Article
Crystallization of Amino Acids on a 21-well Circular PMMA Platform Using Metal-Assisted and Microwave-Accelerated Evaporative Crystallization
Anginelle M. Alabanza 1, 2, Muzaffer Mohammed 1, Kadir Aslan 1*
1 Morgan State University, Department of Chemistry, 1700 East Cold Spring Lane, Baltimore, MD, 21251, USA.
2 The College of New Jersey, Department of Chemistry, 2000 Pennington Road, Ewing, NJ, 08628, USA.
* Corresponding author: Kadir Aslan (Kadir.Aslan@morgan.edu)
Citation: A.M. Alabanza et al. Crystallization of Amino Acids on a 21-well Circular PMMA Platform using Metal-Assisted and Microwave- Accelerated Evaporative Crystallization. Nano Biomed. Eng. 2013, 5(4),147-154.
DOI: 10.5101/nbe.v5i4.p147-154.
Abstract
We describe the design and the use of a circular poly(methyl methacrylate) (PMMA) crystallization platform capable of processing 21 samples in Metal-Assisted and Microwave-Accelerated Evaporative Crystallization (MA-MAEC). The PMMA platforms were modified with silver nanoparticle films (SNFs) to generate a microwave-induced temperature gradient between the solvent and the SNFs due to the marked differences in their physical properties. Since amino acids only chemisorb on to silver on the PMMA platform, SNFs served as selective and heterogeneous nucleation sites for amino acids. Theoretical simulations for electric field and temperature distributions inside a microwave cavity equipped with a PMMA platform were carried out to determine the optimum experimental conditions, i.e., temperature variations and placement of the PMMA platform inside a microwave cavity. In addition, the actual temperature profiles of the amino acid solutions were monitored for the duration of the crystallization experiments carried out at room temperature and during microwave heating. The crystallization of five amino acids (L-threonine, L-histidine, L-leucine, L-serine and L-valine-HCl) at room temperature (control experiment) and using MA-MAEC were followed by optical microscopy. The induction time and crystal growth rates for all amino acids were determined. Using MA-MAEC, for all amino acids the induction times were significantly reduced (up to ~8-fold) and the crystal growth rates were increased (up to ~50-fold) as compared to room temperature crystallization, respectively. All crystals were characterized by Raman spectroscopy and powder x-ray diffraction, which demonstrated that the crystal structures of all amino acids grown at room temperature and using MA-MAEC were similar.
Keywords: Evaporative Crystallization, Crystallization, Amino Acids, Microwave-Induced Temperature Gradient, Microwave Heating, Silver Nanoparticle Films.
1 Introduction
Amino acids are manufactured by fermentation [1], protein hydrolysis [2], or chemical synthesis methods [3], and are widely used in industrial food [4], cosmetics [5] and pharmaceutical products [6]. Crystallization of amino acids, one of the important steps in their production process, has been the subject of numerous publications. In this regard, several crystallization methods, including concentration, neutralization [7], cooling [8], or evaporative crystallization have been employed [9]. At the laboratory bench scale, the crystallization of amino acids has been carried out using engineered surfaces, such as, nanoscale cylindrical pores [10], platforms for microdroplet solvent evaporation and self-assembled monolayers of alkane thiols on patterned surfaces or commercially available platforms. Microfluidic platforms were also used to screen for pharmaceutical salts and polymorphs of heat sensitive active pharmaceutical ingredients through antisolvent crystallization, as an alternative method [11-13]. However, evaporative crystallization based on the traditional room temperature crystallization method still takes hours to days to occur in laboratory bench scale [9,14,15]. Subsequently, there is still a need for crystallization platforms that offer rapidity and control over crystal size and morphology of amino acid crystals. In response to the aforementioned needs, the Aslan Research Group has described and demonstrated the use of Metal-Assisted and Microwave-Accelerated Evaporative Crystallization (MA-MAEC) for the rapid crystallization of several amino acids [9,13,16,17] and a pharmaceutical compound [18]. In MA-MAEC, the combined use of plasmonic nanostructures (i.e., silver nanoparticle films, SNFs) and microwave heating affords for the rapid crystallization of amino acids without altering the physical properties of the crystal. In this regard, the use of MA-MAEC technique was demonstrated for the rapid crystallization of glycine [9], L-arginine acetate [17], L-alanine [14] and acetaminophen [18]. The success of the MA-MAEC technique can be attributed to the presence of plasmonic nanostructures, which serve as selective and heterogeneous nucleation sites for the growth of amino acid crystals and a medium to generate a microwave-induced temperature gradient between the solvent and themselves [9]. In MA-MAEC, the microwave heating of amino acid solution results in the mass transfer of amino acids from the warmer solution to cooler plasmonic nanostructure surface. As the microwave heating continues, amino acids assemble onto SNFs, where heterogeneous nucleation and crystal growth occurs until the solvent is completely evaporated [9]. One can also harvest the amino acid crystals during solvent evaporation without the complete evaporation of solvent. In our previous works [19], we have demonstrated the use of a 16-well circular poly(methyl methacrylate) (PMMA) platform, which is designed specifically for use in MA-MAEC applications. The 16-well circular PMMA crystallization platform was designed to reduce the effects of temperature variations caused by microwaves by allowing the electric field component of the microwaves to flow around the circular surface rather than reflect them. Due to the small size of the circular PMMA crystallization platform (5 cm diameter) relative to the wavelength of the microwaves (~12.2 cm) employed, the interactions of the PMMA platforms with the heterogeneous electric field component of a conventional microwave oven was significantly reduced. The homogenous heating of the circular PMMA crystallization platform also afforded for high throughput small-scale production of L-alanine crystals. However, the 16-well version of the PMMA platform was constructed from commercially available silicon isolators that were designed for rectangular glass microscope slides. Although, the 16-well design was demonstrated to be effective in use for rapid crystallization of amino acids, the number and the actual design of the PMMA platform was limited by the shape of silicon isolators. Moreover, it was also shown that the optimum of microwave power level for amino acid crystallization using a commercially available microwave cavity was 1 (10% duty cycle) [19]. In this work, using theoretical calculations we have designed our own silicon isolator (not commercially available) with the following specifications: 1) affords for homogeneous temperature profile across its surface during microwave heating, 2) 21 well capacity based on symmetric placement on the surface, 3) one-piece, ready-to-use construction and 4) each well can hold up to 40 μl sample.Subsequently, the PMMA platform was constructed by combining a SNFs-deposited PMMA circular disk and the 21-well capacity silicon isolator. SNFs were deposited only to regions corresponding to the wells on the silicon isolator to ensure that SNFs are not destroyed during microwave heating. Theoretical calculations were carried out to predict the electric field and temperature distributions in a commercially available microwave equipped with our PMMA platform. In addition, the actual temperature variations of the amino acid solution during the crystallization experiments carried out at room temperature and using MA-MAEC were measured. Optical microscopy was employed to collect visual evidence for the crystallization of five amino acids (L-threonine, L-histidine, L-leucine, L-serine and L-valine-HCl), which were also used to determine the induction times and crystal growth rates for all amino acids. Raman spectroscopy and powder XRD methods were also used to further characterize and compare the crystals grown at room temperature and using MA- MAEC. These results demonstrated that our PMMA crystallization platform with 21-well capacity affords for reduced induction times (up to ~8-fold) and increased crystal growth rates (up to ~50-fold) as compared to room temperature crystallization, respectively.
2 Materials and Methods
2.1 Materials
L-Histidine, L-Leucine, L-Serine, L-Threonine, and L-Valine were purchased from Sigma-Aldrich (USA) and were used without further purification. Hydrochloric acid (HCl) was obtained from Sigma-Aldrich (USA). PMMA disks were attained from McMaster-Carr (USA). Silver target (57 mm diameter) was purchased from Electron Microscopy Sciences (USA). Silicon isolator wells were designed by the Aslan Research Group and produced by Grace BioLabs for Aslan Research Group (Oregon, USA).
2.2 Preparation of SNFs
SNFs were prepared as previously described [19]. First, PMMA discs were cleaned using a Harrick PDC32G Plasma Cleaner. A sputter coater (EMS, Model No: 150RS) was used subsequently to coat the PMMA disks with a ~1 nm thick layer of silver through a patterned mask. Silicon isolators were then applied to PMMA disks, matching silver-coated areas with well sites. SNFs deposited on PMMA platforms were used no later than one day after they were prepared. Blank PMMA platforms those were intended for control experiments followed the same procedure without applying silver to the disks.
2.3 COMSOL simulations and temperature measurements during microwave heating.
A 21-well PMMA platform model was designed using COMSOL software, and simulations were performed for a model microwave cavity of dimensions same as those of 900 W Frigidaire microwave cavity used in the crystallization experiments. Initial temperature of the amino acid solutions was set at 30-50°C. Duration of microwave heating was set at 3 sec and temperature profile of model was obtained and the predicted temperature of each well was obtained. Actual temperature of the samples inside the wells was measured using an infrared thermal gun during microwave heating up to 90 min.
2.4 Preparation of solutions
Aqueous solutions of amino acids were prepared separately. A saturated solution (pH=6.45) of L-Histidine (0.032 g/ml) and deionized water (Millipore, a minimum resistivity of 18.2 MΩ/cm) was heated to 40 ±2°C and used as is. Similarly, saturated aqueous solutions of L-Leucine (0.019 g/ml, pH= 6.60), L-Serine (0.440 g/ ml, pH=5.31), and L-Threonine (0.049 g/ml, pH=4.90) in deionized water were heated to 40, 50, and 35±2°C, respectively. L-Valine-HCl solution (pH= 3.13) was prepared by dissolving 0.069 g L-Valine in 1 ml water at 45°C, then adding 6.87 ml HCl in a 2:1 Val:HCl stoichiometric ratio at 50°C (final temperature of the mixture). No buffer was added to the saturated solutions and pH values were measured after the preparation of the saturated solutions.
2.5 Crystallization of amino acids
Solutions were crystallized on SNFs-deposited and blank PMMA platform, as described previously [19]. 20 µL of solutions were placed in the isolated wells. Blank PMMA platform were left undisturbed at room temperature for the duration of the control crystallization experiments. MA-MAEC-based crystallization experiments were carried out on the SNFs-deposited PMMA platforms in a 0.9 cubic feet, 900 W conventional microwave oven (Frigidaire Model No. FCM09Z03KB). Total evaporation time was recorded when the complete evaporation of solvents was achieved.
2.6 Characterization of crystals
Raman spectra for all crystals were obtained using a Raman spectrometer (i-Raman, BW&Tek). XRD data was collected with RigakuMiniFlex Optical images of crystals were recorded with a Swift Digital M10L monocular microscope. The size of the crystals was determined using the software (Motic Images Plus 2.0) for the microscope provided by vendor. Digital photographs were taken using an 8 MP digital camera.
3 Results and Discussion
Figure 1 shows the real-color photographs of SNFs-deposited and blank PMMA platforms. The PMMA platforms are also modified with single-piece silicon isolators (designedby the Aslan Research Group), which affords for the crystallization of 21 samples (up to 30 μl each well) simultaneously. The blank PMMA is optically clear (between 400-800 nm) and the presence of silver (only on selected areas corresponding to the position of the wells) can be observed visually as shown in the real- color photographs. Figure 1 also shows the absorption spectrum of 1 nm thick SNFs deposited on to PMMA platform. The absorption spectrum displays a major peak around 600 nm and decreases thereafter, which suggests that silver was deposited as a semi-continuous film. The semi-continuous nature of the SNFs is also evident from the dark blue-gray color of the silver film. It should be noted that SNFs were deposited on to PMMA platform in a homogeneous manner and any silvered area that displayed variance in color was not used in the crystallization experiments. Since our PMMA platforms are designed for use in MA-MAEC, the heating characteristics of our PMMA platform placed in a conventional microwave cavity using COMSOLTM software was investigated. It is thought that homogenous heating throughout the wells can be achieved during the entire heating cycle due to the small size and circular shape the current PMMA platform design. It was previously shown that the duty cycle of the conventional microwave oven used in this study was ~3 sec (Figure S1, Supporting Information) [19]. The duty cycle corresponds to: at power level 1 setting the full microwave power on for 3 sec, and is followed by ~30 sec of period of no microwave power. Subsequently, theoretical calculations were carried out for 3 sec of microwave heating for the following initial conditions for temperature: 30, 40 and 50°C, which corresponds to the initial temperature of the amino acid solutions used in this study. Figure 2 and S2 (Supporting Information) show the predicted temperature variation and electric field (z-component) distribution after 3 sec of microwave heating in a conventional microwave oven with the current design for the circular PMMA platform. The temperature variation between all 21 wells of the PMMA platform at different initial temperatures was predicted to differ by <0.2°C after 3 sec periods of microwave heating. In addition, the temperature of the first column of wells on the right side of the PMMA platform close to the microwave source (right-middle of the cavity) was predicted to increase in a similar fashion. This trend continued for the other column of wells, which implies that all wells were homogeneously heated in 3 sec. It is important to note that the PMMA platform is allowed rotate in the conventional microwave cavity for the duration of the crystallization experiments (24-90 min), and subsequently, all 21 wells were expected to have the same average temperature variation. The minimal variation in temperature between the wells also implies that circular PMMA platforms are appropriate for MA- MAEC technique [18]. Figure 2 also shows the predicted intensity of the z-component of the electric field. As expected from a conventional oven, z-component of the electric field is heterogeneous throughout the microwave cavity, which is alleviated by the rotation of the circular PMMA platforms during the microwave heating cycle. As described in the Introduction, the success of the MA-MAEC depends on the generation and maintenance of a microwave-induced temperature gradient between the bulk and SNFs. In this regard, we measured the actual temperature of amino acid solutions placed in the well of a PMMA platform at room temperature and during microwave heating as shown in Figure 3 and Figure S3 (Supporting Information). The temperature of the amino acid solutions (initially at 35, 40 and 50°C) placed on blank PMMA at room temperature rapidly decreased to 22°C within 10 min. This implies that the temperature gradient between the bulk and the surface of the PMMA platform is diminished in the first 10 min of the crystallization experiments carried out at room temperature. In MA-MAEC, the temperature of the amino acid solutions (initially at 35, 40 and 50°C) decreased at a slower rate within 10-20 min of microwave heating at power level 1 to: 28.5, 33 and 40°C, respectively. In addition, a >50% loss of solvent was observed only after 10 min, followed by a subsequent increase in the temperature of the amino acid solutions. The increase in the temperature of the amino acid solutions (>10 min) was more pronounced for the solution at initial temperature of 50°C, which can be attributed to the higher rate of heating than the cooling of the amino acid solutions. The higher rate of heating can also be attributed to the heating of the SNFs and PMMA by microwave heating as seen in Figure 3 and Figure S3 (Supporting Information). These results imply that microwave heating of amino acid solutions resulted in the generation and maintenance of temperature gradient between the bulk and SNFs over the duration of the crystallization experiments, and the temperature gradient plays an important role in achieving rapid crystallization of amino acids using MA-MAEC. In our previous publications related to the crystalliza- tion of amino acids [14,17], we have demonstrated that the total time needed for the complete evaporation of solvent decreases in the following order of experimental conditions: blank PMMA_RT (room temperature) >SNFs_RT>blank PMMA_microwave heating >SNFs_ MA-MAEC. Subsequently, for the sake of brevity and to demonstrate the most observable discrepancies in the solvent evaporation time for the crystallization of amino acids, only two experimental conditions have been selected for use in this study: blank PMMA_RT(for the longest evaporation time) and SNFs_MA-MAEC (for the shortest evaporation time). It is important to note that the use of SNFs_RT and blank PMMA_microwave heating will also reduce the solvent evaporation time. Five amino acids (L-leucine, L-histidine, L-serine, L-threonine, and L-valine-HCl) were crystallized from aqueous solutions, both at room temperature and using MA-MAEC and these results are summarized in Table 1. As shown in Table 1, the use of MA-MAEC reduces the evaporation time of the solvent up to ~9- fold (for L-serine) as compared to room temperature evaporation process. For example, L-leucine solution required an average of 203 min on blank PMMA at room temperature and required only ~45 min on SNFs using microwave heating. While L-leucine crystals grown at room temperature were observed to be only 58 - 555 µm in length, L-leucine crystals produced using MA-MAEC ranged from 78 to 699 µm. On average, the crystallization of L-serine took 218 and 24 min on blank PMMA at room temperature and on microwaved SNFs, respectively. The size of the L-serine crystals grown on blank PMMA at room temperature ranged between 476-1995 µm and between 195 and 926 µm on SNFs using MA- MAEC. Similar observations were made for L-threonine, L-histidine and L-valine-HCl as shown in Table 1. We note that the initial temperature of the amino acid solutions were adjusted to those values reported in literature and we did not attempt to investigate the effect of temperature on the crystallization of a single amino acid. As expected, the total crystallization (at the end of complete evaporation of solvent) time was decreased as the initial temperature of the solvent was increased. Subsequently, the crystals of all amino acids after complete evaporation of the solvent were characterized by optical microscopy and are shown in Figure 4. While the crystallization of L-leucine and L-threonine at room temperature and using MA-MAEC resulted in the growth of most identical crystals, the physical appearance of L-histidine, L-serine, and L-valine-HCl crystals seems to be slightly different in both crystallization conditions. Detailed descriptions of the optical microscope images for amino acids are given in the following paragraphs. Optical images (Figure 4) reveal that L-leucine crystals were optically transparent, thin and fragmented both on blank PMMA at room temperature and using MA-MAEC. L-leucine crystals were grown in a multilayer fashion, where single crystals were not easily identifiable. In addition, L-leucine crystals were noted to be very brittle during the recovery of these crystals for characterization studies. Using MA-MAEC, L-serine crystals were grown as single crystals of the α-form and covered most of the available PMMA surface in a monolayer fashion. At room temperature, larger L-serine crystals were grown in a multilayer fashion, which was attributed to the significant coverage of the available PMMA surface by large single L-serine crystals. L-serine crystals grown at room temperature also contained visually distinguishable ridges (i.e., microsteps) on the macrosteps of the crystals [20]. The microsteps were less visible in the L-serine crystals grown using MA-MAEC. Moreover, L-serine crystals grown at room temperature appeared to be thicker than those grown using MA-MAEC, though exact thickness were not measured. The observations of multi- layer formation, the presence of microsteps and growth of thicker L-serine crystals at room temperature on blank PMMA can be partially attributed to the lack of selective nucleation sites on blank PMMA for crystal growth. That is, after the random occurrence of nucleation on the blank PMMA, large L-serine crystals grow on those nucleation sites and cover significant extent of the available PMMA surface. Additional crystals grow on top of the existing crystals (and on the microsteps of the crystals) on the PMMA surface. In MA-MAEC, since L-serine molecules selectively chemisorb onto silver, multiple nucleation sites on SNFs were available for crystal growth. Subsequently, single L-serine crystals were grown throughout the PMMA surface using MA-MAEC. As shown in Figure 4, L-threonine crystals grown on blank PMMA at room temperature and using MA- MEAC visually appeared to be of the γ-form. At room temperature, optically opaque L-threonine polycrystals, originating from the same nucleation sites and the macrosteps on the previously formed crystals, were formed. Using MA-MAEC, L-threonine polycrystals were formed on different locations on the PMMA surface, which is attributed to the selective nucleation process due to chemisorption of L-threonine on silver surface. The growth of L-histidine crystals on blank PMMA surfaces at room temperature occurred similar to the growth of L-serine and L-threonine crystals. Only a few large and optically opaque L-threonine polycrystals with linear and planar defects were grown at room temperature. Conversely, using MA-MAEC, larger extent of L-threonine crystals were grown on the identical surface area. In addition, these crystals were smaller in size as compared to those grown at room temperature and appeared to be of the γ-form. Optical images of the L-valine crystals grown at room temperature show the presence of the α-form and slight imperfections near the edges of the crystals. Those grown using MA-MAEC had a mixture of the α-form and were optically transparent. The number of L-valine crystals grown using MA-MAEC was significantly higher than those grown at room temperature. Therefore, it is suggested that partial evaporation of solvent for the growth of L-valine crystals using MA-MAEC is a suitable technique for the rapid production of perfect crystals. In our previous publications on MA-MAEC [9,17,21] we have also demonstrated that the growth of amino acid crystals on blank glass slides and SNFs-deposited glass slides can be observed before the complete evaporation of the solvent using optical microscopy. In this study, we show that the amino acids were grown on the PMMA platform during the evaporation process and these crystals can be harvested when the crystals reach the desired size. Figure 5 shows the growth progression of a selected L-threonine crystal at room temperature on blank PMMA and SNFs using MA-MAEC. As observed from the optical microscope images, at room temperature L-threonine crystals appeared in the partially evaporated solvent in ~200 min, and continue to grow until the solvent completely evaporated in ~340 min. Using MA- MAEC, L-threonine crystals appeared as early as in ~60 min on SNFs, where the crystals were completely immersed in the solvent. The size of the initial L-threonine crystals grown using MA-MAEC was larger than those grown at room temperature. Subsequent to the observation that amino acid crystals can be grown during the evaporation of solvent at room temperature and microwave heating, we have tracked the size of amino acid crystals during the crystallization experiments as shown in Figure 6. For each amino acid, the size of only one crystal was tracked for the designated amount of time (that is, these plots are not average sizes of the particular amino acid). The exact size distribution of crystals of each amino acid are given in Figures S4-S5 (sample size n=30). In addition, we have calculated the crystal growth rate for each amino acid crystals as shown in Table 2. The crystal growth rate was calculated using the minimum and maximum size of each amino acid crystals measured between the initial crystallization time and complete evaporation time listed in Table 1. At room temperature, the shortest initial crystallization time (~60 min) was observed for L-serine and L-valine solutions, which had an initial temperature of 50°C. However, the observed L-serine crystal had the fastest growth rate (0.4- 27 nm/min) and largest size (~1995 nm) after complete evaporation of the solvent. The initial crystallization time for other amino acids from solutions at initial temperature <50°C, was further extended up to ~238 min. Using MA-MAEC, the crystal growth rate for each amino acid increased significantly as compared to room temperature evaporation. An increase in crystal growth rate up to ~50- fold was observed for L-serine, which can be attributed to 1) the increased solvent evaporation time (for example ~9-fold for L-serine) the increased mass transfer rate (not measured) due to microwave-induced temperature gradient and 3) selective nucleation of amino acid crystals on SNFs. In addition, the initial crystallization time was also significantly reduced between 2.5- to 8-fold (~ 8-fold, L-leucine). These observations imply that the use of MA- MAEC not only reduces the crystallization time, but also affords for the growth of amino crystals of larger size at a higher rate without the need for complete evaporation of solvent. All amino acid crystals were characterized using Raman spectroscopy (Figures 7, S6-S9 in Supporting Information) and powder XRD (Figures 8, S10-S13 in Supporting Information) to determine the differences in the crystals grown at room temperature and using MA- MAEC. In Figure 7, the Raman spectra for L-threonine crystals grown at both room temperature and microwave heatingare shown. The Raman spectra are almost identical to each other, which imply the molecular structure L-threonine crystals grown using both techniques are similar. The same observation was made for L-leucine, L-serine, L-histidine, and L-valine-HCl crystals as shown in Figures S6-S9 (Supporting Information). These observations are in agreement with our previous publications [14,17], where we have reported that the crystals of other amino acids were structurally similar when grown at room temperature and using MA-MAEC. Although Raman spectroscopy is a useful tool for the characterization of amino acid crystals, powder XRD studies provide direct evidence for the differences in the crystal structures. The powder XRD patterns and corresponding faces of L-threonine crystals grown at room temperature and using microwave heating(Figure 8) show that the crystal structure for L-threonine were indistinguishable which confirms the visual evidence provided by optical microscopy (Figures 4 and 5). Figures S8-S11 (Supporting Information) show that the XRD patterns of other amino acids grownat room temperature and using MA-MAEC are identical and were comparable to the results obtained by other research groups [22- 24]. Our research group is currently working on the crystallization of amino acid mixtures to control crystal morphology and size using MA-MAEC. These results will be reported in due course.
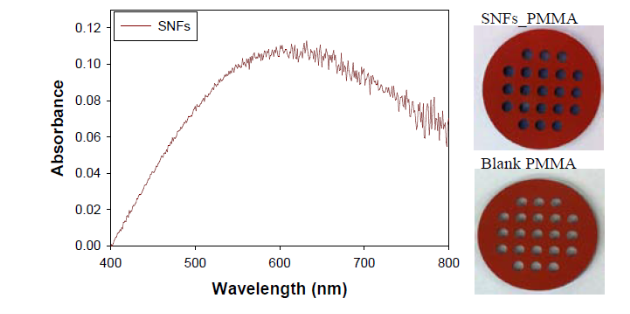
Fig. 1 Absorbance spectra of SNFs-deposited and blank PMMA crystallization platforms. Blank PMMA is used as background. Real- color photographs of the SNFs-deposited and blank PMMA platforms.
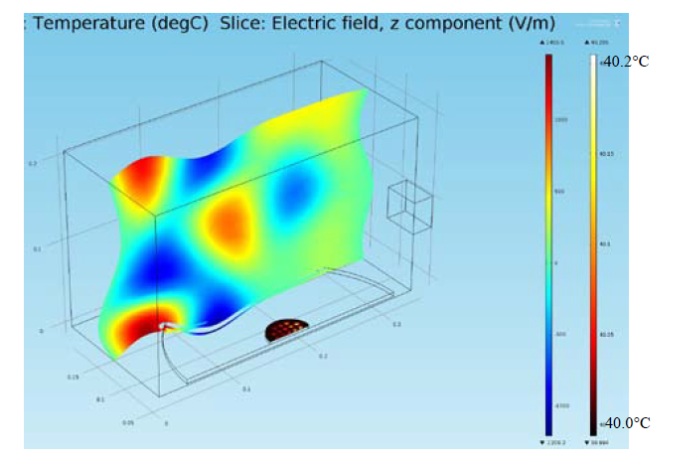
Fig. 2 Theoretical calculation of heating pattern in a conventional microwave oven containing 21-well PMMA platform after 3 sec. Initial temperature (T, right bar) of the solution inside wells was 40°C and increased to 40.2°C after 3 sec of microwave heating. Z-component of the electric field (Ez: V/m) is also shown (left bar).
Table 1. Summary of crystallization time results.
|
Amino Acid Initial Temp |
Complete Evaporation Time (min) |
*Size Range (μm) |
||
|
PMMA_RT |
MA-MAEC |
PMMA_RT |
MA-MAEC |
|
|
L-Threonine 35°C |
397 ± 1 |
86 ± 8 |
110-1516 |
280-1728 |
|
L-Histidine 40°C |
308 ± 3 |
80 ± 6 |
250-1664 |
235-1261 |
|
L-Leucine 40°C |
203 ± 3 |
45 ± 2 |
58-555 |
78-699 |
|
L-Serine 50°C |
218 ± 1 |
24 ± 4 |
476-1995 |
195-926 |
|
L-Valine-HCl 50°C |
201 ± 1 |
29 ± 3 |
228-1529 |
256-1322 |
*Please see Figures S3-S4 (Supporting Information) for crystal size distributions for all 5 amino acids.
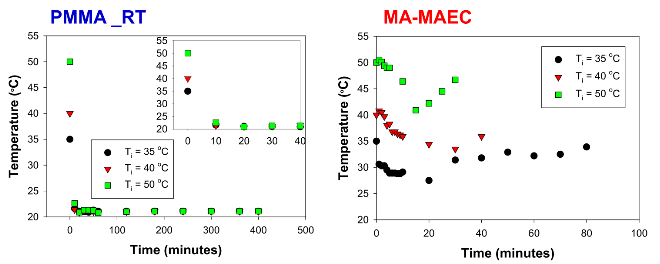
Fig. 3 Observed temperature profile of a water drop on the SNFs-deposited PMMA platform microwave heated in a 900 watt microwave cavity at power level 1. In MA-MAEC, the arrows indicate the time of significant loss (>50%) of solvent due to evaporation. During this period, the rate of cooling for solutions were: 0.33, 0.35 and 0.67 °C / min for solutions initially at 35, 40 and 50 °C, respectively.
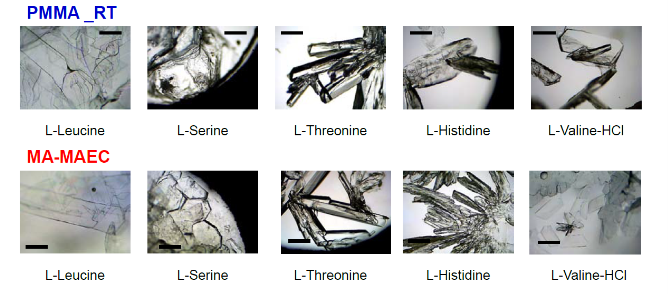
Fig. 4 Optical images of amino acid crystals formed using room temperature evaporation and MA-MAEC. The solvent in each sample was completely evaporated. Scale bar = 500 μm.
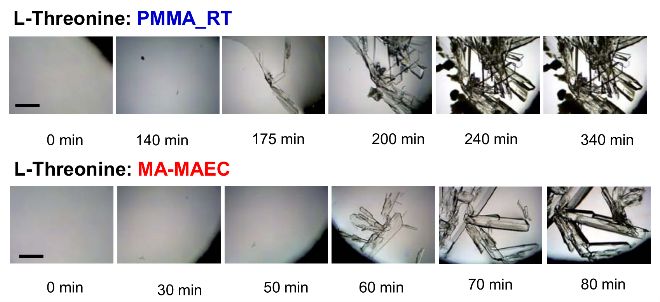
Fig. 5 Growth progression of L-Threonine crystals at room temperature on blank PMMA (Top) and on SNFs-deposited PMMA (Bottom). Scale bar = 500 μm
Table 2 Summary of crystallization time results
|
Amino Acid |
Initial Crystallization Time (min)* |
Crystal Growth Rate (Minimum) Maximum (μm / min) |
||
|
PMMA_RT |
MA-MAEC |
PMMA_RT |
MA-MAEC |
|
|
L-Threonine |
160 |
60 |
(2.0) 8.2 |
(10) 94 |
|
L-Histidine |
238 |
60 |
(0.2)14 |
(5.0)99 |
|
L-Leucine |
80 |
10 |
(2.5) 4.4 |
(8.0) 13 |
|
L-Serine |
60 |
20 |
(0.4)27 |
(20)79 |
|
L-Valine-HCl |
60 |
12 |
(1.1) 5.2 |
(2.4) 26 |
* Initial crystallization time refers to the time that initial amino acid crystals appeared on the surface. The minimum and maximum size of each amino acid crystals were measured between the initial crystallization time and complete evaporation time listed in Table 1.
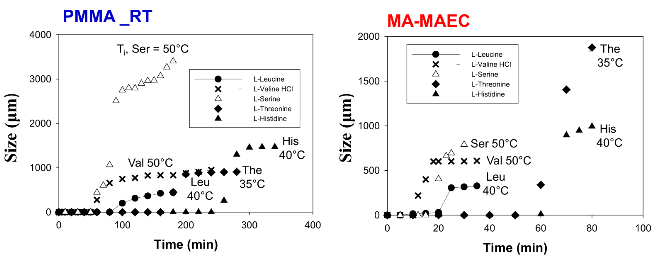
Fig. 6 Typical growth progression of amino acid crystals at room temperature on PMMA at room temperature and in a microwave cavity on SNFs-deposited PMMA. *Crystallization time refers to the time that initial amino acid crystals appeared on the surface. Initial temperature (Ti) of each amino acid solution is also shown
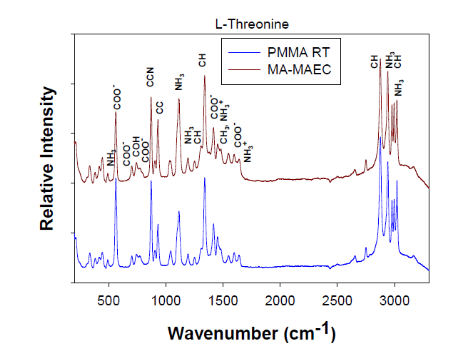
Fig. 7 Raman spectra and peak assignments of L-Threonine crystals grown on blank PMMA at room temperature and using MA-MAEC
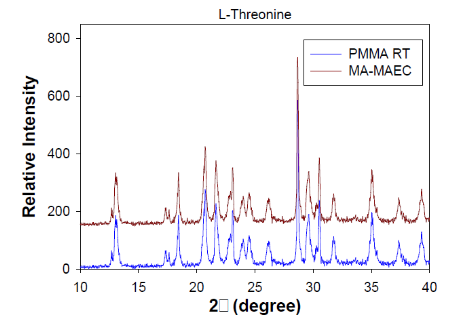
Fig. 8 XRD patterns of L-Threonine crystals grown at room temperature on blank PMMA and in the microwave on SNFs. Miller indices of corresponding peaks are labeled above peaks.
4 Conclusions
The use of a 21-well PMMA platform for the MA- MAEC-based crystallization of a variety of amino acids with wide range of chemical properties was demonstrated. It was shown that the use of MA-MAEC significantly reduced the crystallization time in addition to affording for the growth of well-developed amino acid crystals. Temperature profile of the amino acid solutions during the crystallization process was measured, which showed that a temperature gradient is maintained during the microwave heating of amino acids solutions. On the other hand, the initial temperature gradient at room temperature based crystallization experiments was diminished very rapidly. The observation of reduction of crystallization time was partially attributed to the maintenance of the microwave-induced temperature gradient for a longer period of time. All amino acid crystals were characterized by optical microscopy, which afforded the determination of the induction time and the calculation of the crystal growth rates. Using MA-MAEC, for all amino acids the induction times were significantly reduced (up to ~8-fold) and the crystal growth rates were increased (up to ~50- fold), respectively. Raman spectroscopy and powder XRD studies of the amino acids crystals grown using MA- MAEC proved that their crystal structures are identical to those grown at room temperature.
Acknowledgements
The project described was supported by Maryland Innovation Initiative (Phase 1) Award from Technology Development Corporation (TEDCO). Additional support was provided by Award Number 5-K25EB007565-05 from the National Institute of Biomedical Imaging and Bioengineering. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Biomedical Imaging and Bioengineering or the National Institutes of Health.
Appendix A. Supplementary data
Supplementary data associated with this article can be found in the online version at the link at:
http://nanobe.org/Assets/userfiles/sys_eb538c1c-65ff-4e82-8e6a-a1ef01127fed/files/5%284%29_p140-147SI.pdf
References
Copyright: (c) 2013 A.M. Alabanza et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.