Review
A Review: Biological Synthesis of Silver and Copper Nanoparticles
Ratnika Varshney1*, Seema Bhadauria1, Mulayam S. Gaur2
1Microbiology and Nanotechnology Research Lab, R. B. S. College, Agra, India.
2Department of Physics, Hindustan College of Science & Technology, Mathura, India.
* Corresponding author. Email: ratnika_bt@rediffmail.com Tel/fax.: +919696691824
Citation: Ratnika Varshney , et al. A Review: Biological Synthesis Of Silver And Copper Nanoparticles. Nano Biomed. Eng. 2012, 4(2), 99-106.
DOI: 10.5101/nbe.v4i2.p99-106.
Abstract
The antimicrobial properties of silver nanoparticles were known since ancient times and silver ions are widely used as bactericidal agent. Researchers have also recommended the use of silver and copper ions as superior disinfectants for wastewater generated from hospitals containing infectious microorganisms. A wide range of nanophasic and nanostructured particles are being fabricated globally with the aim of developing clean, nontoxic and eco-friendly technologies. Use of ambient biological resources in this area of science is rapidly gaining importance owing to its growing success and simplicity. Currently, simple prokaryotes to complex eukaryotic organisms including higher angiospermic plants are used for the fabrication of NPs. One area of untapped potential is the use of microbes to fabricate copper nanoparticles. We are working on this aspect and successfully fabricated spherical copper nanoparticles of size 4-10 nm. This article presents a review of the ambient biological systems for fabrication of these nanoparticles and development of an updated knowledge base.
Keywords: Bioreduction, Biosynthesis, Silver, Copper, Bacteria, Fungi, Algae.
1. Introduction
Microbial contamination of water poses a major threat to public health. With the emergence of microorganisms resistant to multiple antimicrobial agents, there is an increased demand for improved disinfection methods [1]. The antimicrobial properties of silver nanoparticles are well-established since ancient times and silver ions are widely used as bactericide in catheters, burn wounds and dental work [2, 3] and several mechanisms for their bactericidal effects have been proposed. Although only a few studies have reported the antibacterial properties of copper nanoparticles, they show copper nanoparticles have a significant promise as bactericidal agent [4]. Yoon et al. reported the antibacterial effects of silver and copper nanoparticles using single representative strains of E. coli and Bacillus subtilis, where the copper nanoparticles demonstrated superior antibacterial activity compared to the silver nanoparticles [5]. Researchers have also recommended the use of silver and copper ions as superior disinfectants for wastewater generated from hospitals containing infectious microorganisms [6, 7]. However, residual copper and silver ions in the treated water may adversely affect human health [8].The emergence of nanoscience and nanotechnology in the last decade presents opportunities for exploring the bactericidal effect of metal nanoparticles. The bactericidal effect of metal nanoparticles has been attributed to their small size and high surface to volume ratio, which allows them to interact closely with microbial membranes and is not merely due to the release of metal ions in solution [9]. Metal nanoparticles with bactericidal activity can be immobilized and coated on to surfaces, which may find application in various fields, i.e., medical instruments and devices, water treatment and food processing. Metal nanoparticles may be combined with polymers to form composites for better utilization of their antimicrobial activity. Metal nanoparticles are also finding application in various other fields, i.e., catalysis and sensors [10, 11]. Nature has elegant and ingenious ways of creating the most efficient miniaturized functional materials. In attempts to create miniaturized structures, significant achievements have been achieved and structures of micron and nano-dimensions can now be routinely fabricated though the complexity manifested by thenature is yet a distant goal. In the current context, importance is being given to the fabrication of a wide range of nanomaterials for developing environmentally benign technologies in material synthesis [12]. Despite of their minute structure, they trigger the chemical activity due to their distinctive crystallographic nature that increases surface area, hence the scope of reactivity [13, 14]. In recent times, synthesis of inorganic nanoparticles has been demonstrated by many physical and chemical means. But the importance of biological synthesis is being emphasized globally at present because chemical methods are capital intensive, toxic, non eco-friendly and have low productivity [15]. Utilizing potential biological systems from higher angiospermic plants or microbes, biosynthesis of NPs is currently under wide exploration [16, 17]. These ambient biological systems provide excellent examples of nanophasic materials with highly optimized characteristics resulting from evolution over a long scale of time [18] and the synthesis of inorganic materials may occur either extracellularly or intracellularly [19]. Exposure to varying temperature, pH and substrate concentration influences, directly or indirectly, the rate of intracellular NPs fabrication [20]. It is important to understand the biosynthetic mechanism involved in the fabrication of metal nanomaterials mediated by a biological system in order to gain better control of the process and products. So far, little is known about the interaction between biomolecules and NPs, though several analyses have been made. It is reported that bioreduction of metal ions and stabilization of Au or Ag-NPs is primarily caused by various terpenoids or alkaloids present in the Geranium, extract itself [21]. It is a need of today’s nanotechnology to develop reliable, non-toxic, clean and eco-friendly experimental protocols for the synthesis of silver and copper NPs over a range of chemical composition, size and synchronized monodispersity, which is possible through ambient biological resources. In the last few years a lot of reviews have been published [12, 22, 23], it is necessary further to elaborate this technology in a consolidated way with an approach that provides an overview of the current trend of research on the biosynthesis of Ag and Cu-NPs for their further applications. The present review highlights the current knowledge regarding the potential organisms for biosynthesis of these NPs and presents a database that future researchers can build upon.
1.1 Fabrication of nanoparticles using bacteria
The most important challenge in nanotechnology today is to cost effectively tailor the optical, electric and electronic property of NPs by controlling the configuration as well as monodispersity. This goal could be achieved using bacterial organisms in an organized manner [20]. In the last few years, fabrication of Ag-NPs has increased extensively owing to its immense applications [9]. Pseudomonas stutzeri AG259 has been reported to fabricate Ag particles [24], which are accumulated within the periplasmic space of bacterialcell of 200 nm. Lactobacillus, a common bacterial strain present in the buttermilk, synthesizes both Au and Ag NPs under standard conditions [25]. Rapid synthesis of metallic NPs of Ag using the reduction of aqueous Ag +has been achieved in the cultural supernatants of Klebsiella pneumonia, Escherichia coli and Enterobacter cloacae [26]. Recently detailed studies confirmed that synthesis of Ag can be triggered through the liquid mixing process developed in the visible light spectrum by Klebsiella pneumonia [27]. Extracellular biosynthesis of 40 nm Ag NPs by the culture supernatant of Bacillus licheniformis has been customized as an easy way to work out the process [28]. Varshney et al. have reported a rapid biological synthesis technique for the synthesis of spherical Cu nanoparticles of in the size range of 8-15 nm using non-pathogenic Pseudomonas stutzeri [29] (Fig. 2).
1.2 Fabrication of nanoparticles using yeast
Among the simple eukaryotic organisms, yeasts are explored mostly for the fabrication of Cd-NPs and also known as ‘Semiconductor Crystals’ or ‘Quantum Semiconductor Crystals’ [32]. Ag-NPs have been fabricated extracellularly using an Ag tolerant yeast strain MKY3 [15]. They have demonstrated that MKY3, a silver-tolerant yeast species, when challenged withsoluble silver in the log phase of growth, majority of silver precipitate extracellularly as elemental nanoparticles. They used differential thawing methods for the sample, for separation of the metallic nanoparticles from the medium. Studies are still being carried on to search further for diverse groups of beneficial yeasts.
1.3 Fabrication of nanoparticles using algae
An alga is a diverse group in plant kingdom that is being explored for application in nanotechnology. Besides the production of NPs, algae are also being explored for determining its nutritional value, efficacy in bio-diesel improvement as well as its vast potential for therapeutic application. Govindaraju et al. reported extracellular synthesis of silver nanoparticles by a brown seaweed, Sargassum wightii [33]. Their recorded antibacterial effect against bacteria isolated from the infected silkworm, was found more potent when compared to the chemically synthesized silver nanoparticles and it is expected to be biocompatible. Mohseniazar et al. demonstrated that Nannochloropsis oculata and Chlorella vulgaris have the potential of nanosilver production in a culture medium containing 1mM of AgNO3 within 24 h. The size range of particles was approximately less than 15 nm [34].
1.4 Fabrication of nanoparticles using fungi
The fungal mediated green chemistry approach towards the fabrication of NPs has many advantages. This includes easy and simple scale up method, economic viability, easy downstream processing and biomass handling, and recovery of large surface area with optimum growth of mycelia. It has been observed that most of the fungal genera are coupled with the synthesis of Ag-NPs either intracellularly or extracellularly showing the onset of deep brown coloration [35]. Aqueous Ag ions exposed to Fusarium oxysporum leads to the fabrication of extremely stable Ag hydrosol. The particles are stabilized in solution by the proteins excreted through the fungus [36]. Extracellular biosynthesis of Ag-NPs in the 5-25 nm range using Aspergillus fumigatus is found to be quite fast and manifested the production of dense fungal biomass [37]. White rot fungus, scientifically known as Phaenerochaete chrysosporium has also been used for biomimetics of Ag-NPs [38]. When Aspergillus flavus has been challenged with AgNO3 solution it accumulated Ag-NPs [39] on the surface of its cell wall. The TEM micrographs of dislodged NPs in aqueous solution confirmed the fabrication of convincingly monodispersed NPs of size 8.92 ± 1.61 nm. FTIR confirmed the existence of proteins adjoining the Ag-NPs with a distinctive emission peak at 553 nm excited at 420 nm in photoluminescence spectrum [40]. Phytochelatin and NADPH dependent nitrate reductases for in-vitro production of Ag-NPs have been isolated from Fusarium oxysporum and been elucidated [17]. Fusarium acuminatum has been studied intensely for the formation of Ag-NPs [41]. Fusarium semitectum fabricated 10-60 nm spherical NPs and the colloidal suspensions are stable for several weeks but after thatstability. Possible medicinal applications of these Ag-NPs are envisaged [42]. Extracellular biosynthesis of Ag-NPs by Fusarium solani (USM-3799), a phytopathogen, has produced nanoparticles with an average diameter of 16.23 nm. FTIR analyses provide evidence for the presence of proteins as a capping agent that enhanced the stability of synthesized Ag-NPs [43]. Further, it has been reported that functional groups, e.g. ≡C-O-C≡, =C=O, ≡C-O-R and =C=C= are being derived from heterocyclic compounds like proteins that are present in the fungal extract and act as capping ligands of NPs [44]. The genus Penicillium seems to have extremely good candidates for the fabrication of Ag-NPs. Production proceeds via extracellular mechanism with high negative zeta potential and stable at pH above 8.0 because of electrostatic repulsion [45]. Penicillium fellutanum, isolated from coastal mangrove sediment, is found to be highly efficient in synthesis of Ag-NPs. Production is optimized at 0.35 % NaCl, pH 6.0, incubated at 5 ºC and treated with AgNO3 (1mM) for 24 h. A protein band with a molecular weight of 70 KDa is detected when analyzed by PAGE [46]. As Penicillium is a very common biomass waste from pharmaceutical industry that possesses a potential to fabricate particles, it would enhance the opportunity for cost-effective preparation of various Ag based nanostructures. Controlling the scale-up process for biosynthesis of Ag-NPs with fungal proteins of Coriolus versicolor has been carried out [44]. Fungal biomass accumulated Ag-NPs in 72 h, which is reduced to 1 h in customized reaction conditions. FTIR studies revealed that amino groups bound to particles account for the stability of NPs and established the existence of protein as the stabilizing and capping agent. Extracellular synthesis took place whereby other than the fungal proteins, glucose is found to be responsible for the reduction. While in fungal mycelium, intracellular development of Ag-NPs could be modified to give both intracellular and extracellular Ag-NPs under alkaline conditions, wherein the surface - S-H groups of the fungus play a major role. Extracellular synthesis of Ag-NPs from Phoma glomerata (MTCC-2210) has been traced and its efficacy against Escherichia coli, Staphylococcus aureus and Pseudomonas aeruginosa has been evaluated [47]. Experimental evidence suggests that the extracellular production of NPs is stabilized by proteins as well as reducing agents secreted by the fungus itself. NPs have been found to be coupled with at least four high molecular weight proteins released from the biomass of fungus. FTIR indicates the native forms of these proteins [48]. Reduction of metal ions and surface binding of proteins to the NPs has been elucidated little far [17]. Studies further indicate that genera Fusarium, Aspergillus and Penicillium have large potential for the fabrication of different metal NPs. Recently Ag-NPs synthesis and its antimicrobial activity against gram positive and negative bacteria has been reported by Pleurotus sajor caju fungi [49]. We have also reported a novel fungi Hormoconis resinae for the synthesis of silver nanoparticles within the range of 20-80 nm [50].Very recently Li et al. demonstrated a method for AgNPs synthesis by reduction of aqueous Ag+ with the culture supernatants of Aspergillus terreus at room temperature. The synthesized Ag-NPs were polydispersed spherical particles ranging in size from 1 to 20 nm and stabilized in the solution (Fig. 4). Reduced NADH was found to be an important reducing agent for the biosynthesis, and the formation of Ag-NPs might be an enzyme-mediated extracellular reaction process. Their antimicrobial potential was systematically evaluated and found that they could efficiently inhibit various pathogenic organisms, including bacteria and fungi [51].
1.5 Fabrication of nanoparticles using higher angiospermic plants
Several microorganisms such as bacteria, fungi and yeasts have come up as nanofactories synthesizing metal NPs of Ag. However, use of plants for the fabrication of NPs has drawn attention of workers because of its rapid, economical, eco-friendly protocol and it provides a single step technique for the biosynthesis process [52]. In the extract of Geranium sp. fabrication of Ag-NPs as well as Au-NPs has been reported [53]. The leaf broth of Azadirachta indica extracellularly fabricates pure metallic Ag- and Au- NPs and bimetallic Au/Ag NPs. Competitive reduction of Au 3+ and Ag + ions present simultaneously in solution during exposure to plant leaf extract generates bimetallic Au core and Ag shell NPs. TEM revealed Ag particles have been adsorbed on Au-NPs and constructed the former structure. Flavonone and terpenoid components of leaf broth are being predicted to stabilize the formation of NPs in contrast to high molecular weight proteins of fungal biomass [54]. The fruit extract of Emblica officinalis enhances the extracellular synthesis of highly stable Ag and Au-NPs [55]. Utilizing leaf extract of Aloe vera as a reducing agent, fabrication of Au nano triangles and Ag-NPs in single triangular form has been achieved [56]. Cinnamomum camphora has also been revealed to fabricate Au and Ag-NPs [52]. Ag-NPs have also gained significance due to their broad-spectrum activity against bacterial infections. The roots of Medicago sativa accumulate Ag ions insidethe plant tissue from the enriched medium and undergo nucleation and fabricate Ag-NPs [57]. Using Pelargonium graueoleus leaf extract, Ag + is reduced to Ag-NPs that are crystalline as well as highly stabilized [58]. Bioreduction activity of leaf extracts of Helianthus annus, Basella alba, and Saccharum officinarum resulted in the fabrication of Ag-NPs in which Helianthus annus is found to exhibit strong potential for quick reduction of Ag + [59]. The polyol components and the water soluble heterocyclic components were mainly responsible for reduction of Ag + as well as stabilization of NPs. Information regarding the activity of reductases in NP fabrication were well illustrated by Kumar et al [17]. No correlation is observed between the color development and increase in abundance exhibited by the synthesized nano metal. Differences in morphology of nanoparticles synthesized, is one possible reason for variation in optical properties [60]. Further studies reflect that several parameters together determine the NP synthesis including plant source, the organic compounds in the crude leaf extract, the concentration of AgNO 3, the temperature and the pigments of the corresponding leaf extract [59]. Recently, fabrication of Ag-NPs using the callus extract of Carica papaya has been reported [61]. Brown coloration of the MS medium and analyses of FTIR confirmed the presence of proteins and other ligands that are mandatory for the synthesis and stabilization of derived spherical NPs. The use of latex of Jatropha curcas as reducing as well as capping agent in the fabrication of Ag-NPs has recently been reported [62]. Jha et al. reported the synthesis of Ag-NPs by Eclipta leaf fabricating 2-6 nm size particles [63]. Biosynthesis of Ag-NPs using Glycine max (soybean) leaf extract and its different varieties has been reported by Vivekanandan et al. and yield organic free particles64. Very recently several researchers exploited plant extracts for the synthesis of silver nanoparticles using Coriandrum sativum leaf extract [65]; leaf and seed extract of Syzygium cumini [66]; Cycas leaf [67], Argimone Mexicana leaf extract [68]; onion extract [69]. Varshney et al. reported a novel biological method for the synthesis of rod and cubes shaped silver nanoparticles by exploiting sundried Stevia rebaudiana leaves at ambient conditions and synthesized silver nanoparticles ranging from 80-200 nm diameter and 400-800 nm height [70]. Govindaraju et al. reported the biological synthesis of silver nanoparticles using Solanum torvum and its antimicrobial activity. They obtained silver nanoparticles of average size 14 nm and antimicrobial activity of S. torvum mediated silver nanoparticles was performed against pathogenic bacteria and fungi of silkworm Bombyx mori[71]. With the aim of developing new methods of producing metallic nanoparticles from materials safe for the environment, Guajardo-Pacheco et al. reported a method of producing metallic nanoparticles of copper by using soybeans extract as a chelating agent [72]. Recently Singh et al. reported biosynthesis of gold and silver nanoparticles using leaf extract of ginger (Zingiber officinale). Ag-NPs produced were spherical and with dimensions ranging from 10.1 to 65.91 nm with an average size of 30.31nm (Fig. 5) [73]. Lee et al. reported biologically synthesized copper nanoparticles using plant leaf extract Mangolia as reducing agent. On treatment of aqueous solutions of CuSO 4.5H2O with leaf extract, stable copper nanoparticles were formed ranged in size from 40-100 nm. Their antibacterial properties were tested by counting viable Escherichia coli cells after 24 h growth and showed higher antibacterial activity compared with untreated samples [74].
1.6 Fabrication of nanoparticles using weeds
Among the above bio-based protocols, Parashar et al. exploited the synthesis method of silver nanoparticles from weed Parthenium leaf extract [75]. Recently the bioreduction property of three aquatic weed leaves extracts such as Ipomoea aquatica (Convolvulaceae), Enhydra fluctuans (Asteraceae) and Ludwigia adscendens (Onagraceae) in the synthesis of silver nanoparticles has been investigated by Roy et al [76].
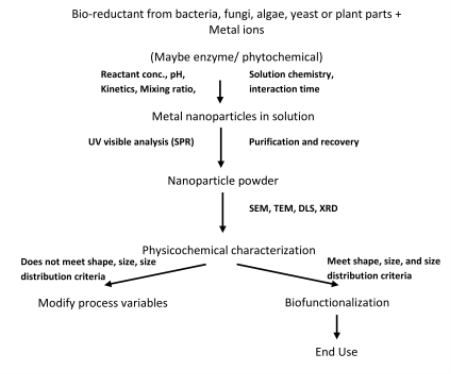
Fig. 1 Generalized Flow Chart for Biosynthesis of Nanoparticles
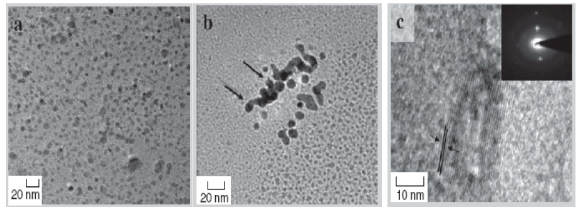
Fig. 2 HRTEM images of Cu nanoparticles formed by bacteria Pseudomonas stutzeri shows: (a) spherical Cu nanoparticles; (b) a thin bacterial layer around nanoparticles which acts as capping agent; (c) close-up view of nanoparticles showing lattice fringes; inset shows selected electron diffraction pattern. [Adapted from Ref. 29]
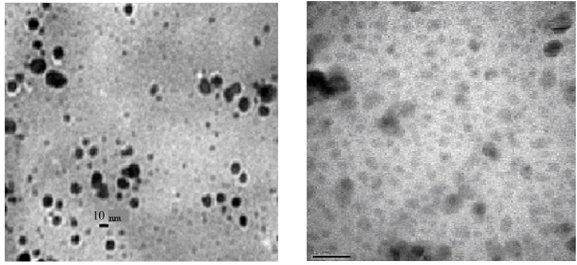
Fig. 3 TEM photographs of AgNPs from Bacillus cereus at different magnifications. [Adapted from Ref. 31]
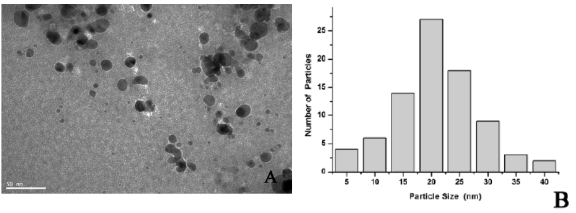
Fig. 4 (A) Representative images of AgNPs synthesized by the reduction of AgNO 3 solution with the dialyzed cell filtrate of Aspergillus terreus and NADH; (B) Size distribution of the AgNPs from TEM analysis. [Adapted from Ref. 51]
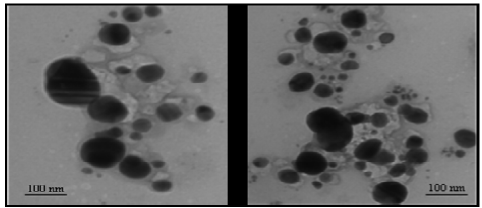
Fig. 5 Representative TEM images illustrating the formation of silver nanoparticles biologically synthesized by reduction of Ag+ ions using leaf broth of Zingiber officinale. [Adapted from Ref. 73]
2. Conclusions
This paper has reviewed recent knowledge and built a data base of bioreductive approaches to Ag-NPs and Cu-NPs using different biological systems. There are several reports of physical and chemical synthesis of copper and copper based nanomaterials. But no reports are available on the biological synthesis of copper nanomaterials with size and shape control. It is, therefore, important to develop synthetic strategies which are simple, cost-effective, environment friendly, easily scalable and at the same time with parameters to control size and shape of the materials. The exact mechanism for the fabrication of NPs in biological resources is still being investigated and several possible ways have been proposed [21, 52[. Microorganisms where proteins [44] and angiosperms where carboxylic groups, amino groups, proteins and carbohydrates [52] are present in the source extract have been proposed to play a key role in the biosorption and bioreduction process for the fabrication of NPs.
References
2005.184
Copyright: (c) 2012 Ratnika Varshney, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.