Article
Biosynthesis of Silver Nanoparticles From Dictyota Bartayresiana Extract And Their Antifungal Activity
P. Senthil Kumar1, S. Sudha1*
1Molecular Diagnosis and Drug Discovery Laboratory, Department of Biotechnology, School of Life Sciences, Karpagam University, Coimbatore-640 021, Tamilnadu, India.
* Corresponding author: Sudha S (sudhasellappa@gmail.com)
Citation: P. Senthil Kumar, S. Sudha. Biosynthesis of Silver Nanoparticles From Dictyota Bartayresiana Extract And Their Antifungal Activity.Nano Biomed. Eng. 2013, 5(2), 72-75.
DOI: 10.5101/nbe.v5i2.p72-75.
The synthesis, characterization and application of biologically synthesized nanomaterials are an important aspect in nanotechnology. The present study deals with the synthesis of silver nanoparticles (AgNPs) using aqueous extract of brown seaweed Dictyota Bartayresiana as the reducing agent. The formation of AgNPs was confirmed by UV-Vis Spectroscopy, Scanning electron microscopy (SEM) and Transmission electron microscopy (TEM). The synthesized AgNPs was predominately crystalline in shape, polydispersed and ranged from 15 to 40 nm in size. Fourier Transform Infra-Red (FT-IR) spectroscopy analysis showed that the synthesized AgNPs was capped with bimolecular compounds which are responsible for reduction of silver ions. The antifungal effects of these AgNPs were studied against Humiclo insulans (MTCC 4520) and Fusarium dimerum (MTCC 6583). The present study indicates that AgNPs has considerable antifungal activity in comparison with other antifungal drugs.
Keywords: Dictyota Bartayresiana, Brown seaweed, Silver nanoparticles, Antifungal activity.
Recently, nanotechnology research is raising advanced technology from the past few decades, because of their appliances in the areas of physics, chemistry, biology and medicine. Commonly, metal nanoparticles are synthesized and stabilized by means of chemical methods such as chemical reduction [1-4], electrochemical techniques [5], photochemical reactions in reverse micelles [6] and nowadays via green chemistry [7]. Synthesis of nanoparticles through biological methods is a good, environment friendly and economically alternative method. Appliance of green chemistry to the synthesis of nanomaterials has an essential value in medicinal and scientific aspects [8,9]. Biologically synthesized silver nanoparticles (AgNPs) have wide range of applications because of their notable physical and chemical properties. There is a very limited data on the extra cellular biosynthesis of AgNPs using plants and their isloated pure compounds [10-12] and extracellular synthesis of AgNPs by using seaweed is very little [13]. In this study, we report on the biosynthesis of AgNPs by the reduction of aqueous Ag+ with methanolic extract of Dictyota bartayresiana at direct sunlight condition.The amount of nanoparticles synthesized and qualitative differences between synthesized nanoparticles were also investigated by a range of analytical methods. Dictyota bartayresiana J.V. Lamouroux (Class: Phaeo- phyceae, Order: Dictyotales, Family: Dictyotaceae) is an abundantly growing brown seaweed in coastals of south India. It is commonly occurs in inter tidal region of Gulf of Mannar Southeast coast of India. We have recently reported its antioxidant activity and antibacterial activity [14]. Keeping the biological perspectives in mind, the results reported here covers the biological synthesis of AgNPs and their antifungal activity.
In the present study, Dictyota bartayresiana J.V. Lamouroux a brown seaweed was collected from Mandapam coastal region (78°8’E, 9°17’N), Gulf of Mannar, Tamilnadu, South India. Samples were brought to laboratory in polythene bags and cleaned thoroughly with fresh water to remove adhering debris and associated biota. The algae were cleaned using brush for the removal of the epiphytes with distilled water. After cleaning, algae were dried in shade at room temperature for one week.
Silver nitrate (AgNO3) as analytical grade (AR) was purchased from E. Marck (India). In the typical synthesis of silver nanoparticles, 10 ml of the aqueous extract of Dictyota bartayresiana was added to 90 ml of 1 mM aqueous AgNO3 solution in 250 ml conical flask and kept at room temperature for 48 hours at 120 rpm. Suitable controls were maintained throughout the conduct of experiments.
The colour change in reaction mixture (metal ion solution + seaweed extract) was recorded through visual observation. The bio reduction of silver ions in aqueous solution was monitored by periodic sampling of aliquots (0.5 ml) and subsequently measuring UV-vis spectra of the solution. UV-vis spectra of these aliquots were monitored as a function of time of reaction on UV-Vis spectrophotometer UV-2450 (Shimadzu).
SEM analysis was done using Hitachi S-4500 SEM machine. Thin films of the sample were prepared on a carbon coated copper grid by just dropping a very small amount of the sample on the grid, extra solution was removed using a blotting paper and then the film on SEM grid were allowed to dry by putting it under a mercury lamp for 5 min.
The structural characterization of the AgNPs was carried out by TEM. The sample was prepared by air- drying drops of diluted solutions of the preparations on carbon films supported by copper grids.
To remove any free biomass residue or compound that is not the capping ligand of the nanoparticles, the residual solution of 100 ml after reaction was centrifuged at 5000 rpm for 10 min. The supernatant was again centrifuged at 10000 rpm for 60 min and the pellet was obtained. This is followed by redispersion of the pellet of AgNPs into 1 ml of deionized water. Thereafter, the purified suspension was freeze dried to obtain dried powder. Finally, the dried nanoparticles were analyzed by FTIR Nicolet Avatar 660 (Nicolet, USA).
2.8 Antifungal activity
The antifungal activity of AgNPs was evaluated against the following strains: Humiclo insulans (MTCC No. 4520) and Fusarium dimerum (MTCC No.6583). Cultures were maintained on potato dextrose agar (Hi Media, India) slants and they were subculture before use. The fungi studied were clinically important ones causing several infections and it is essential to overcome them through some active therapeutic agents. Agar well diffusion assay method was followed, which involves swabbing the cultures in pre-sterilized nutrient agar plates potato dextrose agar plates and three wells were cut in the same using sterile cork borer. Each well was loaded with 50 µl of solution in the following order: positive control, aqueous seaweed extract of Dictyota bartayresiana solution of AgNPs, and test sample methanolic extract. Then the sample loaded in potato dextrose agar plates were incubated at 37 ℃ for 48 hours. Then the formation of zone of inhibition was observed.
The formation of AgNPs by reduction of aqueous Ag+ during exposure to the aquous extract of Dictyota bartayresian showed reddish-brown colour, suggested the formation of AgNPs in solution which agreement with the statement reporting that AgNPs display reddish-brown in water [15]. These colours occur due to excitation of outer plasmon vibrations in the AgNPs [16]. This fundamental examination indicates the decline of Ag+ and biosynthesis of the AgNPs. The Formation of metal nanoparticles by reduction of the aqueous metal ions during exposure of Dictyota bartayresian extract was followed by UV-Vis spectroscopy. UV-Vis absorption spectrum of AgNPs in the presence of extract is shown in Fig. 1. The Surface plasmon band in the AgNPs solution remains close to 419 nm throughout the reaction period, suggesting that the AgNPs were dispersed in the aqueous solution with no evidence for aggregation of them in UV-Vis absorption spectrum. The SEM image (Fig. 2) showing the high density AgNPs synthesized by the D. bartayresiana further confirmed the development of silver nanostructures. The SEM micrographs of nanoparticle obtained in the filtrate showed that AgNPs are well distributed without any aggregation in solution. The synthesized AgNPs was 21 nm and seems to be spherical in morphology as shown in TEM image (Fig. 3). FT-IR analysis was used for the characterization of the extract and the resulting nanoparticles, absorption spectra of water soluble extract before and after reduction of Ag+. Absorbance bands seen at 3444.87 cm−1 and 2939.52 cm−1 were assigned to the stretching vibrations of primary and secondary amines respectively. These bands at 1955.82 cm−1 and 1643.35 cm−1 were known to be associated with the bending vibrations. In particular, 1535.34 cm−1 and 1400.32 cm−1 arises most probably from C=O stretching–β dike tone and CH3 and CH2 bending vibrations (Fig. 4). The result revealed that the capping ligand of the AgNPs may be an aromatic compound or alkanes or amines. Many studies have shown the antimicrobial effects of AgNPs [17-19]. However, only limited literatures supports the effects of AgNPs against fungal pathogens. Further the nanoparticles synthesis by green route by using Dictyota bartayresian extract was found highly toxic against clinically important fungal species at a concentration of 50 μl AgNPs shown high antifungal activity against Muco circinelloids and a transitional activity was shown against Fusarium dimerum (Fig. 5). Similarly our previous study proved that the AgNPs synthesized from Gelidiella acerosa as an effective antifungal agent against pathogenic fungal strains [20]. The inhibitory activities in culture media of AgNPs reported were comparable with standard antifungal agent Clotrimazole. The present study showed a simple, rapid and economical route to synthesize AgNPs from brown seaweeds.
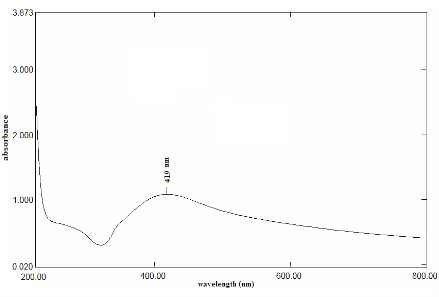
Fig. 1 UV-Vis absorption spectra of AgNPs synthesized by treating 1mM AgNO3 solution with extract of Dictyota bartayresiana after 48 hours.

Fig. 2 SEM micrograph of AgNPs synthesized by treating 1mM AgNO3 solution with extract of Dictyota bartayresiana after 48 hours.
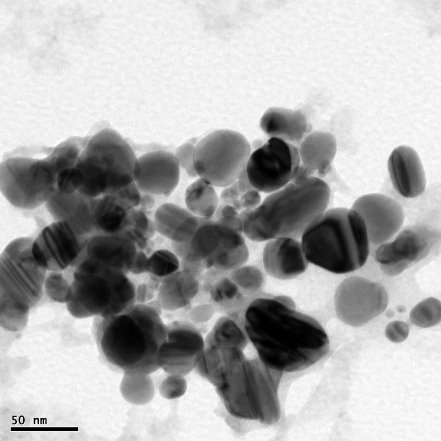
Fig. 3 TEM micrograph of AgNPs synthesized by treating 1mM AgNO3 solution with extract of Dictyota bartayresiana after 48 hours.
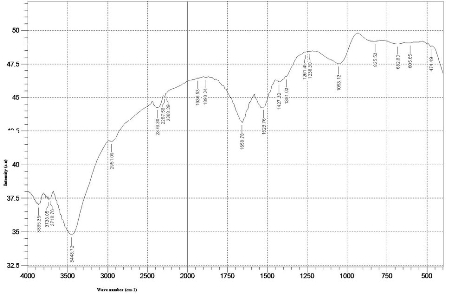
Fig. 4 FT-IR spectra of AgNPs synthesized by treating 1mM AgNO3 solution with extract of Dictyota bartayresiana after 48 hours.
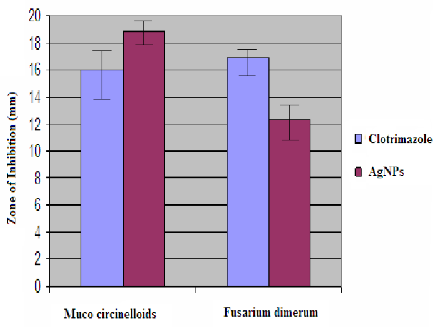
Fig. 5 Antifungal activity of AgNPs synthesized by reduction of AgNO3 with Dictyota bartayresiana extract against some selected fungal pathogens. Error bars refer to standard deviation by means of three replicates.
4 Conclusions
Quick and green synthesis demonstrates that the environmentally benevolent Dictyota bartayresiana can be used as an effective capping as well as reducing agent for synthesis of AgNPs. The spectroscopic characterizations from UV-Vis spectroscopy, FT-IR, SEM and TEM support the formation and stability of the biosynthesized AgNPs. Further, the above AgNPs revealed to possess an effective antifungal property against Muco circinelloids and Fusarium dimerum. The present study emphasizes the use of seaweeds for the synthesis of AgNPs with potent antimicrobial effect. Synthesis of metallic nanoparticles using green resources like Dictyota bartayresiana is a substitute to chemical synthesis, since this novel green synthesis is a pollutant free and eco-friendly synthetic route for the biosynthesis.
The authors are grateful to the authorities of Karpagam University, Coimbatore, Tamilnadu, India for providing facilities and for their encouragement. Authors also thank Dr. M. Ganesan, Scientist, CSMCRI- Marine Algal Research station, Mandapam camp, Tamilnadu, India for the species identification.The authors would like to acknowledge Department of Nono Science and Technology, Bharathiar University for the SEM analysis.
Copyright:(c) 2013 P. Senthil Kumar, S. Sudha. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.