Article
Time-Resolved Fluorescence Immunoassay of Mouse IgG using Europium(Ш) Chelate-doped Silica Nanoparticles
Dongguang Yin*, Li Zhang, Binhu Liu, Le Zhang, Hu Yan
College of Environmental and Chemical Engineering, Shanghai University, Shanghai 200444, P. R. China
* Corresponding author: ydg@shu.edu.cn
Abstract
Nanoparticle labes conjuaged with biomolecules have been used in a variety of different assay application. We investigated the possibility of using europium(III) chated-doped silica nanoparticle conjugated with streptavidin (SA) to detect mouse IgG by time-resolved fluorescence immunoassay (TRFIA). Results demonstrate that the nanoparticle is a novel kind of superior fluourscent probe which could be effectively applied in time-resolved fluorescent immunoassay with high detective sensitivity. In the present study, the lowest detection limit of mouse IgG is 34 pg mL-1.
Keywords: Europium chelate, Fluorescent probe, Silica nanoparticle, Time-resolved fluorescence, Mouse IgG
Citation: D. Yin, et al. Fluorescent Immunoassay of Mouse IgG using Europium(Ш) chelate-doped Silica Nanoparticles. Nano Biomed. Eng. 2011, 3(1), 25-28.
DOI: 10.5101/nbe.v3i1.p25-28.
1.Introduction
Traditional fluorescent probes, such as fluorescein, rhodamine, Cy3 and Cy5, have been widely applied in immunoassay and other biological detection systmes. But they are not capable to be used for super- sensitivity detection and real time monitoring due to their comparative low fluorescence intensity and low photostability. Recently, fluorescent nanoparticles including quantum dots and fluorescent silica particles, as fluorescent probes, have been developed and shown a lot of advantages [1-11]. As a lot of fluorescent dye molecules encapsulated in the silica matrix that also severs to protect dye molecules from photodamaging oxidation, the silica fluorescent nanoparticles are extremetly bright, photostable and chemical stable [12,13]. Time-resolved fluorescence immunoassay (TRFIA) is one of the most sensitive immunoassay techniques due to its big stokes displacement and long lifetime of fluorescence signal. Developing novel rare-earth fluorescent probe is one of the most important techniques for TRFIA. Using nanoparticle as luminescent probe is very favorable for TRFIA [14,15]. In this study, an ultrasensitive TRFIA of mouse IgG using a novel fluorescent probe of DPPDA-Eu3+dopedsilica nanoparticle has been developed. The results are desirable which demonstrate that the nanoparticles, as a novel fluorescent probe, could be applied in super-sensitivity fluorescent immunoassay.
2. Experimental
2.1 Instrumentation
The 1H NMR spectra were recorded on a Bruker AVANCE 500 spectrometer(Switzerland). UV-vis absorption spectra were measured on a Hitachi 3010 UV- vis spectrophotometer (Japan). Fluorescence intensities were measured on a Hitachi F-7000 spectrophotometer (Japan). The transmission electron microscopy (TEM) was measured on JEOL -200CX transmission electron microscope(Japan). TRFIA was measured on a Thermo Varioskan Flash Multifunction Microplate Reader (USA). Elemental analyses were performed at a Elementer Vario EL Ш Elemental analyzer (Germany). The 96-well plates were from NuNc, Danmark. The 1296-026 DELFIA plate shaker was from Pekin Elmer-Wallac.
2.2 Reagents and chemicals
Eu2O3, tetraethyl orthosilicate (TEOS), n-hexanol, bathocuproine(98%), N-chlorosuccinimide, triton X-100, APTMS, cyclohexane and ammonium hydroxide (28- 30 wt %) were purchased from Alfa aesar. All of these regents were analytical grade. SA, mouse IgG stands, goat anti-mouse IgG monoclonal antibody, goat anti-mouse IgG polyclonal antibody, Bovine serum albumin (BSA), biotin-N-hydroxysuccinimide (B•NHS), were purchased from Sigma. The coating buffer was 0.05 mol L-1 sodium carbonate buffer (pH 9.5).The blocking buffer was 0.05 mol L-1 phosphate buffer (pH 7.4), containing 0.9% NaCl, 1% BSA, 0.04% NaN3. The assay buffer was 0.1 mol L Tris-HCl (pH 8.6), containing 0.05% normal mouse sera, 0.9% NaCl, 0.1% BSA, 0.05% NaN3, 0.03% Tween-20. The washing buffer was 0.01mol L-1 phosphate buffer (pH 8.0), containing 0.9% NaCl, 0.05% Tween-20. The labeling buffer was 0.1 mol L-1 NaHCO3(pH 9.0). The purifying buffer was 0.1 mol L-1 phosphate buffer (pH 7.0), containing 0.9% NaCl.
2.3 Preparation of europium(Ш) chelate- doped silica nanoparticles and nanoparticles- labeled SA
The europium(Ш) chelate -doped silica nanoparticles were prepared as our previous report [4].To conjugate with SA, the nanoparticles were coated with BSA first. 1.0 mg nanoparticles ultrasonic dispersed in 1.0 mL 0.1 M phosphate buffer (pH 7.0) was mixed with 4.0 mg of BSA and 0.3 mL of 1% (v/v) glutaraldehyde and stirred for 24 h at 4◦C. After centrifuging and washing with the phosphate buffer two times, the BSA coated nanoparticles were suspended in 1.0 mL of the phosphate buffer again, then 200 µg of SA and0.2 mL of 1% glutaraldehyde were added. After stirring at 4◦C for 24 h, 2 mg of NaBH4 was added, the reaction was allowed to continue for 2 h. After being centrifuged and washed with the phosphate buffer and water, the nanoparticles-labeled SA was further purified by size- exclusion chromatography on a Sephadex G-50 column, eluting with mobile phase of 0.05 mol L-1 NH4CO3 (pH 8.0) The fractions containing the nanoparticles-labeled SA were collected and stored at 4°C after diluting with 0.1 mol L-1 phosphate buffer (pH 7.4) containing 0.1% BSA, 0.05% NaN3 and 0.9% NaCl.
2.5 Preparation of biotinylated goat anti- mouse IgG polyclonal antibody
1.0 ml 0.5 mg mL-1 goat anti-mouse IgG polyclonal antibody was first dialyzed against labeling buffer overnight at 4°C. The goat anti-mouse IgG polyclonal antibody was then transferred to a clean brown glass bottle. Then, 3.0 mg B•NHS were added into the bottle and the mixture was incubated under vortexing for 24 h at 4°C. The solution was dialyzed against purifying buffer overnight at 4°C and stored at −20°C after adding 1% BSA.
2.6 Preparation of surface antibody
To each microwell, 200 µl coating buffer containing10 µg goat anti-mouse IgG monoclonal antibody was added and incubated for 24 h at 4°C. After twice washing with washing buffer, the microwells were blocked by incubation with 250 mL of blocking buffer for 2 h at room temperature. After decanting the solution, the microwells were allowed to thoroughly dry, placed in plastic bags, and stored at 4°C.
2.7 Mouse IgG TRFIA
After careful optimization, the mouse IgG TRFIA based on nanoparticle-labeled and streptvaidin- biotin-separated-enhanced was performed as follows: biotinylated goat anti-mouse IgG polyclonal antibody and nanoparticle-labed SA were first diluted with assay buffer to the ratio of 1:300 and 1:100, respectively. Duplicate 100µL of mouse IgG standards and 100µL of biotinylated goat anti-mouse IgG polyclonal antibody diluted solution were pipetted into the microwells coated with goat anti- mouse IgG monoclonal antibody. The mixture was incubated for 1 h at 37°C with shaking. The microwells were washed 4 times with washing buffer. Then, 100 µL nanoparticle-labed SA diluted solution was added to each well and incubated 30 min at 37°C with shaking. After washing 6 times, the fluorescence was measured on the Thermo Varioskan Flash Multifunction Microplate Reader.
3. Results and Discussion
3.1 Preparation and characterization of DPPDA-Eu3+-doped silica nanoparticles
The TEM image of the DPPDA- Eu3+/SiO2 nanoparticles was shown in Fig. 1. The nanoparticles are spherical, monodisperse and uniform in size (80+8 nm in diameter). As shown in Fig. 2, maximum excitation and emission wavelengths of fluorescent spectra of the nanoparticles are 300 and 615 nm, respectively. The nanoparticles have a wide excitation wavelength and a sharp emission peaks with 10 ~ 15 nm of half-peak width. 300nm of large stokes displacement is favorable to effectively eliminate short-lived scattering light and background noises.
3.2 Preparation of nanoparticle-labeled SA
Active amino groups were introduced to the surface of the nanoparticles through a copolymerization of TEOS and APTMS during the preparation process of the nanoparticles. The nanoparticles were directly used to conjugate with biological molecules with no need of complicated surface modification. To prepare nanoparticle-labeled SA, BSA was first conjugated to the surface of nanoparticle to form a layer of BSA by coupling the amino groups of the nanoparticle and BSA by glutaraldehyde coupling method [10], and then the BSA-coated nanoparticle was conjugated to SA with the same conjugating reaction. Since a layer of BSA was coated on the surface of the nanoparticle and the layer of BSA just acts as a flexible bridge, it keeps the activity of the SA without variety and thereby greatly improves the assay sensitivity. Moreover, the flexible BSA bridge enables the nanoparticle-labeled SA more easily to react with biomolecules.
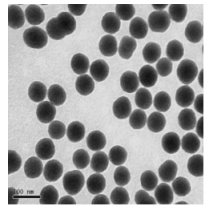
Fig. 1 TEM image of the DPPDA-Eu3+/SiO2 nanoparticles
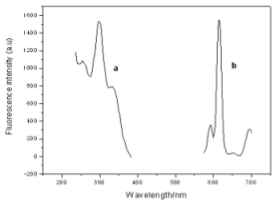
Fig. 2 Excitation and emission spectra of DPPDA-Eu3+/SiO2 nanoparticles (a: excitation spectra; b: emission spectra)
3.3 Mouse IgG TRFIA
This assay system is identical to that of routine TRFIA except that the nanoparticle takes the place of traditional rare-earth fluorescent probe. A typical standard curve is shown in Fig. 3. The fluorescent intensity increases with increasing mouse IgG concentration, showing a typical characteristic of sandwich immunoassays (non- competitive immunoassays), while the mouse IgG standard concentration was in the range of 0.01–500 ng mL-1. The linear correlation coefficient is 0.99754. The lowest detection limit of the assay was 34 pg ML-1, defined as the mouse IgG concentration corresponding to the mean fluorescent reading of zero standard (n= 20) minus two times the standard deviation. The within-run precision of the assay was determined by assaying three mouse IgG standards, corresponding to different levels of mouse IgG (mean concentrations of 1.0, 50, and 200 ng mL-1), in 12 replicates in a single assay. For the determination of the between-run precision, duplicate measurements of these mouse IgG standards were performed in 12 different runs. The within-run CVs were 3.4, 5.6, and 3.6% (mean), and the between-run CVs were 5.5, 10.1, and 6.7%, respectively.
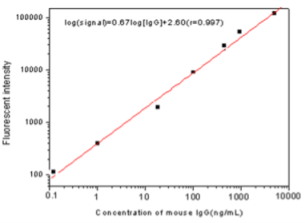
Fig. 3 TRFIA standard curve of mouse IgG
4. Conclusions
In summary, an ultrasensitive fluorescent immunoassay of mouse IgG using a novel fluorescent probe of DPPDA- Eu3+-doped silica nanoparticle was described in this paper. Comparing with the tradition organic dye and rare-earth fluorescent probe, the nanoparticle is extremely bright, photostable and chemical stable. When the nanoparticles applied as a novel fluorescent probe combining with streptavidin (SA)-biotin separation and enhanced techniques, ultrasensitive fluorescent immunoassay could be achieved. These results demonstrate the advantages of the nanoparticles over traditional biomarkers when they are applied in fluorescent immunoassay.
Acknowledgements
Financial support from the National 973 Program (No.2010CB933901), Shanghai Leading Academic Discipline Project (No. S30109), Shanghai Pujing Program, Shanghai Nano Program (No. 0752nm024) is gratefully acknowlegded.
References
1.Cui D X, Han Y D, Li Z M, Song H, Wang K “Fluorescent Magnetic Nanoprobes for in vivo Targeted Imaging and Hyperthermia Therapy of Prostate Cancer”. Nano Biomed. Eng. 2009,1, 61-74. doi: 10.51 01/nbe.v1i1.p61-74.
2.Cui DX, Li Q, Huang P, Wang K, Kong YF, Zhang H, You XG, He R, Song H, Wang JP, Bao CC, Asahi T, Gao F, Osaka T. “Real time PCR based on Fluorescent Quenching of Mercaptoacetic Acid- Modified CdTe Quantum Dots for Ultrasensitive Specific Detectionof Nucleic Acids”. Nano Biomed. Eng.2010, 2(1),44-54. doi:10.5101/nbe.v2i1.p44-54
3.Xie CJ, Yin DG, Li J, Zhang L. “ Preparation of a Novel Amino Functionalized Fluorescein-doped Silica Nanoparticle for pH Probe”. Nano Biomed. Eng. 2009,1, 27-31. doi: 10.5101/nbe.v1i1. p27-31
4.Yin DG, Zhang L, Xie CJ, Liu BH. “Preparation and Characterization of DPPDA-Eu3+ Doped Silica Fluorescent Nanoparticles” Nano Biomed. Eng. 2010, 2, 39-43. doi: 10.5101/nbe.v2i1.p39-43
5.Yin DG, Liu BH, Zhang L, Xie CJ, Zhang L. .Synthesis of Ru(bpy)3-doped Silica Nanoparticle and Its Application in Fluorescent Immunoassay Nano Biomed. Eng.2010, 2, 117-120. doi: 10.5101/nbe.v2i2.p117-120
6.Moronne, M.; Gin,P. Semiconductor Nanocrystals as Fluorescent Biological Labels. Science.1998, 281,2013-2016.doi:10.1126/ science.281.5385.2013
7.Choi, J.H.; Chen, K.H.; Strano,M.S. Aptamer-Capped nanocrystal quantum dots: A new method for Label-Free protein detection. J. Am. Chem. Soc.2006,128, 15584-15585. doi:10.1021/ja066506k
8.Peng,H.;Zhang,L.J. DNA hybridization detection with blue luminescent quantum dots and dye-labeled single-stranded DNA. J.Am.Chem.Soc.2007,129,3048-3049. doi:10.1021/ja0685452
9.Cui,D.X.;Pan,B.F.;Zhang,H. Self-Assembly of quantum dots and carbon nanotubes for ultrasensitive DNA and antigen detection. Anal. Chem.2008,80,7996-8001. doi:10.1021/ac800992m
10.Santra, S.;Zhang,P.; Wang, K.M. Conjugation of biomolecules with luminophore-doped silica nanoparticles for photostable biomarkers. Anal. Chem.2001,73,4988-4993. doi:10.1021/ac010406+
11.Zhao, X.J.; Dytioco, R.T.; Tan, W.H. Ultrasensitive DNA detection using highly fluorescent bioconjugated nanoparticles. J. Am.Chem. Soc.2003,125,11474-11475. doi:10.1021/ja0358854
12.Ye,Z.Q.;Tan,M.Q.;Wang,G.L.;Yuan,J.L. Preparation, Characterization, and Time-Resolved Fluorometric Application of Silica-Coated Terbium(III) Fluorescent Nanoparticles. Anal. Chem.2004,76,513-518.
13.Tan,M.Q.; Ye,Z.Q.; Wang,G.L.;Yuan,J.L. Preparation and Time- Resolved Fluorometric Application of Luminescent Europium Nanoparticles. Chem. Mater.2004,16,2494-2498. doi:10.1021/cm0 30305n
14.Soukka, T. ; Antonen, K. ; Härmä, H. Harma.Highly sensitive immunoassay of free prostate-specific antigen in serum using europium(Ш) nanoparticle label technology. Clin Chim Acta,2003,328,45-58. doi:10.1016/S0009-8981(02)00376-5
15.Yuan, J.L.;Wang, G.L.;Kimura, H.;Matsumoto, K.Highly sensitity time-resolved fluoroimmunoassay of human immunoglobulin E by using a new europium fluorescent chelate as a label. Anal. Biochem.1997, 254, 283-287. doi:10.1006/abio.1997.2444
Copyright:(c) 2011 D. Yin, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.