation of CaCO3 crystal polymorphism by the highly acidic molluscan shell protein Aspein. FEBS. Lett. 2008;582:591-596. doi:10. 1016/j.febslet.2008.01.026
29. Cusack M, Pérez-Huerta A, Dalbeck P. Common crystallographic control in calcite biomineralization of bivalved shells. Cryst. Eng. Comm. 2007;9:1215-1218. doi:10.1039/b708795k
30. Miyamoto H, Miyashita T, Okushima M, Nakano S, Morita T, Matsushiro A. A carbonic anhydrase from the nacreous layer in oyster pearls. Proc. Natl. Acad. Sci. U. S. A 1996;93:9657- 9560. doi:10.1073/pnas.93.18.9657
31. Westbroek P, Marin F. A marriage of bone and nacre. Nature. 1998;392:861-362.doi:10.1038/31798
32. Dauphin Y. Soluble organic matrices of the calcitic prismatic shell layers of two pteriomorphid bivalves: pinna nobilis and pinctada margartifera. J. Biol. Chem. 2003;278:15168-15177. doi:10.1074/jbc.M204375200
33. Bezares J, Asaro R, Hawley M. Macromolecular structure of the organic framework of nacre in Haliotis rufescens:Implications for growth and mechanical behavior. J. Struct. Biol. 2008; 163:61-75. doi:10.1016/j.jsb.2008.04.009
34. Sudo S, Fujikawa T, Nagakura T, Ohkubo, Sakaguchi K, Tanaka M, et al. Structures of mollusc shell framework proteins. Nature.1997;387:563-564. doi:10.1038/42391
35. Marin F, Amons R, Guichard N, Stigter M, Hecker A, Luquet G, et al. Caspartin and Calprismin, two proteins of the shell calcitic prisms of the mediterranean fan mussel pinna nobilis. J. Biol. Chem. 2005;280:33895-33908. doi:10.1074/jbc.M506 526200
36. Tsukamoto D, Sarashina I, Endo K. Structure and expression of an unusually acidic matrix protein of pearl oyster shells. Biochem. Bioph. Res. Co. 2004;320:1175-1180. doi:10.1016/ j.bbrc.2004.06.072
37. Kong Y W, Yan Z G, Li C Z, Gong N P, Zhu F J, Li D X, et al. Cloning and characterization of prisilkin-39, a novel matrix protein serving a dual role in the prismatic layer formation from the oyster pinctada fucata. J. Biol. Chem. 2009;284:10841- 10854. doi:10.1074/jbc.M808357200
38. Marin F, Corstjens P, Gaulejac B D, Vrind-De Jong E D, Westbroek P. Mucins and molluscan calcification.J.Biol. Chem. 2000;275:20667-20675. doi:10.1074/jbc.M003006200
39. Dauphin Y, Cuif J P, Doucet J, Salomé M, Susini J, Williams C T. In situ mapping of growth lines in the calcitic prismatic layers of mollusc shells using X-ray absorption near-edge structure (XANES) spectroscopy at the sulphur K-edge. Marine. Biology. 2003;142: 299-304. doi: 10.1007/s00227- 002-0950-2
40. Raz S, Hamilton P C, Wilt F H, Weiner S and Addadi L. The transient phase of amorphous calcium carbonate in sea urchin larval spicules: the involvement of proteins and magnesium ions in its formation and stabilization. Adv. Funct. Mater. 2003;13:480-486. doi:10.1002/adfm.200304285
41. Wirth R. Focused ion beam (FIB) combined with SEM and TEM: advanced analytical tools for studies of chemical composition,microstructure and crystal structure in geomaterials on a nanometre scale . Chem. Geology. 2009; 261:217-229. doi:10.1016/j.chemgeo.2008.05.019
42. Velázquez-Castillo R R, Reyes-Gasga J, García-Gutierrez D I,Jose-Yacaman M. Crystal structure characterization of nautilus shell at different length scales. Biomaterials. 2006; 27: 4508-4517. doi:10.1016/j.biomaterials.2006.04.003
43. Mann S. Molecular recognition in biomineralization. Nature. 1988;332:119-124. doi:10.1038/332119a0
44. Weiner S, Addadi L. Design strategies in mineralized biological materials. J. Mater. Chem.1997;7:689-702.doi:10. 1039/a604512j
45. Gower LB. Biomimetic model systems for investigating the amorphous precursorpathway and its role in biomineralization Chem. Rev 2008;108:4551-4627. doi:10.1021/cr800443h
Received 10 November, 2010; accepted 6 December, 2010; published online 16 December, 2010.
Copyright: (c) 2010 D. Yang, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.