Article
The Radiosensitization of Melanoma Cells by Gold Nanorods Irradiated with MV X-ray
Wencai Xu 1 ,Teng Luo 2 , Bo Pang 4 , Ping Li 1 , Chuanqing Zhou 2 , Peng Huang 3 , Chunlei Zhang 3 , Qiushi Ren *, Wenbin Hu 5*, Shen Fu 1*
1 Department of Radiation Oncology, Shanghai Sixth People's Hospital, Shanghai Jiao Tong University, Shanghai 200030, China.
2 School of Biomedical Engineering, Shanghai Jiao Tong University, Shanghai 200240, China.
3 National Key Laboratory of Nano/Micro Fabrication Technology, Key Laboratory for Thin Film and Microfabrication of Ministry of Education, Institute of Micro-Nano Science and Technology, Shanghai Jiao Tong University, Shanghai 200240, China.
4 Department of Biomedical Engineering, College of Engineering, Peking University, Beijing 100871, China.
5 State Key Laboratory of Metal Matrix Composites, Shanghai Jiao Tong University,Shanghai 200240, China.
* Corresponding authors. Email: wbhu@263.net; fushen1117@gmail.com
Abstract
Melanoma is known to be radioresistant and traditional treatments have been intractable, and therefore, novel approaches are required to improve therapeutic efficacy. Gold nanoparticles (GNPs) have been explored as radiosensitizers, while most of the research in the area has focused on the enhancement occurred in the kilovoltage (kV ) range. The present study investigated the possible application and biological mechanism of gold nanorods (GNRs) for sensitization at clinically relevant MV X-ray energies. A375 melanoma cells were treated by gold nanorods (GNRs) with or without irradiation. The anti-proliferative impacts of the treatments were measured by MTT assay. The cellular uptake and intracellular localization were analyzed by transmission electron microscopy. Radiosensitizing effects were determined by a colony formation assay. Apoptosis and cell cycle data were measured by flow cytometry. DNA damage was estimated by γ-H2AX expression measured with immunofluorescent staining. Results showed that the addition of GNRs enhanced the radiosensitivity of A375 cells with a dose-modifying factor (DMFSF2) of 1.14, increasing more radiation-induced DNA double-strand breaks and apoptosis. DNA flow cytometric analysis indicated that GNRs plus irradiation significantly induced G2/M phase arrest in A375 cells. In Conclusions: GNRs could sensitize melanoma A375 cells to 6 MV X-ray irradiation, and this was mainly through increasing the DNA double-strand breaks, in addition to the induction of a higher proportion of cells within the G2/M phase. The interaction of GNRs and high energy commonly used in the clinic may provides another rational for the potential application of GNRs in the treatment of cancer..
Keywords: Melanoma cells, Gold nanoparticles, Irradiation;
Citation: W. Xu, et al. The radiosensitization of melanoma cells by gold nanorods irradiated with MV X-ray. Nano Biomed. Eng. 2012, 4(1), 6-1.
DOI: 10.5101/nbe.v4i1.p6-11.
1. Introduction
The incidence of melanoma is increasing worldwide with an estimated age-standardized incidence rate (World 2000 population) of 40.2 cases per 100,000 population in 2008 [1]. Although surgery remains the primary treatment for patients with localized melanoma, available data indicate that there is a need for improved local-regional control in situations where complete surgical resection may be difficult or when high-risk features are noted pathologically, therefore, radiation therapy is one of the commonly utilized treatments for melanoma cancer [2], but malenoma cells are well known to be resistant to radiation [3, 4], and delivering a curative dose of radiation to tumor tissues while sparing normal tissues is still a great challenge in radiation therapy. Therefore, any new strategies that overcome the relative radioresistance of melanoma tumors could prove to be useful and beneficial to the patients. Nanotechnology is an emerging technique for improved cellular targeting and radio-sensitization. Nanoparticles (NPs) are widely used to treat cancer [5, 6]. Since nanoparticles are definition smaller than a typical cutoff size of the pores (e.g., up to 400 nm) in the tumour vasculature [7] , so they can take full advantage of the so-called ‘leaky’ vasculature of tumours [8]. Thus, nanoparticles may have a better chance ofpenetrating into the tumour interstitium. However, most studies have focused on low-energy radiation because high atomic number (Z) materials, which preferentially absorb kilovoltage x-rays, the effect of a defined dosage is increased when a high-Z material is in the targeted zone through a photoelectric effect [9, 10], of which the enhancement is proportional to the amount of the high-Z material. Although kV energies like brachytherapy are still used in treatment of some cancer patients, megavoltage x-rays are more commonly used in the clinic, particularly for deep-seated tumors. Therefore, it is necessary to study the radiosensitizing effect of MV x-rays since it cannot be attributed to high-Z materials alone. Other material nanopartilcles like iodine, gadolinium, etc, have been used to enhance the dose of radiation [11], but gold as nanoparticle constituent has shown more promise because gold nanoparticles have low cytotoxicity, robust stability, biocompatibility, and suitable physiochemical parameters [12]. The use of structurally modified gold nanoparticles is less toxic to normal tissue during delivery, and at the molecular level, could traverse biologic barriers and preferentially accumulate in cancer cells [13-14]. The present study investigated the benefit of combing gold nanorods with MV irradiation to treat radioresistant melanoma cancer cells, as well as assessing some possible mechanisms for the radiosensitizing effects of GNRs.
2. Materials and Methods
2.1 Preparation of gold nanorods
The GNRs were synthesized using the seed-mediated template-assisted protocol [15,16], by reducing gold salt in the presence of surfactant directed synthesis. First, 20 mL aliquot of GNRs stock solution (Sinopharm Chemical Reagent Co., Shanghai, China) was centrifuged and redispersed in 20 mL deionized water (Millipore, Shanghai, China). Then, 1.1 mL of the TEOS ethanol solution (10 mM) (J & K Chemical Ltd , Shanghai, China) was added to the 20 mL of aqueous GNRs (pH adjusted to 10 ~ 11 by NH4OH). After vigorous stirring for 10 h at room temperature, an approximately 31 nm thick silica layer formed on the surface of the GNRs through hydrolysis and condensation of TEOS [17]. The silica-coated nanoparticles were isolated by centrifugation, washed with deionized water and ethanol several times, and then dispersed in DMEM medium (Hyclone, Carlsbad, CA, USA) and stored at 4˚C for later cell experiments.
2.2 Cell lines and Culture
The human melanoma A375 cells were purchased from the Shanghai Institute of Cell Biology & Chinese Academy of Sciences (Shanghai, China) and grown in DMEM medium containing 10% heated-inactivated fetal bovine serum (Hyclone, USA). Cells were incubated at 37 °C in a humidified atmosphere with 50 ml/L CO2 .
2.3 TEM analysis of cells with internalized Gold nanorods
A375 cells incubated with GNRs were washed three times with PBS and fixed with 2.5% glutaraldehyde for 6 hours. The cells were then postfixed in 1% osmium teroxide for 2 hours, dehydrated in ethanol and embedded in agar resin. Thin section of 60-70 nm were collected on copper grids and stained with methanol and lead citrate. The grids were visualized using transmission electron microscopy (Philips CM120).
2.4 Cellular proliferation assay
Exponentially growthing cells were incubated with different concentrations of GNRs varying lengths of time. Then, 15 μL of MTT solution (5 mg/mL) (Sigma-Aldrich, USA) was added into each well for 4 h of incubation. The reaction was stopped by removal of MTT, and 100 μl dimethylsulfoxide (DMSO) (Sigma-Aldrich, USA) was added into each well in order to dissolve the formazan crystals, and the plates were read at enzyme-labeling analyzer. All measurements were done in triplicate.
2.5 Irradiation
Cells in a monolayer were irradiated at room temperature using 6MV x-rays from linear accelerators (Siemens, Germany) with a dose rate of 3 Gy/min 1.5 cm bolus were used as compensators.
2.6 Clonogenic Assay
A375 cells were seeded and grown in 6 well culture plates. 24 hours after incubation, GNRs (50 µg/ml concentration solution) were introduced and kept for 1 hour before X-ray irradiation. After irradiation, the dishes were trypsinized, and different of detached cells were seeded and grown in 6 well culture plates. Cells were incubated for 2 weeks to form colonies. The colonies were stained with 0.4% crystal violet and the colonies containing >50 cells were counted for calculating the surviving fraction (SF). Values were expressed as the mean ± standard deviation (SD). The survival curves were fitted to the Linear Quadratic (LQ) Model [S= exp(-αD-βD2)] using least-squares regression in Prism 5.0 (GraphPad Software, CA). Dose modifying factor (DMF) values were calculated to quantify the radiosensitizing effect of the cells.
2.7 Immunofluoresent assay for γ-H2A.X
Cells were seeded on cover slides in a 6-well culture dish. After seeding the cells for 24 hours, GNRs (50 µg/mL) were introduced and incubated for 1 hour before X-ray irradiation. After irradiated, the cells were further incubated for 1 hour, then fixed in 1 % paraformaldehyde for 30 min and permeabilized in 0.5 % Triton X-100/PBS for 30 min at room temperature. Cover slips were washed three times with PBS, and the cells were blocked with 10 % FBS for 30 min at room temperature. Subsequently the flourescein isothiocyanate ( FITC)-labeled mouse monoclonal antibody against γ-H2A.X (Millipore, USA) with 1:200 dilution in 10 % FBS was incubated for 1.5 hat room temperature, and then the cells were washed three times in PBS. Cells then were incubated in the dark with 5µg mL -1 4`,6-diamidino-2-phenylindole(DAPI) (Sigma-Aldrich, USA) in PBS for 10 min to stain the cell nuclei and coverslips were mounted on glass slides. The cells were imaged usng a Leica fluorescent microscope. For each treatment condition, γ-H2A.X foci were counted by eye in at least 50 cells from the stored images [18].
2.8 Cell cycle and apoptosis assays by flow cytometry (FCM)
A375 cells, (106 cells/mL) fixed in 95 % ethanol at -20 ˚C for 24 h, were washed with cold phosphate-buffered saline (PBS), re-suspended, and stained with propidium iodide (50 µg/mL PBS; Invitrogen, Shanghai, China) for 15 min at 4 °C. Analysis was performed using a FACSCalibur flow cytometer (Becton-Dickinson, Franklin Lakes, NJ, USA). Cellular DNA content and cell cycle data were analyzed by FCM using multicycle system 2.0 software. For apoptosis assay, the cells were stained with Annexin-V-fluorescein isothiocyanate/propidium iodide (Annexin-V-FITC/PI; Invitrogen) and measured by FCM. All tests were repeated three times.
2.9 Statistical analysis
The SPSS statistical software(13.0) was used for statistical analyses. The results were expressed as the means±standard deviation. The One-Way ANOVA was performed to compare the means between either two test groups with p value of < 0.05 considered statistically significant.
3. Results
3.1 Cellular toxicity and uptake of gold nanorods on A375 cells
The GNRs coating with about 13 nm thick silica layer were used to reduce the biotoxicity derived from a large amount of the surfactant cetyltrimethylammonium bromide (CTAB) molecules during GNRs (GNRs, length: 44.44±4.7 nm; width: 15.10±1.7 nm) synthesis, which had a uniform silica shell (thickness ≈13 nm), as shown in Fig. 1B. MTT assays showed that GNRs were biologically nontoxic within the concentration of 50 µg mL -1 in 24 hours (p<0.05) (Fig. 2). A375 cells treated with GNRs (50 µg mL -1 ) for 1 hours, internalized GNRs into the cells by endocytosis (Fig. 1A).
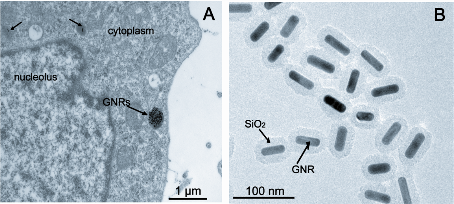
Figure 1: Gold nanorods image and distribution on human melanoma A375 cells. A: TEM show A375 cells with internalized GNRs. B: transmission electron microscopy (TEM) image and structure of gold nanorods. (abbreviation: GNRs: gold nanorods)
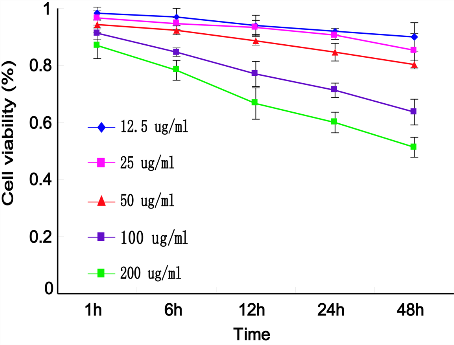
Figure 2: Effects of GNRs on cell viability using MTT assay. A375 cells were treated with different concentrations of GNRs for variable times, and the A570 values following incubation with MTT stop solution were measured by an enzyme-labeling analyzer. Data were compared to the control group (assumed to be 1.0), and the relative viability of each experimental group was plotted.
3.2 The radiosensitization of melanoma cells by gold nanorods
As illustrated in Fig. 3, when cells were treated with GNRs (50 µg/ml), the cell survival fraction at 2 Gy dropped from 55.4 % of control group to 48.7 %. Significant radiosensitization was observed at GNRs with the DMF (SF2) of 1.14, compared with the control group (p=0.012).
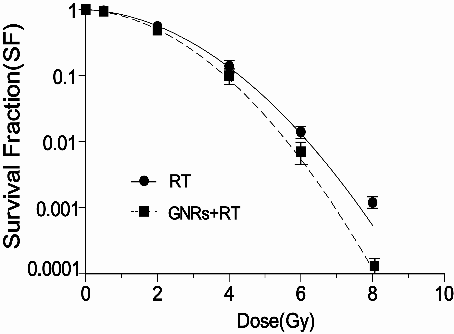
Figure 3: The radiosensitiziting effect by GNRs . A375 cells were treated with GNRs (50 µg/ml for 1h) in combination of radiation with indicated doses, then cells were trypsinized, counted, seeded at different dilutions and incubated for colony formation for 14 days. Data points shown represent the mean survival fraction of three independent experiments, Bars, SD. (abbreviation: RT: radiotherapy).
3.3 Analysis of DNA double-strand break
γ-H2A.X foci was assessed as indicator of DNA double-strand breaks (DSBs) induced by ionizing radiation. As shown in Fig. 4A, γ-H2A.X foci could be clearly distinguished after irradiation(2Gy) of A375 cells. The average number of γ-H2A.X foci per cell were counted in the micrographs and the results are presented in Fig. 4B. The average number of γ-H2A.X foci per cell in cultures receiving the combined GNRs treatment was significantly greater compared with the irradiation alone (p<0.05).
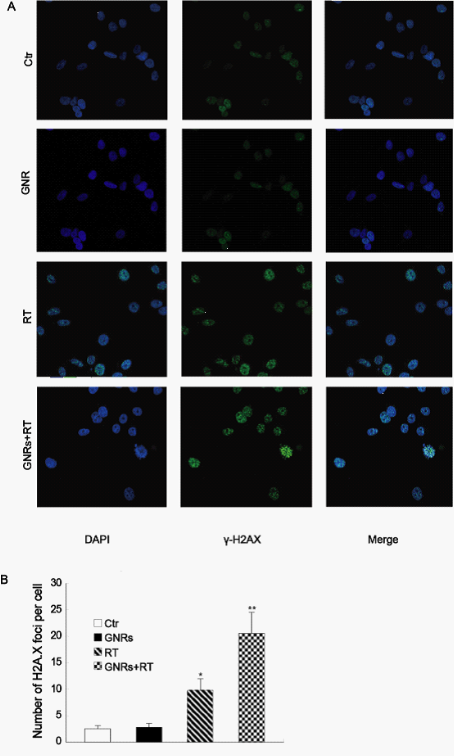
Figure 4: Quantitative analysis of radiation-induced γ-H2AX foci. The A375 cells were incubated with GNRs (50 µg/ml) for 1 h prior to irradiation with 6MV X-rays with a dose of 2 Gy. The cells were fixed at 1 h after the irradiation and stained with the flourescein isothiocyanate (FITC)-labeled mouse monoclonal antibody against γ-H2A.X and DAPI for analysis of the nuclear γ-H2AX foci by fluorescent microscope. A: the immunofluorescence images of the nuclear γ-H2AX foci . B: quantitative analysis of the nuclear γ-H2AX foci in the cells with different treatments. Columns, mean from three repeated experiments; bars, SD. *p< 0.05 compared to control group; **p< 0.05 compared to RT group.
3.4 Enhanced radiation-induced apoptosis by gold nanorods in melanoma cells
The apoptotic percentage of the A375 cells after variable treatments were shown in Fig. 5. The cell incorporation of GNRs did not significantly increase the amount of cell apoptosis compared to control values. Radiation alone (6MV X-rays at a dose of 4 Gy) or combined with GNRs, significantly enhanced apoptosis (4.97 ± 0.83 % and 7.67±0.31 %, respectively), compare to control values (2.23 ± 0.42 %) (p<0.05).
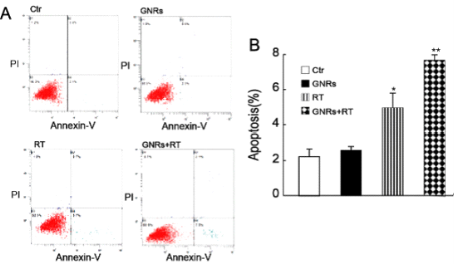
Figure 5 Enhancement of radiation-induced apoptosis by GNRs. A375 cells were treated with GNRs (50 µg/ml) for 1 h prior to irradiation (6MV X-rays at a dose of 4 Gy). The cells were cultured for 24 h then stained with Annexin V and propidium iodide (PI), and apoptosis analyzed by flow cytometry. A: Flow cytometry plots (Ctr- control; GNRs- gold nanorods without radiation; RT- radiation alone; GNRs+RT- GNRs plus radiation). B: Data from Fig. 5A were quantified shown (mean ± SD; n= 3 experiments). *, p <0.05 vs. control group; **, p <0.05 compared to other treated groups.
3.5 The enhancement of radiation-induced G2 arrest by gold nonrods
GNRs (50 µg/mL) treating A375 cells for 1 hour caused cells to accumulate in the radiosensitive G2/M phase of the cell cycle, when compared with the control group. In our study, 25.84 ± 0.49 % of control cells were in the G2/M phase, while GNRs or radiation alone (6MV X-rays with a dose of 4 Gy) significantly increased the fraction of cells in the G2/M phase to 35 ± 2.65 % and 40.9 ±0.35 %, respectively) (p<0.05). The cells treated with a combination of GNRs plus radiation further enhanced the cells arrest at G2/M phase (46.5 ± 1.2 %). One-way ANOVA analysis has also shown that there were significant differences between the GNRs plus radiation group and both untreated and single treatment groups (p < 0.05).
4. Discussion
Nanotechnology has been intensively explored in the treatment of cancer. Nanotherapeutics are rapidly progressing and being implemented to solve some limitations of cancer radioresistances [19]. Melanoma cells are well known to be resistant to radiation; therefore, any new strategies like nanotechnology that overcome the relative radioresistance of melanoma tumors could prove to be useful and beneficial to many patients. In the present study, GNRs coated with SiO2 were used as sensitizer. Since cetyltrimethyl ammonium bromide (CTAB), which is a necessary structure-directing agent in the GNRs synthesis method, had severe cytoxicity and low stability when coated with CTAB, in order to solve the two problems, an additional coating material (SiO2) has been deposited on the surface of CTAB. SiO2 has been reported to be stable and low toxicities, and is very suitable for using as a coating material for GNRs [20-21], and it also is very biocompatible, GNRs coating with silica can easily be covalently conjugated with antigen like peptides, antibody, etc [22-23], therefore, GNRs may have the potential to be used for biologically targeted imaging and therapy. Our results showed that radioresistant melanoma cells treated with GNRs could synergistically enhance the cytotoxicities of radiation in terms of apoptosis and mitotic death (Fig. 5), about three fold enhancement of radiosensitivity was achieved compared to radiation alone. The mechanisms whereby GNRs enhanced the response to radiation were associated with multiple factors. It is believed that the role of the cell cycle phase is critical to the response of cancer cells to radiation, GNRs or radiation alone resulted in an increase in the percentage of cells in the G2/M phase of the cell cycle , and this effect was enhanced when the two treatments were combined (Fig. 6). Because tumor cells are more radiosensitive in the G2 /M phase compared to the G0 /G1 or S phase [24], increased radiation-induced cytotoxicities were seen following the cell being treated with a combination of GNRs and radiation in terms of apoptosis and mitotic death. Likewise, increased cell numbers in the G2 /M phase could also explain some of the increase in susceptibility to radiation-induced DNA damage reflected by the increased expression of γH2AX, compared to cells treated with radiation alone [25]. Our data showed that melanoma cells treated by GNRs plus radiation had more γH2AX foci compared to radiation alone (Fig. 4). Based on these findings, the disruption of the cell cycle phases by GNRs might determine the relative radiosensitivity of A375 cells. Another possible mechanism for GNRs-mediated radiosensitization is that GNRs enhance radiation-induced DNA damage in the cells. It is well established that the primary focus of radiation damage is nuclear DNA, with DSB formation is the most lethal lesion to the DNA. γ-H2AX expression has been shown as a sensitive indicator of DSBs induced by clinically relevant doses of ionizing radiation [26]. At sites of radiation-induced DNA DSBs, the histone H2AX becomes phosphorylated rapidly (γ-H2AX), forming nuclear foci that can be visualized by immunofluorescence microscopy [27]. Although the specific role of γ-H2AX in the repair of DSBs has not been defined, recent reports indicate the dephosphorylation of γ-H2AX and dispersal of γ-H2AX foci in irradiated cells correlates with the repair of DNA DSBs and cellular radiosensitivity [28, 29]. Our data showed in Fig 4 that melanoma cells treated by GNRs plus radiation had two fold higher of the average number of γ-H2A.X foci per cell than radiation alone (20.6± 3.9 Vs 9.8±2.2), the quantitation of DSBs using γ-H2A. X foci was consistent with GNRs-mediated radiosensitization in terms of apoptosis and mitotic death. Since GNRs have the interesting properties such as increasing the absorption of radiation energy and their preferential accumulation in cancer cells[29-32]. It has long been realized that the effect of a defined dosage is increased when a high-Z material is in the targeted zone through a photoelectric effect [33], and the enhancement is proportional to the amount of the high-Z material. This explained why GNRs, which have high atomic number (Z), preferentially absorb kilovoltage x-rays. The radiosensitizing effect of MV x-rays cannot be attributed to high-Z materials alone. The possible mechanism is that GNRs to enhance the cytotoxicities of MV x-rays, when irradiated in the cell, produce a high level of intracellular reactive oxygen species (ROS), which leads to elevated levels of oxidative stress and is manifested as an increased level of apoptosis compared to irradiation alone [34]. An additional hypothesis proposed on the basis of DNA plasmid models , suggested that sensitization occurs due to short-range electrons produced by the interaction between GNRs and MV X-rays [25]. The increased production of low-energy electrons close to the DNA causes more damage than radiation alone. Our data confirmed that GNRs plus radiation induced more apoptosis and mitotic death in A375 cells than radiation alone. In summary, we demonstrated that GNRs have remarkable potential to enhance radiotherapy of melanoma cancer cells. GNRs, in combination with 6 MV irradiation, enhance the radiosensitivity of the cells. This radiosensitizing effect may be attributable to alterations of cell’s capacity to resist apoptosis caused by the GNRs, mainly through enhancement of radiation-induced DSBs and arresting the cell cycle at the G2/M phase. As the success of radiotherapy for patients with melanoma largely depends on tumor radiosensitivity, the combination of GNRs and radiation needs further investigation.
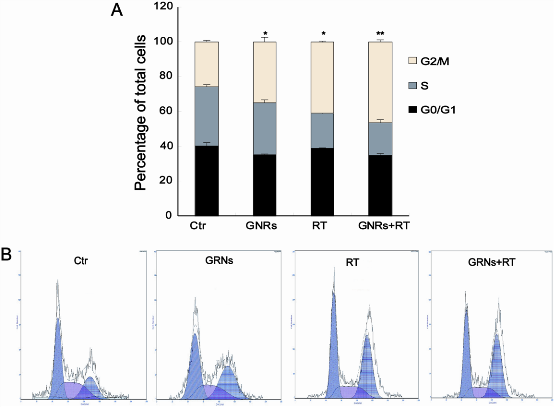
Figure 6: GNRs induce cell -cycle arrest in the G2/M phase of the cell cycle. The A375 cells were incubated with GNRs (50 µg/ml) for 1 h, then the cells were stained with propidium iodide, and analyzed by flow cytometry 24 h after radiation. Experiments were done in triplicates. Columns, mean from three repeated experiments; bars, SD. A: the percetage of different cell phase. B: histogram of flow cytometry. (abbreviation: Ctr: control)
Acknowledgements
This article was funded by research grants (Grant No.09JC411900, 09411951100, 08411960600) from the Science and Technology Commission of Shanghai, and National Nation Science Foundation of China (81171377), National Basic Research Program of China [No.2011CB707504, No.2010CB933903], People’s Republic of China. The authors thank Dr. T. FitzGibbon for comments on earlier drafts of the manuscript.
References
Copyright: (c) 2012 W. Xu, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.12