Article
A facile green synthesis of silver nanoparticles using the medicinal plant Leucas aspera and their antibacterial activity
D Vijayaraj 1, J Anarkali 1, K Rajathi 2, S Sridhar 1*
1 Department of Botany, Govt. Arts College, Thiruvannamalai – 606603, Tamil Nadu, India.
2 Department of Chemistry, Govt. Arts College, Thiruvannamalai – 606603, Tamil Nadu, India.
* Corresponding author. Email: sekarsridhar@rediffmail.com Tel/fax.: +91 9443105935
Citation: D Vijayaraj, et al. A facile green synthesis of silver nanoparticles using the medicinal plant Leucas aspera and their antibacterial activity. Nano Biomed. Eng. 2012, 4(2), 95-98.
DOI: 10.5101/nbe.v4i2.p95-98.
Abstract
The junction of nanotechnology and biomedical sciences unfasten the possibility for a wide range of biological research topics and medical uses at the molecular and cellular level. In the present study silver nanoparticles were synthesized by using Leucas aspera leaf extract support of biological process. The extract incubated with 1mM AgNO 3 solution showed gradual change in the color of the extract from greenish to reddish brown. Reduction of silver ions by the leaf extract was examined by UV-visible spectroscopic technique. Further, it was confirmed by Fourier Transformations Infrared spectroscopic (FTIR) analysis. The purity of the synthesized silver nanoparticles was examined through X-Ray Diffraction (XRD) analysis. The nanoparticles were examined for the antibacterial activities of antibiotic (Tetracycline), silver nanoparticles and the combined effect of both antibiotic and nanoparticles against the multi drug resistant human pathogens.
Keywords: Leucas aspera, Silver Nanoparticles, X-Ray Diffraction, FTIR, Antibiotic
Green chemistry is intended for the implementation of chemical products and processes to reduce or eliminate the use and generation of substances hazardous to human health and environment in the synthesis of metal nanoparticle by the reduction of the corresponding metal ion salt solutions. Nanoparticles are often referred to as clusters, nanospheres, nanorods and nanocups that are just a few of the shapes at the small end of the size ranges from 1 to 100 nm. Nanoparticles reveal a number of special properties relative to bulk material and often have unique visible properties because they are small enough to confine their electrons and produce quantum effects [1]. The biomedical applications of silver nanoparticle can be effective by the use of synthesized nanoparticles which minimize the factors such as toxicity and cost and are found to be exceptionally stable like other nanomaterials. Hence the development of better experimental procedures for the synthesis of nanoparticles of different chemical compositions, sizes, shapes and controlled polydispersity is vital for its advancement [2]. Synthesisof nanomaterial such as silver, gold, platinum and palladium using plants or plant extracts have been suggested as possible ecofriendly alternatives to chemical and physical methods. Nanoparticles synthesis using plants can be advantageous over other biological processes because it eliminates the elaborate process of maintaining cell cultures and can also be suitably scaled up for large-scale synthesis of nanoparticles [3]. Silver nanoparticles as an archway product from the field of nanotechnology, has gain interest because of distinctive properties, such as good conductivity, chemical stability, catalytic, antibacterial activity, antifungal, anti-viral, anti-inflammatory activity [4]. Silver-based medical products, ranging from topical ointments and bandages for wound healing to coated stents, have been proven to be effective in retarding and preventing bacterial infections [5]. Recently, some studies have shown that specially prepared Ag-NPs have superior antibacterial activity [6]. The bacteria usually are incapable of developingresistance against Ag-NPs, because these nanomaterials can at the same time attack a broad range of targets in microorganisms such as proteins with thiol groups, cell walls and cell membranes. A modern study on Escherichia coli has shown that Ag-NPs react with cell walls and cytoplasmic membranes, resulting in pits in the cell wall of bacteria, and finally killing those [7]. It has been reported that green chemistry synthesized titania (TiO2 ) and silver nanocomposites (TANCs) can easily damage the cell walls of E. coli [8]. In this study, the synthesis and characterization of silver nanoparticles were synthesized by using Leucas aspera leaf extract support of biological process. Ag-NPs were prepared using silver nitrate as silver precursor and extract of Leucas aspera leaf as reducing agent and stabilizer. The antibacterial effect of silver nanoparticles of Leucas aspera was evaluated against four pathogenic bacteria, including Vibrio cholerae, Klebsiella pneumoniae (Gram negative) and Staphylococcus aureus, Micrococcus luteus (Gram positive) using the agar well diffusion method.
2.1 Preparation of Leucas aspera leaf broth
The AR grade silver nitrate (AgNO3 ) was purchased from Sigma-Aldrich chemicals and fresh L. aspera leaves were collected from surroundings of Govt. Arts College, Thiruvannamalai, Tamil Nadu, India. The L. aspera fresh leaf extract used for the reduction of Ag+ ions to Ag° was prepared by taking 20 g of thoroughly washed finely cut leaves in 500 ml Erlenmeyer flask along with 100 ml of distilled water and then boiling the mixture for 5 min. before decanting it. Further, the extract was filtered with Whatman No. 1 filter paper and stored at 4°C and used for further experiments.
2.2 Test organisms
The bacterial strains Staphylococcus aureus, Vibrio cholerae, Micrococcus luteus and Klebsiella pneumoniae were collected from the Microbial Type Culture Collection (MTCC), The Institute of microbial Technology, Chandigarh, India. Thirty Six grams of Muller Hindon Media (Hi-Media) was mixed with distilled water and then sterilized in autoclave at 15lb pressure for 15 minutes. The sterilized media were poured into petri dishes. The solidified plates were bored with 6mm dia cork porer. The plates with wells were used for the antibacterial studies. The antibacterial assays were done by well diffusion method [9].
2.3 Synthesis of silver nanoparticles
In a representative experiment, the leaf extract (0.5 ml) was added to 10 ml of 1 mM AgNO3 aqueous solution. The bioreduced aqueous component (0.5 ml) was used to measuring UV-Vis spectra of the solution. The particle suspension was diluted 10 times with distilled water to avoid the errors due to high optical density of thesolution.
2.4 UV-Vis spectral analysis
The color change in reaction mixture (metal ion solution + plant extract) was recorded through visual observation. Synthesized silver nanoparticles was confirmed by sampling the aqueous component of two hour after reaction and the absorption maxima was scanned by UV-Vis spectrophotometer at the wavelength of 325 – 825 nm on Beckman Du-50 Spectrophotometer.
2.5 X-ray diffraction studies
The formation and quality of compounds were checked by X-ray diffraction (XRD) spectrum. The mixture was centrifuged at 10000 rpm for 10 minutes in a refrigerated centrifuge, followed by redispersion of the pellet in acetone. The dispersed pellets were dried in an incubator at 37 °C for 1 week. The size of the purified Ag nanoparticles was analyzed by X-ray powder diffraction crystallography SEIFERT JSO-DEBYEREX-2002 (Germany) diffractometer with Cu-Kα radiation (λ=1.540 nm) with a scan rate of 0.04o per second and a scan range between 10 -70o , 2 theta in flat plate geometry with Cu radiation.
2.6 FTIR analysis of silver nanoparticles
To remove any free biomass residue or compound that is not the capping ligand of the nanoparticles, the residual solution of 100 ml after reaction was centrifuged at 5000 rpm for 10 min. The supernatant was again centrifuged at 10000 rpm for 60 min and the pellet was obtained. This is followed by redispersion of the pellet of Ag-NPs into 1 ml of deionized water. Thereafter, the purified suspension was freeze dried to obtain dried powder. Finally, the dried nanoparticles were analyzed by ALPHA FT-IR Spectrometer (from Bruker, Germany).
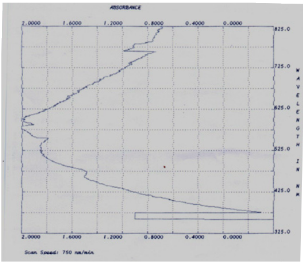
Fig. 1 UV-Vis Absorption Spectrum of nanoparticles synthesized from Leucas aspera extract
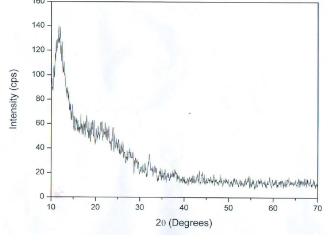
Fig. 2 XRD pattern of silver nanoparticles formed after reaction of Leucas aspera extract
Table 1 Measurement of the size of AgNPs of Leucas aspera by using Debye-Scherrer’s equation
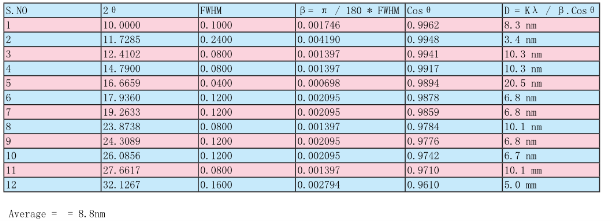
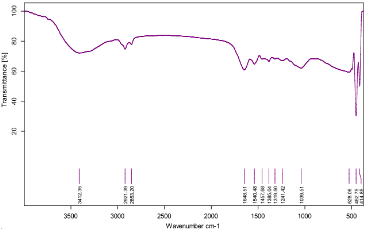
Fig. 3 FTIR spectra of silver nanoparticles synthesized by Leucas aspera plant extract
Table 2 Antibacterial activity of the silver nanoparticles synthesized from Leucas aspera

3. Results and Discussion
The L. aspera leaf extract was utilized for the synthesis of silver nanoparticles. The reaction started within one hour of the incubation with 1 mM silver nitrate. This was confirmed by the color changes from green to reddish brown in the reaction mixture. Present findings showed similarity to the results reported of the extract of Capsicum annum [10] and the extract of Aloe vera [11]. Fig. 1 shows the UV-Vis spectra recorded from the reaction medium after 4 hours. Absorption spectra of silver nanoparticles formed in the reaction media has absorbance peak at 575 nm, broadening of peak indicated that the particles are polydispersed. The biosynthesized silver nanoparticles by employing L. aspera leaf extract was further established and confirmed by the characteristic peaks observed in the XRD image (Fig 2). The XRD pattern showed twelve intense peaks in the whole spectrum of 2θ 10.00, 11.73, 12.41, 14.79, 16.67, 17.94, 19.26, 23.87, 24.31, 26.09, 27.66 and 32.13. The mean size of silver nanoparticles was calculated using the Debye-Scherrer’s equation. An average size of the AgNPs synthesized by L. aspera was 8.8 nm with size ranging from 3.4 to 20.5 nm (Table 1) with cubic and hexagonal shape. The typical XRD pattern revealed that the sample contains a mixed phase structures of silver nanoparticles. Debye-Scherrer’s equation, D = Kλ / β. Cosθ Where, β= π / 180 * FWHM (FWHM= Full Width Half Maximum) K= 0.94 λ= 1.540598 Ao Kλ= 0.94 * 1.540598 Ao = 1.4482 FTIR analysis was used for the characterization of the extract and the resulting nanoparticles (Fig 3). Representative spectra obtained from nanoparticles evident the absorption peaks located at about 3412.35 cm -1 , 2921.39 cm -1 , 2853.20 cm -1 , 1648.51 cm -1 , 1540.48 cm -1 , 1457.68 cm -1 , 1385.04 cm -1 , 1319.50 cm -1 , 1039.51cm -1 in the region of 3500 cm -1 to 500 cm -1 . The FTIR spectra revealed the presence of different functional groups like secondary Alcohol (O-H stretch, H-bonded), Alkanes (C-H stretching), Alkanes (C-H stretching), Alkenes (C=C stretching), Aromatic (C=C stretching), Alkane (-C-H stretching), Alkane (-C-H stretching), Amine (C-N stretching), Amine (C-N stretching), respectively. The peak at 3427 cm -1 indicates polyphenolic OH group along with the peak of 882 cm -1 which represents the aromatic ring C-H vibrations, indicate the involvement of free catechin [12]. The peak at 1624 cm -1 is associated with stretch vibration of –C=C- [13] and is assigned to the amide 1 bonds of proteins. The peak at 1539 cm -1 may be assigned to symmetric stretching vibrations of –COO- (carboxyl ate ion) groups of amino acid residues with free carboxyl ate groups in the protein [14]. The peaks at 1000-1200 cm -1 indicate C-O single bond and peaks at 1620-1636 cm -1 represent carbonyl groups (C=O) from polyphenols such as catechin gallate, epicatechin gallate and theaflavin [15]. The individual and combined effects of the bioreduced silver nanoparticles with antibiotic were investigated against four bacterial strains using the well diffusion method. The diameters of the silver nanoparticle inhibition zones against different pathogenic cultures, antibiotic (Tetracycline) as well as combined result of silver nanoparticles with antibiotic were observed (Table 2). Silver nanoparticles synthesized from L. aspera revealed maximum antibacterial activity against Micrococcus luteus and followed by Vibrio cholerae and Klebsiella pneumoniae. Staphylococcus aureus were not showed activity. Silver nanoparticles were found to be comparatively less active. Combined with the silver nanoparticles, the antibacterial activity of the antibiotic showed wide variation. When combined with Tetracycline, the silver nanoparticles were found to be superior against Micrococcus luteus, showing 21.5±1.4 mm of inhibition zone.
References
Copyright: (c) 2012 S. Sridhar, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.