Review
Biogenic Synthesis of Silver Nanoparticles Using Fruit Extract of Ficus Carica and Study Its Antimicrobial Activity
K. Logaranjan, S. Devi, K. Pandian*
Department of Inorganic Chemistry, University of Madras, Guindy Campus, Chennai 600 025, Tamil Nadu, India.
* Corresponding author. Email: jeevapandian@yahoo.co.uk
Citation: K. Logaranjan et al. Biogenic Synthesis of Silver Nanoparticles Using Fruit Extract of Ficus Carica and Study Its Antimicrobial Activity. Nano Biomed. Eng. 2012, 4(4), 177-182.
DOI: 10.5101/nbe.v4i4.p177-182.
Abstract
Biogenic synthesis of metal nanoparticles is considered to be a cost effective and eco-friendly approach. Here we have demonstrated biogenic synthesis of silver nanoparticles (AgNPs) using Ficus Carica extract at room temperature. Ficus Carica fruit extract can be used as reducing as well as capping agent without adding any external reducing agents. The rate of formation of AgNPs was monitored by using UV-Vis spectroscopy by measuring the absorbance at 420 nm for different molar ratios of plant extract to silver nitrate concentrations. The resulting AgNPs was isolated and characterized with various instrumental methods such as FTIR, XRD and FE-SEM to study the size and shape of particles and functional group of the stabilizing agents. The particles sizes are found to be 20-25 nm ranges with face centered cubic crystal structure of AgNPs. The antimicrobial activity of AgNPs synthesized by biogenic method was tested against gram negative and gram positive micro-organism. An enhanced antimicrobial activity was observed with AgNPs synthesized from fruit extract when compared with AgNPs synthesized by EDTA method.
Keywords: Biogenic synthesis, Silver nanoparticles, Ficus Carica fruit extract, Antimicrobial activity
1. Introduction
Green synthesis of nanoparticles is an easy, efficient and eco friendly approach, where most researchers are looking at the eco-friendly and green synthesis of nanoparticles paves the way for the researchers around the world to explore the ability to use herbs in synthesizing of metal nanoparticles. Silver nanoparticles (AgNPs) can be synthesized from various parts of the herbal plants like bark of cinnamon zeylanicum [1], azadirachta indica (neem) leaves [2], citrus limon [3], tannic acid [4], parthenium hysterophorus leaf [5], murraya koenigii (curry leaf) [6], lantana camara leaf [7] and various plant leaves [8]. It has been shown that tea leaf and coffee extracts can be utilized to synthesize silver and palladium nanoparticles [9]. Thus many groups have been involved on synthesis of metal nanoparticles for various kinds of applications including antimicrobial activity, catalytic reduction of 4-nitrophenol and removal of dye effluents. The influence of nanoparticles on biological functions or the relative toxicity on bacterial origin was not well-understood and they are becomes one of the major public concerns. Biological organisms like bacteria [10], fungi and actinomycetes [11] were also utilized for the synthesis of AgNPs. The synthesis of metal nanoparticles using plant extract (biogenic approach) is still an expanding area of research due to the potential applications in nanomedicines [12]. The fruits of the plant Ficus Carica (Figure S1, Supplementary Information) are the important food and traditional medicine to cure various diseases. The Ficus Carica contains laxative substances, flavonoids, sugars, vitamins A and B, acids and enzymes [13,14]. The major bioactive compounds present in the Ficus Carica extract (FC extract) were flavonoids such as quercetin, luteolin and the compound 6-O-acyl-β-d-glucosyl-β-sitosterols [14,15]. The fruit can be used to treat hypoglycemic, hepatoprotective, hypolipedimic, scavenging and immune response, anti-inflammatory, anticancer, antioxidant, antipyretic, antibacterial and antifungal activities as well as used in Indian homeopathic medicine [16]. Because of its wider potential application, we have taken an initiative to develop a novel method for synthesis of AgNPs using FC extract at room temperature. It has been well established that AgNPs are found to have extensive applications in various areas like optical receptors [17], sensors [18], bioactive materials [19], signal enhancers in SERS based enzyme Immunoassay [20]. AgNPs are known to have antimicrobial activity [21]. Silver is an effective antibacterial agent with low toxicity which is important especially in the treatment of burn wounds. Due to its broad spectrum of antibacterial activity, AgNPs have been the focus of increasing interest and are being used for therapeutic purposes [22]. In the present study, we demonstrated a simple green method for the synthesis of AgNPs using FC extract as reducing agent. The extracellular mechanism of extract to stabilize and binding nature of the nanoparticles were studied. The assynthesized AgNPs were characterized with various characterization techniques to study the formation kinetics, size and shape. The antibacterial activity of AgNPs was tested against various pathogens [23]. The biosynthesis using FC extract endows the AgNPs with low cost and green route for production of antibacterial materials. In recent years, antibacterial materials impregnated with AgNPs such as coatings [24], textiles [25] and wood flooring [26] have attracted much interest.
2. Experimental
2.1 Materials and methods
The fresh Ficus Carica fruits were collected from the well grown trees in and around Madras University, Guindy Campus at Chennai. Silver nitrate (AgNO3) and Acetonitrile (HPLC grade) were purchased from Sigma-Aldrich Chemical Pvt. Ltd, Bangalore, India. All the aqueous solutions were prepared using double distilled deionized water.
2.2 Preparation of FC extract
22 g of fresh Ficus Carica fruits washed with distilled water and crushed with mortar and pestle to fine grind to achieve a wet paste. To this added 100 mL of DI water and kept stand for 2 days at room temperature in a dark room. The extract was filtered and stored at 5°C until further use.
2.3 Synthesis of AgNPs
About 0.5 mL of FC extracts was added to 9.0 mL of 1mM AgNO3 and then made upto 10 mL with deionized water. AgNPs are formed.
2.4 Instrumentation
2.4.1 UV-Vis spectroscopy
The biogenic synthesis of AgNPs was monitored periodically by UV-Vis spectroscopy. The UV-Vis spectra were collected using Shimadzu 1800 spectrophotomer, Japan. All spectra were collected by scanning from 200 to 800 nm ranges. All spectra were corrected against the background spectrum of water as reference.
2.4.2 X-ray diffraction (XRD) analysis
XRD pattern of AgNPs was recorded using X-ray instrument (JSO Debye Flex 2002 Seifert diffractometer) with Cu-Kα1 radiation with λ of 1.5406 Å and nickel monochromator filtering using tube voltage of 40 kV. The scanning was done in the region of 2θ from 0° to 80° at 0.02° min−1 .
2.4.3 Field emission scanning electron microscopy (FE-SEM) analysis
Morphological and structural investigations were carried out with field emission JEOL-JSM-6360 instrument, USA. In a typical measurement, a small amount of sample powder was adhered onto a copper stub using double-sided carbon tape. The sample was then sputtered with platinum to reduce charging effect.
2.4.4 Fourier transform infrared spectroscopy (FTIR) analysis
The FTIR spectra were recorded using Perkin-Elmer 360 model IR double beam spectrophotometer. The AgNPs containing fruit extract solution was centrifuged at 10,000 rpm for 15 min and the powder was washed three times with 20 mL deionized water. After drying, the powdered samples were collected at room temperature. A known amount of sample was ground with KBr and the pellet form of the samples was analyzed with FTIR instrument.
2.4.5 High performance liquid chromatography (HPLC)
A HPLC equipped with gradient elution capability in combination with Photodiode detector and an auto sampler (using Water Alliance system, 2690 and Waters Empower software). Intersil ODS 3V (id 150 mm ×4.6 mm×5 µm) column was used for the separation. Milli-Q water (98%) and filtered Acetonitrile (2%) mixture was used as mobile phase in isocratic mode. The detector was set at 260 nm for all measurements and 20 µL of injection volume of sample was allowed to elute through the system for 15 min at a flow rate of 0.7 mL min−1 and the column oven temperature was set at ambient. Filter the FC extract using 0.45 µm nylon filter and the filtrate was used for HPLC analysis.
2.5 Antibacterial assay
The antibacterial activity of AgNPs was tested by using agar disc diffusion assay as per Clinical Laboratory Standards Institute (CLSI) [27]. Briefly, 0.5 MacFarland of inoculum was made using the overnight grown bacterial strains. Strains used in this study were gram positive bacteria Staphylococcus Aureus (ATCC 29213) and gram negative bacteria Escherichia Coli (ATCC 25922). They were diluted 1:10 to get final inoculum of 1×107 Colony Forming Unit per mL (CFU mL−1) and inoculated on the Mueller Hinton Agar (BD Difco) medium using sterile cotton swabs. Sterile discs of six millimeter width were dipped into extract, AgNO3 solution, extract stabilized AgNPs as well as the assay control imipenem antibiotic. Imipenem was used as positive standard for comparison purpose. Dried discs were placed over the inoculated plates followed by incubation overnight at 37°C. The antibacterial activity was assigned by measuring the diameter of the zone of inhibition around the disc.
3 Results and Discussion
3.1 Synthesis of FC extract stabilized AgNPs
The reaction kinetics of the formation of AgNPs using FC extract was investigated using UV-Vis spectral studies by measuring the increase in absorbance values at 420 nm (Plasmon peak position) with increase of the reaction time. The influence of various factors on the rate of reduction processes under the optimum experimental condition also investigated. Here FC extract is not only act as reducing agent and also act as capping agent. The formation of AgNPs during the chemical reaction between the FC extract and AgNO3 is due to reducing agent ability of biologically important molecules such as reducing sugars, flavonoids, saccharides and proteins present in the FC extract which had been discussed in many works. A spontaneous coloration was observed when the AgNO3 was allowed to react with different concentration of plant extract which is due to the reducing ability of flavonoids and proteins molecules present in the fruit extract. Scheme 1 depicted the optical photograph for the color changes of free extract when the addition of 1 mM AgNO3 A color changes from light yellow to orange color indicates the formation of AgNPs and the reaction mixture was allowed to stand for 24 hrs (the reaction was completed within 24 hrs). A control experiment was carried out without addition of FC extract, but there was no appreciable color changes was noted while standing the reaction mixture for one day. These results proved that FC extract can be used for the synthesis of AgNPs under ambient condition without addition of any external reducing agent.

Scheme 1 Optical photograph for the color changes of fruit extract.
3.2 UV-Vis spectral study
UV-Vis spectroscopy is an important tool to determine the formation, size and shape and stability of AgNPs in aqueous under various experimental conditions. The growth rate of AgNPs was monitored by using UV-Vis spectral studies by measuring the change in absorbance at different time intervals. Also the influence of FC extract concentration on the rate of AgNPs formation was investigated. The rate of reduction of AgNO3 after addition of 0.5 mL concentration of FC extract was monitored by measuring the increase in absorbance values at different time scale. Initially the reaction is very fast and then become slow because of the capping action of the reducing polysaccharides and proteins present in the FC extract. The growth kinetic of AgNPs is shown in Figure 1(A). The reaction was completed within 24 hrs and it was observed that there was no further increase in absorbance values. The influence of FC extract on reduction of AgNO3 was investigated. Different proportion of FC extract to the AgNO3 were prepared and allowed to stand for 24 hrs in order to attain complete reduction of AgNO3 under dark condition. The UV-Vis spectrum of each reaction mixture was recorded and the data are shown in Figure 1(B). The AgNPs gave the sharp intensity peak in visible region of the spectrum. Figure 1(B) shows the effect of concentration of FC extract while maintaining the concentration of AgNO3 as constant on formation of AgNPs. From this observation it is inferred that when the increasing the FC extract concentration from 0.1 to 1.0 mL, the absorbance values also increases. Thus, the synthesis of AgNPs was carried out at room temperature in neutral pH ranges of the medium. It is well known that AgNPs exhibit reddish yellow color due to excitonic vibration electron due to the Surface Plasmon Resonance (SPR) of AgNPs. The AgNPs SPR band occur within the ranges of 419-442 nm [28,29] in an aqueous medium which is gradually shifted to longer wavelength ranges while increasing the concentration of FC extract. The biosynthesis of AgNPs was performed at room temperature for 24 hrs. The AgNPs SPR band consistently obtained at 440 nm with gradual increase in the intensity of absorbance peak. The color of the solution was stable for about one month and then slowly color changes from orange yellow to grey colored. In summary, the FC extracts exhibit a very good reducing agent as well as stabilizing agent and reduction reaction take place extracellularly. This method is considered to be one of the easy ways of synthesis of AgNPs in biogenic approach. The formation of AgNPs has been confirmed from UV-Vis analysis. The fruit containing flavonoids are responsible for the reduction of Ag+ to AgNPs. This concept was proved by many people [30]. The generally acceptable mechanism for the reduction reaction is the presence of polyphenol and flavonoids in the most of the plant extract. Based on the literature report we concluded that the flavonoids play as reducing agent. The following reaction mechanism is given in the most of the recent papers for the reduction of Ag+ to AgNPs (Scheme S1, Supplementary Information). The size and shape and their capping agent nature were derived from FE-SEM and FTIR studies.
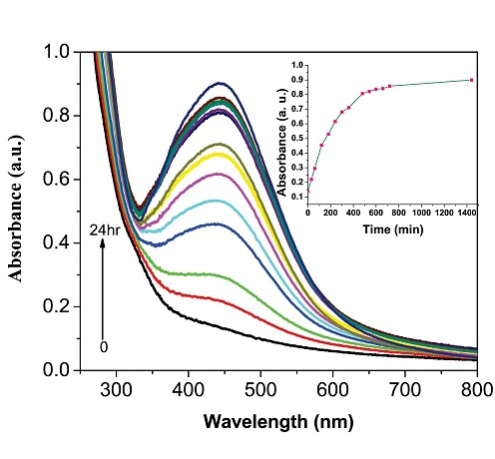
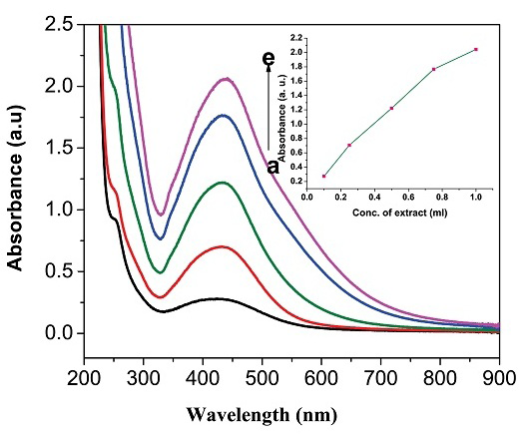
Figure 1 (A) UV-Visible spectrum of FC extract and bio-synthesized AgNPs at different time intervals. The inset a plot of the absorbance values of AgNPs formed at different time interval. (B) UV-Visible spectrum of AgNPs at different FC extracts concentration (a to e: 0.1, 0.25, 0.5, 0.75 and 1 mL). The inset a plot of concentration of fruit extract versus absorbance values.
3.3 HPLC analysis
HPLC analysis of fruit extract was investigated to find out the active component present in the fresh extract (Figure 2). From the previous literature report [31], it is inferred that the presence of phenolic compounds and some of the flavonoids are present in the Ficus Carica. Only three peaks were observed from the HPLC chromatogram with a retention time of 3.5, 4.3, and 7.4 min for the fruit extract. This result reveals that three major components such as quercetin, luteolin and 6-O-acyl-β-D-glucosyl-β-sitosterols are present in the fruit extract in major proportion. All remaining compounds are present in very small quantity. A detail investigation on separation of the active molecules from the FC extract is under progress in our laboratory. The structures of compounds are given in Supplementary Information (Scheme S2).
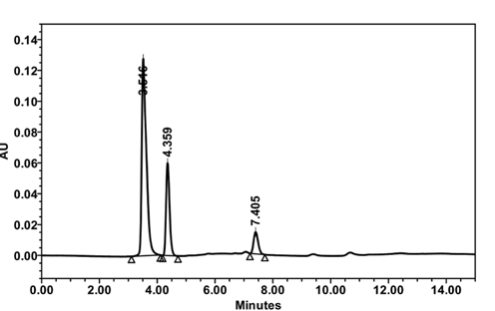
Figure 2 HPLC analysis of FC extract. Mobile phase: H2O:ACN (98:2, v/v) mixture ; Flow rate: 0.7 mL min−1; Injection volume: 20 μL; UV detector at 260 nm.
3.4 FTIR analysis
FTIR analysis was carried out to identify the possible interaction between the biomolecule and Ag+ during the biogenic reduction reactions. The FTIR data for the FC extract (a) and the AgNPs containing FC extract (b) are shown in Figure 3. The FC extract shows the bands at 3413, 2919, 1831, 1640, 1559, 1488, 1397, 1065, 672 and 612 cm−1. The band at 3413 cm−1 is assigned for O-H stretching vibration of alcohol and phenol compounds and the band at 2919 cm−1 is assigned to C-H stretching mode in alkanes. The IR bands observed at 1397, 1065 and 1831 cm−1 are due to the C-O stretching and C=O stretching mode of the carbonyl functional groups in alcohol, ethers, carboxylic acids and esters. The bands at 1640 and 1559 cm−1 were attributed to N-H bending vibration of amides. The bands at 1488 cm−1 is assigned to the C=C stretching mode in aromatic compounds. The peaks observed at 672 and 612 cm−1 are due to O-H bend (out of plane) and C-H bending respectively. The carbonyl bands at 1831 cm−1 was shifted to 1821 cm−1 during the formation of AgNPs. The shifts in bands at 1831, 1065 and 1397 cm−1 were clearly indicating the coordination of carboxylic acid with AgNPs. These results imply that flavonoids, proteins, sugars, and amino acids present in FC extract are play a major role on reduction of Ag+ and which also have strong ability to bind with AgNPs.
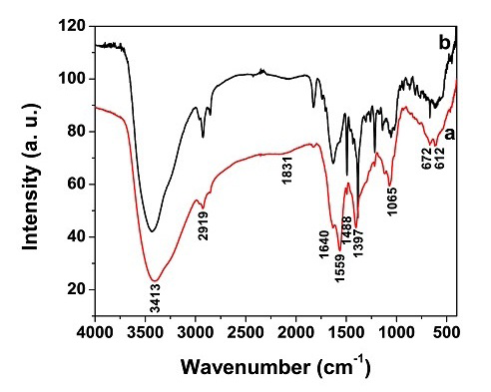
Figure 3 FTIR spectrum of (a) FC extract and (b) AgNPs synthesized from FC extract.
3.5 XRD analysis
The XRD pattern of AgNPs (Figure 4) shows the peaks of face centered cubic (fcc) structure at 40.5° (111), 46.3° (200), 67.4° (220) and 76.7° (311) of plane of index which is referred as JCPDS file No. 03-0921. It is important to know the exact nature of AgNPs formed and this can be deduced from the XRD spectrum of the sample. The slight shift in the peak positions indicated the presence of strain in the crystal structure which is a characteristic of nanocrystallites. The XRD pattern thus clearly illustrated that the AgNPs formed are crystalline in nature.
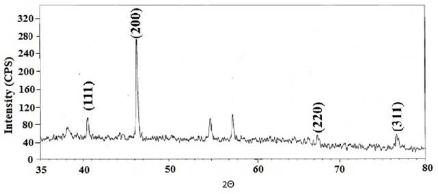
Figure 4 XRD pattern for AgNPs synthesized using FC extract.
3.6 FE-SEM and EDAX analysis
FE-SEM images of AgNPs synthesized by this method are shown in Figure 5. The FE-SEM images provided further insight on morphology and size details of AgNPs synthesized using FC extract. The FE-SEM micrograph revealed that the formation of polydispersive AgNPs with ranges from 20-25 nm in size and the most of the particles are spherical in nature. The elemental analysis of the AgNPs was performed using the EDAX based on selected area EDAX analysis. Figure 6 shows the EDAX spectrum of the AgNPs reduced with FC extract. Apart from silver peaks the presence of Mg, Ca, K and Cl ions are observed as major portion in FC extract.
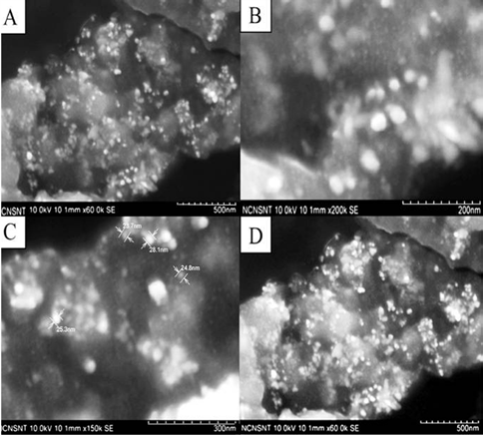
Figure 5 FE-SEM images of AgNPs synthesized using FC extract with different magnification; A) 500 nm; B) 200 nm; C) 300 nm; D) 500 nm.
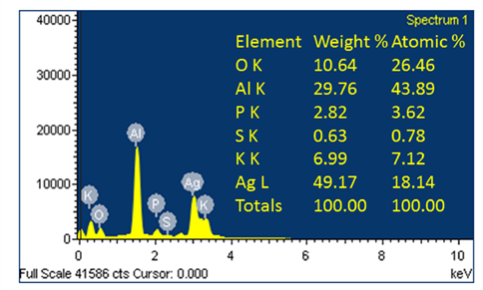
Figure 6 EDAX data for AgNPs synthesized using FC extract. (* indicates aluminum substrate peak, Al K.)
3.7 Antimicrobial analysis
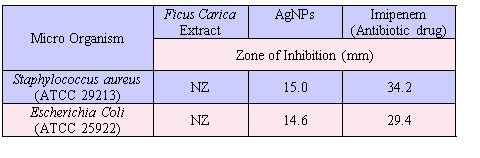
AgNPs synthesized by green route are found highly toxic against gram positive and gram negative pathogenic bacteria [32]. The result of the study presented in Figure 7 and the resulting zone of inhibition values are given in Table I. The FC extract was not shown any inhibition zone and thus its antimicrobial effect is not measurable whereas the FC containing AgNPs exhibit higher antimicrobial effect. The zone of inhibition of AgNPs was compared against the reference antibiotics drug (Imipenem). The present study clearly indicates that the FC extract stabilized AgNPs has excellent antimicrobial activity against gram positive organism of Staphylococcus Aureus and gram negative organism of Escherichia Coli.
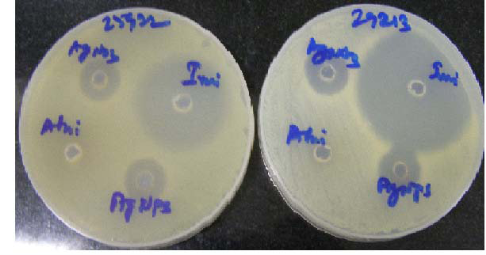
Figure 7 AgNPs show the zone of inhibition against (a) Staphylococcus Aureus (Left side) and (b) Escherichia Coli (Right side).
Table I Antimicrobial activity of fruit extract with AgNPs
4. Conclusion
We have demonstrated that the FC extract can be used for the synthesis of AgNPs at room temperature in a green approach. The major compounds (flavonoids) present in the FC extract were responsible for the reduction of Ag+ to AgNPs. The reduction rate of extract was investigated by measuring the increase in absorbance Plasmon peak intensity and the observed reaction kinetics is depends on the concentration of fruit extract and the duration of reaction interval. This simple procedure can be adopted for the biosynthesis of AgNPs in large scale in a biogenic origin. The main advantages of the proposed method are cost effective, rapid, facile, environmentally safe, and compatible for biomedical and pharmaceutical applications. From the FE-SEM analysis the size and shape of the AgNPs was analyzed. From the antimicrobial studies it is inferred that the AgNPs containing FC extract exhibit excellent bacteriostatic effect against microorganism.
Acknowledgements
One of the authors (K. L) thanks to Orchid chemicals and pharmaceuticals L.t.d., R&D centre, Chennai, providing the necessary infrastructure to carryout the research work, analytical and microbiological support.
References
Copyright:(c) 2012 K. Logaranjan et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.