Research Article
Codelivery of Nanosized Curcumin and Bioenhancer Using Acid Degradable Polymeric Nanoparticles Displayed Enhanced Anticancer Efficacy
Moorthi Chidambaram *, Kathiresan Krishnasamy
Department of Pharmacy, Annamalai University, Annamalai Nagar - 608 002, Chidambaram, Tamil Nadu, India.
* Corresponding author. E-mail: cmoorthitgodu@gmail.com
Received: Jan. 6, 2014; Accepted: Apr. 29, 2014; Published: June 15, 2014.
Citation: Moorthi Chidambaram and Kathiresan Krishnasamy. Codelivery of Nanosized Curcumin and Bioenhancer using Acid Degradable Polymeric Nanoparticles Displayed Enhanced Anticancer Efficacy. Nano Biomed. Eng. 2014, 6(2), 47-59.
DOI: 10.5101/nbe.v6i2.p47-59.
Abstract
Curcumin, a functional food polyphenol reported to inhibit cancer cell proliferation, invasion, angiogenesis and metastasis through interaction with multiple molecular targets. However, the clinical usefulness of curcumin in the treatment of cancer is limited due to poor solubility in water at acidic and neutral pH, hydrolytic degradation in alkaline pH and metabolism in the liver and intestine, resulting in decreased or absence of therapeutic efficacy. Hence, the present study was aimed to overcome the limitations of curcumin in the treatment of cancer by codelivery of nanosized curcumin and bioenhancer using acid degradable polymeric nanoparticles. Modified nanoprecipitation method was used to prepare void, curcumin-piperine, curcumin-quercetin and curcumin-silibinin encapsulated polymeric nanoparticles. Prepared nanoformulations were evaluated for particle size, polydispersity index, zeta potential, surface morphology, drug content, encapsulation efficiency, drug loading, in- vitro release, stability at elevated storage conditions, toxicity on normal liver cells, anticancer activity on various cancer cell lines and on cancer induced rats. Prepared curcumin-bioenhancer encapsulated polymeric nanoparticles were (a) spherical in shape with size <100 nm and displayed excellent uniformity; (b) showed >95% release of curcumin and bioenhancers within 45 minutes in gastric fluid; (c) proved non-toxic to normal liver cells; (d) extremely stable at elevated storage conditions; and (e) demonstrated enhanced anticancer activity against various cancer cell lines and mammary cancer in rats than the pure curcumin. Study concludes that the prepared curcumin-bioenhancer encapsulated polymeric nanoformulations significantly overcome the limitations of curcumin in the treatment of cancer and synergistically enhance its anticancer activity. However, out of three polymeric nanoformulations, curcumin-silibinin polymeric nanoformulation showed superior anticancer activity.
Keywords: Curcumin; Piperine; Polymeric Nanoparticle; Quercetin; Silibinin
Introduction
Chemotherapy is the major remedial approach for the treatment of localized and metastasized cancer but suffers several limitations including poor aqueous solubility, severesy stemictoxicities, and drug resistance. Of all limitations, systemic toxicities pose a major concern [1, 2]. Hence, anticancer functional foods (i.e. biologically active compound) which are devoid of systemic toxicities at the therapeutic dose seem to be better alternative [3]. Curcumin (Fig. 1) is one such functional food, isolated from dried rhizome of turmeric (Curcuma longa Linn). [4, 5]. More than 3000 studies over last 60 years have revealed the multiple cellular targets of curcumin, which are responsible for various pharmacological ctivities including anticancer. Various cell based assays have reported that curcumin inhibit cancer cell proliferation, invasion, angiogenesis and metastasis through interaction with multiple molecular targets and also downregulates the expression of P-glycoprotein, multi-drug resistance protein and breast cancer resistance protein [6-9]. Moreover, curcumin has not shown any toxicity in humans up to 12000 mg/day and has been declared as ‘generally regarded as safe’ by United States Food and Drug Administration [10, 11]. However, the clinical efficacy of curcumin in the treatment of cancer is limited due to limited solubility, hydrolytic degradation, and metabolism via glucuronidation and sulfation leading to reduce the oral bioavailability resulting in decreased or absence of therapeutic efficacy of curcumin [12, 13]. Various pharmaceutical approaches have been tried to overcome the aqueous solubility of curcumin, which have shown significant improvement in oral bioavailability of curcumin. However, there were no reports of nanoparticulate drug delivery system that overcome the metabolism of curcumin. Bioenhancer such as piperine, quercetin and silibinin (Fig. 1) plays a significant role in preventing/minimizing the metabolism of curcumin in liver and intestine. Utilizing these bioenhancers along with curcumin as a dual drug loaded nanoparticles are hypothesized to overcome all the limitations of curcumin such as poor aqueous solubility, hydrolytic degradation, intestinal metabolism, hepatic metabolism and synergistically enhance the anticancer activity of curcumin [14-18]. Hence, the present study was aimed to overcome the limitations of curcumin in the treatment of cancer by codelivery of nanosized curcumin and bioenhancer using acid degradable polymeric nanoparticles.
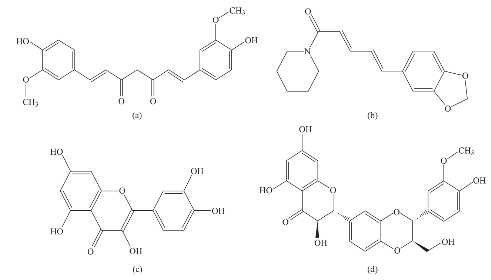
Fig. 1 Illustrate the chemical structure of (a) Curcumin, (b) Piperine, (c) Quercetin and (4) Silibinin.
Experimental section
Material
Curcumin, Piperine, Quercetin, Silibinin, Poloxamer 188, Potassium Bromide, 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide, Sulphorhodamine B, and 7,12-Dimethylbenz(a) anthracene were obtained from Sigma Aldrich, India. Dulbecco’s modified eagle medium, Fetal bovine serum and β-cyclodextrin were obtained from Himedia, India. Poly(butyl methacrylate-co-(2-dimethylaminoethyl) methacrylate-co-methyl methacrylate) polymer was obtained from Degussa, India. Methanol, Propanol and Tris Base were obtained from Merck, India. Ethanol was obtained from Brampton, Canada. Uranyl Acetate was obtained from Electron Microscopy Sciences, India.
Preparation of void and curcumin-bioenhancer encapsulated acid degradable polymeric nanoparticles
Void , curcumin-pipe rine (Cur-Pi ), curcumin - quercetin (Cur-Qu) and curcumin-silibinin (Cur- Si) encapsulated poly(butyl methacrylate-co-(2- dimethylaminoethyl) methacrylate-co-methyl methacrylate) nanoparticles were prepared using nanoprecipitation method with slight modifications [19-22]. Briefly, about 250 mg of poly(butyl methacrylate-co-(2-dimethylaminoethyl) methacrylate- co-methyl methacrylate) polymer with or without 50 mg of various curcumin-bioenhancer combinations [i.e. (25 mg curcumin and 25 mg of piperine for Cur-Pi); (25 mg curcumin and 25 mg of quercetin for Cur-Qu); and (25 mg of curcumin and 25 mg of silibinin for Cur-Si)] were dissolved in 16 mL of 60% ethanol, which was transferred at once into 500 mL beaker containing 49 mL of distilled water, 97 mg of Poloxamer 188 and 54 mg of β-cyclodextrin under mechanical stirring (RQT-124A, Remi) at 520 rpm. Polymeric nanoparticles were formed spontaneously but the stirring process was continued for 1 hour and 20 minutes to aid the size reduction and to evaporate the residual solvent. Prepared polymeric nanoformulations were stored at room temperature for one month to identify any aggregation and post-formulation degradation.
Particle size, polydispersity index and zeta potential analysis
Prepared void and curcumin-bioenhancer encapsulated polymeric nanoformulations were diluted appropriately using ultra pure water (Milli-Q Academic, Milli-Pore). Diluted samples were loaded separately in a disposable zeta cell and measured the average particle size, polydispersity index (PDI) and zeta potential using Zetasizer (ZEN3600, Malvern).
Particle surface morphology analysis
Prepared void and curcumin-bioenhancer encapsulated polymeric nanoformulations were dropped onto Formvar-coated copper grids and air dried. The samples were then negatively stained with 1% uranyl acetate for 10 minutes and air dried again. The samples were then imaged using transmission electron microscope (H-7500, Hitachi).
Drug content estimation
Prepared curcumin-bioenhancer encapsulated polymeric nanoformulations were mixed with equal volume of methanol and sonicated (Ultrasonic cleaner, Lark) for 5 minutes followed by filtration through 0.22 µm membrane. Samples were analyzed in triplicate using the developed high performance liquid chromatographic (HPLC) methods [23-25] and the values were expressed as WTotal.
Encapsulation efficiency and drug loading estimation
Prepared curcumin-bioenhancer encapsulated polymeric nanoformulations were centrifuged using a cooling centrifuge (C-24, Remi) for 45 minutes at 19,000 rpm at -20°C and the supernatant was separated. To 1 mL of supernatant, an equal volume of methanol was added and sonicated (Ultrasonic cleaner, Lark) for 5 minutes followed by filtration through 0.22 µm membrane. Samples were analyzed in triplicate using the developed HPLC methods [23-25] and expressed as Wfree. Encapsulation efficiency (EE) was calculated using formula EE (%) = [(Wtotal - Wfree) / (Wtotal)] X 100. Drug loading (DL) was calculated using formula DL (%) = [(Wtotal - Wfree) / (Wpolymer)] X 100.
Fourier transform-infrared (FT-IR) spectral analysis
Prepared void and curcumin-bioenhancer encapsulated polymeric nanoformulations were loaded in FT-IR grade cell and scanned at moderate scanning speed between 4000-400 cm-1 using FT-IR Spectrometer (Nicolet iS5, Thermo Scientific). The generated signals were monitored and integrated using inbuilt Thermo Scientific OMNIC software.
In-vitro release kinetics study
Prepared curcumin-bioenhancer encapsulated polymeric nanoformulations (weight equivalent to 10 mg of drug) were introduced into 900 mL of simulated gastric fluid (pH 1.2). The medium was maintained at 37±0.5ºC at a rotation speed of 100 rpm. About 5 mL of samples were withdrawn at 5, 10, 20, 30, 45 and 60 minutes, which were replaced with an equal volume of temperature-equilibrated blank media. The samples were centrifuged using cooling centrifuge (C-24, Remi) for 5 minutes at 19,000 rpm at -20°C. The supernatant was separated and filtered using 0.22 µm membrane. The sample was analyzed using developed HPLC methods [23-25].
In-vitro toxicity of void polymeric nanoparticles
Prepared void polymeric nanoformulation was evaluated for cytotoxicity using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay on BRL3A rat liver cells (normal rat liver cells) [26, 27]. Briefly, prepared void polymeric nanoformulation and paclitaxel (positive control) was diluted with Dul be c c o’s m odi fi e d E a gl e m e di um (DME M) su p p l e m e n t e d wi t h 2 % i n a c t i v a t e d f e t a l b o v i n e serum (FBS) to obtain a stock solution of 5 mg/mL concentration, which was sterilized by filtration and finally centrifuged. Serial dilutions (1000, 500, 250, 125, 62.5 µg/mL) were made from the stock solution. About 0.1 mL of the diluted BRL3A cell suspension (approximately 10,000 cells) was added to each well of the 96-well plate. After 24 hours, when a partial monolayer was formed, the supernatant was flicked off, washed the monolayer once with medium and 100 µL of different concentrations of test drugs were added. The plates were then incubated at 37°C for 3 days in 5% CO2 atmosphere and microscopic examination was performed after every 24 hours interval. After 72 hours, the test drug solutions in the wells were discarded and 50 µL of MTT in phosphate buffered saline was added to each well. The plates were gently shaken and incubated for 3 hours at 37°C in 5% CO2 atmosphere. The supernatant was removed and 100 µL of propanol was added and the plates were gently shaken to solubilize the formed formazan. The absorbance was measured using a microplate reader (ELx800, Bio-Tek) at a wavelength of 540 nm. The percentage growth inhibition was calculated using formula GI (%) = [100 – (Mean optical density of test) / (Mean optical density of control)] X 100 and concentration of test drug needed to inhibit cell growth by 50% (CTC50) was generated from the dose-response curves. The experiments were performed in triplicate.
In-vivo acute and 28-day repeated dose oral toxicity study of curcumin-bioenhancer encapsulated polymeric nanoparticles
Animal experiments were performed as per the protocol approved by the Institutional Animal Ethics Committee (160/1999/CPCSEA; Proposal Number 896). Healthy adult female Wistar albino rats (nulliparous; non-pregnant; weighing 250-350 gram) were housed in polypropylene cages layered with husk and maintained in a controlled room at a temperature (i.e. 22±3°C) and light (i.e. 12 hours light/dark cycle). Animals were allowed free access to water and standard pellet diet. In-vivo acute oral toxicity study was performed to evaluate the toxicities of single dose administration of prepared void and curcumin-bioenhancer encapsulated polymeric nanoformulations. Animals were randomly assigned to 5 groups, each containing 5 animals. Animals were withheld of food overnight prior to dosing and 3-4 hours after dosing but not the water. Following the period of fasting, animals were weighed and treated orally as follows (a) Animals in group 1 received water; (b) Animals in group 2 received void polymeric nanoformulation; (c) Animals in group 3 received curcumin-piperine encapsulated polymeric nanoformulation; (d) Animals in group 4 received curcumin-quercetin encapsulated polymeric nanoformulation; and (e) Animals in group 5 received curcumin-silibinin encapsulated polymeric nanoformulation. One animal in each group was gavaged once with respective doses (i.e. equivalent to 2000 mg/kg of body weight) and observed 48 hours for any death. Since there were no death, remaining four animals in each group were gavaged once with respective doses. Animals were observed individually after dosing at least once during the first 30 minutes, periodically during the first 24 hours, with special attention given during the first 4 hours and daily thereafter, for a total of 14 days for clinical signs of toxicity or mortality. Daily observations include changes in skin, fur, eyes, mucus membrane (nasal), respiratory rate, circulatory signs (heart rate), autonomic effects (salivation, lacrimation, piloerection, urinary incontinence and defecation) and central nervous system (drowsiness, gait, tremors and convulsion). The 28-day repeated dose oral toxicity study was performed to evaluate the toxicities of continuous administration of the prepared void and curcumin- bioenhancer encapsulated polymeric nanoformulations. Animals were randomly assigned to 5 groups, each containing 5 animals. Animals were weighed and treated orally as follows (a) Animals in group 1 received water; (b) Animals in group 2 received void polymeric nanoformulation; (c) Animals in group 3 received curcumin-piperine encapsulated polymeric nanoformulation; (d) Animals in group 4 received curcumin-quercetin encapsulated polymeric nanoformulation; and (e) Animals in group 5 received curcumin-silibinin encapsulated polymeric nanoformulation. The animals were gavaged with respective doses (equivalent to 100 mg/kg of body wei ght ) for a peri od of 28 da ys. Ani m a l s we re observed periodically for changes in skin, fur, eyes, mucus membrane (nasal), respiratory rate, circulatory signs (heart rate), autonomic effects (salivation, lacrimation, perspiration, piloerection, urinary incontinence and defecation), central nervous system (drowsiness, gait, tremors and convulsion), body weight, food consumption and mortality for a total period of 28 days. At the end of the treatment period, the animals were bled from the retro orbital sinus for clinical pathology assessment, which included analysis of various haematology parameters and blood biochemistry parameters. Consequently the animals were sacrificed by cervical dislocation and necropsied to facilitate gross pathological examination of vital organs such as brain, liver, kidney and heart.
In-vitro anticancer activity of curcumin-bioenhancer encapsulated polymeric nanoparticles using MTT assay
Prepared curcumin-bioenhancer encapsulated polymeric nanoformulations were evaluated for anticancer activity using MTT assay on human breast adenocarcinoma (MCF-7) cells [26, 28]. Briefly, pure curcumin, prepared curcumin-bioenhancer e n c a p su l a t e d p o l ym e r i c n a n of o r m ul a t i o ns a n d paclitaxel (positive control) were diluted with DMEM supplemented with 2% inactivated FBS to obtain a stock solution of 5 mg/mL concentration, which was sterilized by filtration and finally centrifuged. Serial dilutions were made from the stock solution. About 0.1 mL of the MCF-7 cell suspension (approximately 10,000 cells) was added to each well of the 96-well plate. After 24 hours, when a partial monolayer was formed, the supernatant was flicked off, washed the monolayer once with medium and 100 µL of different concentrations of samples were added. The plates were then incubated at 37°C for 3 days in 5% CO2 atmosphere and microscopic examination was performed after every 24 hours interval. After 72 hours, the samples solutions in the wells were discarded and 50 µL of MTT in phosphate buffered saline was added to each well. The plates were gently shaken and incubated for 3 hours at 37°C in 5% CO2 atmosphere. The supernatant was removed and 100 µL of propanol was added and the plates were gently shaken to solubilize the formed formazan. The absorbance was measured using a microplate reader (ELx800, Bio-Tek) at a wavelength of 540 nm. The percentage growth inhibition was calculated using formula GI (%) = [100 - (Mean optical density of test) / (Mean optical density of control)] X 100 and the concentration of sample needed to inhibit cell growth by 50% (CTC50) was generated from the dose-response curves. The experiments were performed in triplicate.
In-vitro anticancer activity of curcumin-bioenhancer encapsulated polymeric nanoparticles using SRB assay
Prepared curcumin-bioenhancer encapsulated polymeric nanoformulations were evaluated for anticancer activity using Sulforhodamine B (SRB) assay on human ovarian cancer cell (Ovkar-3), human leukaemia cell (HL60), human hepatoma cell (HEPG2), human cervix cancer cell (HeLa), human colon cancer cell (Colo205) and human lung cancer cell (A549) [29]. Briefly, pure curcumin, prepared curcumin- bioenhancer encapsulated polymeric nanoformulations and Adriamycin (i.e. Doxorubicin, a positive control) was diluted with DMEM supplemented with 2% inactivated FBS to obtain a stock solution of 5 mg/ mL concentration, which was sterilized by filtration and finally centrifuged. Serial dilutions (10, 20, 40, 80 µg/mL) were made from the stock solution. About 0.1 mL of the diluted cell suspension (approximately 10,000 cells) was added to each well of the 96-well plate. After 24 hours, when a partial monolayer was formed, the supernatant was flicked off, washed the monolayer once with medium and 100 µL of different concentrations of test drugs were added. The plates were then incubated at 37°C for 3 days in 5% CO2 atmosphere and microscopic examination was performed after every 24 hours interval. The cells were fixed using ice-cold trichloroacetic acid (TCA) for 1 hour at 4°C and then washed using distilled water to remove excess TCA and allowed to dry in the air. After 72 hours, the drug solutions in the wells were discarded and 50 µL of SRB solution was added to each well and allowed to stain at room temperature for 30 minutes. The plate was washed with 1% v/v acetic acid to remove unbound dye and allowed to dry in the air. About 100 µL of 10 mM unbuffered Tris Base (pH 10.5) was added to each well and the plates were gently shaken for 5 minutes on a shaker platform to extract the bound SRB. The absorbance was measured using a microplate reader (ELx800, Bio-Tek) at a wavelength of 492 nm. Parameters such as GI50 (Concentration of the drug that produces 50% inhibition of the cells), TGI (Concentration of the drug that produces total inhibition of the cells) and LC50 (Concentration of the drug that kills 50% of the cells) were calculated. The experiments were performed in triplicate.
In-vivo anticancer activity of curcumin-bioenhancer encapsulated polymeric nanoparticles
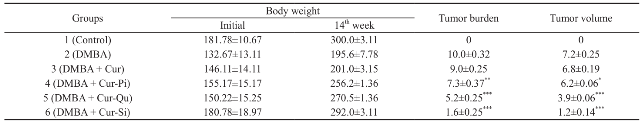
Prepared curcumin-bioenhancer encapsulated polymeric nanoparticles were evaluated for anticancer activity using cancer induced rat. The animal experiment was performed as per the protocol approved by the Institutional Animal Ethics Committee (160/1999/CPCSEA; Proposal Number 896). Briefly, healthy adult female Sprague dawley rats (120-200 gram body weight) were procured and randomly assigned to 6 groups, each containing 6 animals in polypropylene cages layered with husk and maintained in a controlled room at a temperature (22±3°C) and light (12 hours light/dark cycle). Animals were allowed free access to water and standard pellet diet. The 7,12-Dimethylbenz(a)anthracene (DMBA) solution was freshly prepared at a dose of 25 mg/kg in 0.75 ml of sunflower oil and 0.25 ml of physiological saline. Animals (except animals in control group) received a single dose of 25 mg/kg of DMBA through intraperitoneal route and were allowed to develop tumor, which was identified by palpitation and tumor induced animals were selected for 60 days treatment through oral route. Animals in group 1 and 2 received no other treatment, animals in group 3 received pure curcumin (100 mg/kg of body weight), animals in group 4 received curcumin-piperine encapsulated polymeric nanoformulation (equivalent to 100 mg/kg of body weight), animals in group 5 received curcumin- quercetin encapsulated polymeric nanoformulation (equivalent to 100 mg/kg of body weight); and animals in group 6 received curcumin-silibinin encapsulated polymeric nanoformulation (equivalent to 100 mg/ kg of body weight). Anticancer efficacy of the test samples were assessed using tumerogenesis parameter such as body weight, tumor burden and tumor volume.
Statistical analysis
Statistical analysis was performed using SPSS 18.0 (SPSS, Inc., Chicago, IL) statistical package. Data were expressed as mean±standard deviation. One way analysis of variance (ANOVA) followed by Duncan multiple comparison method was used to correlate the difference between the variables. Data were considered statistically significant if P value was < 0.001, < 0.01 and < 0.05.
Stability study
Prepared void and curcumin-bioenhancer encapsulated polymeric nanoparticles were packed in a clear glass vial and stored at 40±2°C and 75±5% relative humidity in a stability chamber (SS134, Shivani Scientific) for 6 months. At the end of storage period, samples were analysed for average particles size, polydispersity index, zeta potential and drug content. The experiments were performed in triplicate.
Result and Discussion
Preparation of void and curcumin-bioenhancer encapsulated polymeric nanoparticles
Void and curcumin-bioenhancer encapsulated polymeric nanoparticles were prepared using modified nanoprecipitation method. Prepared polymeric nanoformulations were stored at room temperature for one month but there were no visual aggregation and post-formulation degradation.
Particles size, polydispersity index and zeta potential analysis
Void polymeric nanoparticles have shown an average particle size of 64.42 nm with PDI of 0.234 and zeta potential of 29.3 mV. However, encapsulation of Cur-Pi and Cur-Qu has increased the average particle size (86.87 nm and 71.03 nm), increased the polydispersity index (0.239 and 0.281) and altered the zeta potential (22.2 mV and 38.8 mV). Curcumin and piperine possess cationic charge on its structure and after encapsulation these phytochemicals are accommodated in the polymeric matrix and expected to repel each which would have increased the size. Similarly, curcumin possess strong cationic charge and quercetin possess week anionic charge on its structure and after encapsulation these phytochemicals are accommodated in the polym eric matrix and expected to attract each other. Hence, curcumin- quercetin combination has produced lesser particle size than the curcumin-piperine combination. Whereas, encapsulation of Cur-Si has decreased the average particle size (46.95 nm), decreased the zeta potential (26.6 mV) and decreased the PDI (0.142). Curcumin possess strong cationic charge and silibinin possess strong anionic charge on its structure and after encapsulation these phytochemicals are accommodated in the polymeric matrix and expected to attract each other strongly, which might have caused significant size reduction than void, curcumin-quercetin & curcumin-piperine combination. Particles size spectrum of prepared curcumin-bioenhancer encapsulated polymeric nanoformulations was displayed in Fig. 2. Prepared polymeric nanoparticles (Void, Cur-Pi, Cur- Qu, Cur-Si) displayed significant size reduction with excellent uniformity (i.e. <100 nm and <0.282 PDI) in comparison to pure curcumin (i.e. 38.617 µm and 0.551 PDI).
Particle surface morphology analysis
Prepared void and curcumin-bioenhancer encapsulated polymeric nanoparticles were spherical in shape. Hence, curcumin and bioenhancers encapsulated in the polymer matrix will also be in spherical shape and expected to enhance the basic function, release of from the polymer matrix, transport of in the body and internalization by many folds than the pure curcumin and bioenhancers.
Drug content, encapsulation efficiency and drug loading estimation
The results of drug content, encapsulation efficiency and drug loading were summarized in Table 1. Drug content in all curcumin-bioenhancer encapsulated polymeric nanoformulations was within ±2% from the actual quantities, which shows that there was no significant post-formulation drug degradation or drug loss. However, encapsulation efficiency of the drug in all curcumin-bioenhancer encapsulated polymeric nanoformulations was greater than 74% but less than 98.5, which shows that major volume of drugs were encapsulated in the polymer matrix and only negligible quantity of curcumin and bioenhancer were available as a free drug. Whereas, drug loading in all curcumin- bioenhancer encapsulated polymeric nanoformulations was greater than 7% but less than 9.9%, which shows that the polymer added to the formulation was optimal.
FT-IR spectral analysis
![]()
![]() Void polymeric nanoparticles showed characteristic absorption bands at (a) 2924 cm-1 (CH3 stretch, dimethylamino groups); (b) 1737 cm-1 (C=O stretch); (c) 1479, 1467, 1451 and 1383 cm-1 (CHX stretch); (d) 1119 cm-1 (C-N stretch); and (e) 1020 cm-1 (CH3 rocking). The obtained spectrum was comparable with poly(butyl methacrylate-co-(2-dimethylaminoethyl) methacrylate-co-methyl methacrylate) spectrum. Hence, formation of poly(butyl methacrylate-co- (2-dimethylaminoethyl) methacrylate-co-methyl methacrylate) nanoparticles was confirmed. Similarly, Cur-Pi, Cur-Qu and Cur-Si encapsulated polymeric nanoparticles also demons trated characteristic absorption bands between (a) 2923 to 2924 cm-1 (CH3 stretch, dimethylamino groups); (b) 1736 to 1737 cm-1 (C=O stretch); (c) 1478 to 1479, 1461 to 1468, 1450 to 1451 and 1350 to 1383 cm-1 (CHX stretch); (d) 1106 to 1121 cm-1 (C-N stretch); and (e) 1020 to 1030 cm-1 (CH3 rocking), which confirm the formation of poly(butyl methacrylate-co-(2-dimethylamino-ethyl) methacrylate-co-methyl methacrylate) nanoparticles and encapsulation of curcumin and bioenhancers in the polymeric nanoparticles. FT-IR spectrum of Cur-Pi encapsulated polymeric nanoparticles were displayed in Fig. 3.
Void polymeric nanoparticles showed characteristic absorption bands at (a) 2924 cm-1 (CH3 stretch, dimethylamino groups); (b) 1737 cm-1 (C=O stretch); (c) 1479, 1467, 1451 and 1383 cm-1 (CHX stretch); (d) 1119 cm-1 (C-N stretch); and (e) 1020 cm-1 (CH3 rocking). The obtained spectrum was comparable with poly(butyl methacrylate-co-(2-dimethylaminoethyl) methacrylate-co-methyl methacrylate) spectrum. Hence, formation of poly(butyl methacrylate-co- (2-dimethylaminoethyl) methacrylate-co-methyl methacrylate) nanoparticles was confirmed. Similarly, Cur-Pi, Cur-Qu and Cur-Si encapsulated polymeric nanoparticles also demons trated characteristic absorption bands between (a) 2923 to 2924 cm-1 (CH3 stretch, dimethylamino groups); (b) 1736 to 1737 cm-1 (C=O stretch); (c) 1478 to 1479, 1461 to 1468, 1450 to 1451 and 1350 to 1383 cm-1 (CHX stretch); (d) 1106 to 1121 cm-1 (C-N stretch); and (e) 1020 to 1030 cm-1 (CH3 rocking), which confirm the formation of poly(butyl methacrylate-co-(2-dimethylamino-ethyl) methacrylate-co-methyl methacrylate) nanoparticles and encapsulation of curcumin and bioenhancers in the polymeric nanoparticles. FT-IR spectrum of Cur-Pi encapsulated polymeric nanoparticles were displayed in Fig. 3.
In-vitro release kinetics study
Prepared curcumin-bioenhancer encapsulated polymeric nanoparticles showed >40% release of drugs within 5 minutes, >60% release of drugs within 10 minutes, >85% release of drugs within 20 minutes, >92% release of drugs within 30 minutes and >95% release of drugs within 45 minutes (Table 2 and Fig. 4). Released nanosized curcumin and bioenhancers displayed enhanced aqueous solubility in gastric fluid. Hence, the movement of undissolved curcumin to the intestine will be prevented; thereby hydrolytic degradation of curcumin in the intestine will also be prevented.
In-vitro toxicity of void polymeric nanoformulation
Positive control paclitaxel displayed CTC50 of 31.25 µg/mL on BRL3A cells. However, at the same concentrations prepared void polymeric nanoformulation has shown CTC50 >1000 µg/mL (Table 3). Hence, prepared void polymeric nanoformulation does not induce any significant toxicity on normal liver cells.
In-vivo acute and 28-day repeated dose oral toxicity study of curcumin-bioenhancer encapsulated polymeric nanoformulations
In the acute oral toxicity study, the animals in various treatment groups were observed individually for significant changes in skin, fur, eyes, mucus membrane (nasal), respiratory rate, circulatory signs (heart rate), autonomic effects (salivation, lacrimation, piloerection, urinary incontinence and defecation) and central nervous system (drowsiness, gait, tremors and convulsion. However, none of the animal in the treatment groups has shown significant changes (Table 1 in supplementary file). In the 28-day repeated dose oral toxicity study, no animal mortalities were observed in any of the study groups. Animals in various treatment groups were observed individually for significant changes in skin, fur, eyes, mucus membrane (nasal), respiratory ra t e , c i r c ul a t o ry si g n s ( he a r t ra t e ), a u t o no m i c effects (salivation, lacrimation, piloerection, urinary incontinence and defecation) and central nervous system (drowsiness, gait, tremors and convulsion. However, none of the animal in the treatment groups has shown significant changes and no significant differences (P>0.05) were observed in body weights (Table 2 in supplementary file) or food consumption (Table 3 in supplementary file) of the animals of the treatment groups when compared with that of the control groups. Similarly, haematology parameters (Table 4 and Table 5 in supplementary file) and blood biochemistry parameters (Table 6 and Table 7 in supplementary file) of the animals in various groups were within normal limits. On completion of the treatment, animals were sacrificed, necropsied and pathological examination of vital organs such as liver, heart, kidney and brain were performed and result showed that cells were within normal histopathological limits. These observations preclude any effects of the polymeric nanoformulations on the general systemic well-being of the animals.
In-vitro anticancer activity of curcumin-bioenhancer encapsulated polymeric nanoformulations using MTT assay
Pure curcumin, paclitaxel and curcumin-bioenhancer encapsulated polymeric nanoformulations were study for its in-vitro anticancer efficacy against human breast adenocarcinoma (MCF-7) cells using MTT assay and the results were summarized in Table 4. Curcumin-bioenhancer encapsulated polymeric nanoformulations displayed lower CTC50 than the pure curcumin. However, none of the curcumin- bioenhancer encapsulated polymeric nanoformulations were comparable with positive control paclitaxel. Out of three curcumin-bioenhancer encapsulated polymeric nanoformulations, Cur-Si encapsulated polymeric nanoformulation displayed significant cytotoxicity on MCF-7 cells.
In-vitro anticancer activity of curcumin-bioenhancer encapsulated polymeric nanoformulations using SRB assay
Prepared curcumin-bioenhancer encapsulated polymeric nanoformulations were evaluated for anticancer activity using SRB assay on Ovkar-3, HL60, HEPG2, HeLa, Colo205 and A549 and the results are summarized in Table 5. Cur-Qu nanoformulation displayed comparable anticancer activity on Ovkar-3, HL60, HEPG2, HeLa, Colo205 and A549 in comparison with Adriamycin. Similarly, Cur-Pi nanoformulation displayed comparable anticancer activity on HEPG2, HeLa and Colo205 in comparison with Adriamycin. Likewise, Cur-Si nanoformulation displayed comparable anticancer activity on HeLa in comparison with Adriamycin. However, prepared c urc um i n-bi o e n ha nc e r e nc a p sul a t e d po l ym e r i c nanoformulations (i.e. Cur-Pi, Cur-Qu and Cur- Si) displayed enhanced anticancer activity against Ovkar-3, HL60, HEPG2, HeLa, Colo205 and A549 than the pure curcumin.
In-vivo anticancer activity of curcumin-bioenhancer encapsulated polymeric nanoformulations
Prepared curcumin-bioenhancer encapsulated polymeric nanoformulations were evaluated for anticancer activity using cancer induced rat and the results are summarized in Table 6. Prepared Cur- Pi, Cur-Qu and Cur-Si nanoformulations displayed e nha nc e d a nt i c a nc e r a c t i vi t y a ga i nst m a m m a ry cancer in comparison with pure curcumin. However, out of three nanoformulations, Cur-Si polymeric nanoformulation showed significant anticancer activity. Enhancement of anticancer property of prepared Curcumin-Piperine, Curcumin-Quercetin and Curcumin-Silibinin encapsulated polymeric nanoformulations than the pure curcumin might be due to (a) natural bioenhancers (Piperine, Quercetin and Silibinin) might have synergistically enhanced the anticancer activity of curcumin; (b) natural bioenhancer piperine, quercetin and silibinin might have suppressed the drug metabolizing enzyme cytochrome P450 3A, hepatic & intestinal glucuronidation and sulfation of the curcumin; (c) natural bioenhancer piperine, quercetin and silibinin along with curcumin might have reversed the multidrug resistance by modulating ATP-binding cassette transporter proteins such P-gp, MDRP1, MDRP2 and BCRP and enhanced anticancer activity in multidrug resistant human ovarian cancer (Ovkar3) cells; (d) nanosizing not only reduces the size but also increase the surface area, which in turn increased the aqueous solubility of the drug, which might have increased the anticancer activity of the prepared polymeric nanoformulation; (e) prepared curcumin-piperine, curcumin-quercetin, curcumin- silibinin encapsulated poly(butyl methacrylate-co- (2-dimethylamino ethyl) methacrylate-co-methyl methacrylate polymeric nanoparticles released nanosized curcumin and natural bioenhancers in gastric fluid, which might have increased the aqueous solubility and permeability. Hence, the movement of undissolved curcumin to the intestine might have prevented, thereby hydrolytic degradation of curcumin in the intestine might have prevented; (f) released nanosized curcumin and natural bioenhancers might have targeted the cancer cells by passive targeting mechanism via enhanced permeability and retention (EPR) effect and thereby enhance the anticancer activity.
Stability study
Prepared void and curcumin-bioenhancer encapsulated polymeric nanoformulations were subjected to stability studies at 40±2°C and 75±5% relative humidity for 6 months (Table 8 in supplementary file). However, prepared curcumin- bioenhancer encapsulated polymeric nanoformulations showed insignificant change in average particles size, polydispersity index, zeta potential and drug content.
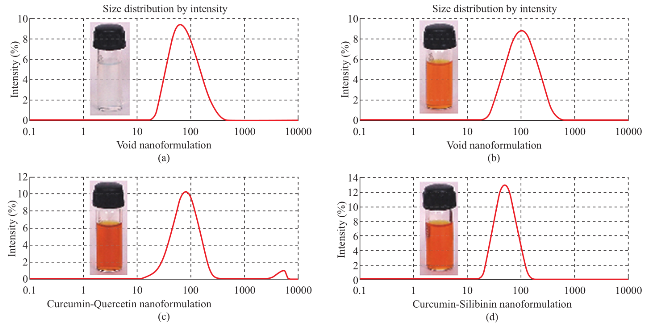
Fig. 2 Illustrate the prepared curcumin-bioenhancer encapsulated polymeric nanoformulations (a) Void polymeric nanoformulation, (b) Curcumin-Piperine polymeric nanoformulation, (c) Curcumin-Quercetin polymeric nanoformulation and (d) Curcumin-Silibinin polymeric nanoformulation.
Table 1: Drug content, encapsulation efficiency and drug loading of the prepared curcumin-bioenhancer encapsulated polymeric nanoformulations
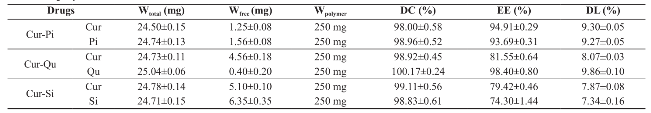
Cur: Curcumin; Pi: Piperine; Qu: Quercetin; Si: Silibinin; Wtotal: Amount of drug in the formulation; Wfree: Amount of drug in the supernatant; Wpolymer: Amount of polymer in the formulation; DC: Drug content; EE: Encapsulation efficiency; DL: Drug loading
Table 2: Cumulative release of curcumin and bioenhancer from the nanoparticles
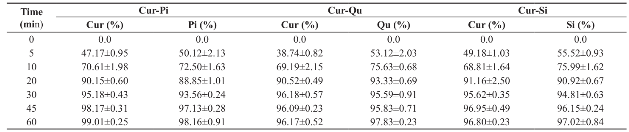
Table 3: In-vitro toxicity of void polymeric nanoformulation on BRL3A cell line

@P<0.01 as compare to Paclitaxel
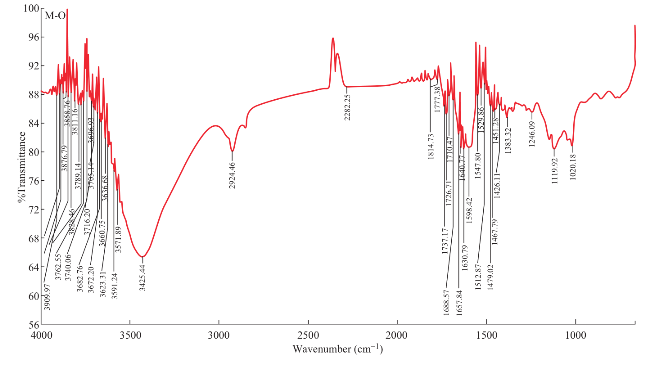
Fig. 3 Illustrate the FT-IR spectrum of prepared Cur-Pi encapsulated polymeric nanoparticle.
Table 4: Anticancer activity of curcumin and curcumin-bioenhancer encapsulated polymeric nanoformulations using MTT assay
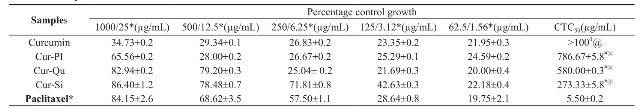

Fig. 4 In-vitro drug release of curcumin and bio-enhancer from the nanoparticles.
Table 5: In-vitro anticancer activity of curcumin-bioenhancer encapsulated polymeric nanoformulations using SRB assay
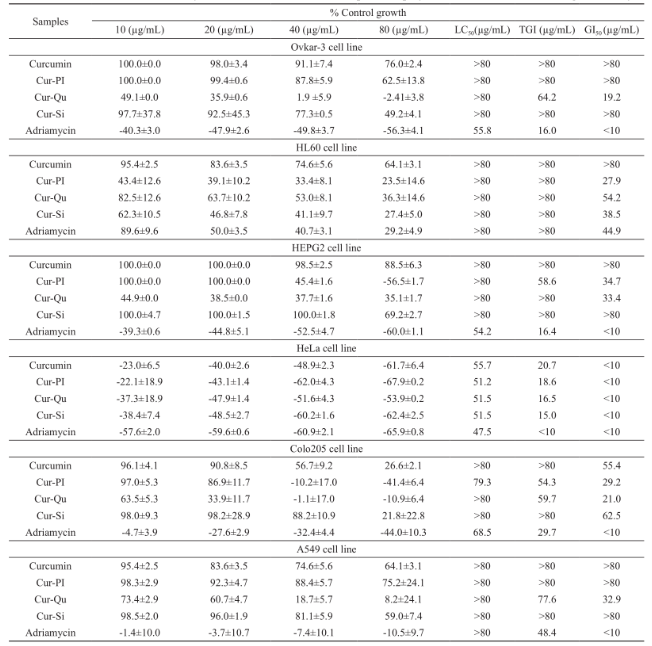
Table 6: In-vivo anticancer activity of curcumin-bioenhancer nanoformulations
Conclusions
Curcumin-Piperine, Curcumin-Quercetin and Curcumin-S ilibinin encaps ulated poly(butyl methacrylate-co-(2-dimethylaminoethyl) methacrylate- co-methyl methacrylate) nanoformula-tions were successfully prepared using modified nanoprecipitation method. Prepared curcumin-bioenhancer encapsulated polymeric nanoparticles were spherical in shape and displayed significant size reduction with excellent uniformity, which in turn results in >50 fold increase in the aqueous solubility than the pure curcumin. Prepared polymeric nanoformulations showed >95% release of drugs within 45 minutes in gastric fluid. Prepared void polymeric nanoformulation does not induce any significant toxicity on normal liver cells. Pre pa re d po l ym e ri c na nof orm u l a t i on di spl a y e d enhanced anticancer activity against various cancer cell lines and mammary cancer in rats than the pure curcumin. However, out of three nanoformulations, curcumin-silibinin polymeric nanoformulation showed significant anticancer activity.
Acknowledgments
C. Moorthi is thankful to UGC, Government of India, for providing UGC-BSR fellowship.
Appendix A. Supplementary data
Supplementary data associated with this article can be found in the online version at the link at: http://www.nanobe.org/index. php?journal=nbe&page=rt&op=suppFiles&path[]=284&pa th[]=0
References
[1] Moorthi C., Manavalan R., Kathiresan K., Nanotherapeutics to overcome conventional cancer chemotherapy limitations. J. Pharm. Pharm. Sci. 2011; 14: 67-77.
[2] Zhou Y, Wang X. Study on synergistic effect of new function-alized Ag nanoparticles for intracellular drug uptake in cancer cells. Nano Biomed. Eng. 2010, 2(4), 208-213.
[3] Guangchang P., Junbo X., Qingsen C., Zhihe H., How functional foods play critical roles in human health. Food Science and Human Wellness. 2012; 1(1): 26-60.
[4] Aggarwal B.B., Sundaram C., Malani N., Ichikawa H., Curcumin: the Indian solid gold. Adv. Exp. Med. Biol. 2007; 595: 1-75.
[5] Moort hi C., Kat hire san K., Fa bri ca ti on of hi ghl y stable sonication assisted curcumin nanocrystals by nanoprecipitation method. Drug Invention Today. 2013; 5: 66-69.
[6] Aggarwal B.B., Harikumar K.B., Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int. J. Biochem. Cell Biol. 2009; 41(1): 40-59.
[7] Moorthi C., Senthil kumar C., Mohan S., Kathiresan K., SLS / βCD-curcumin nanosuspension: Preparation, characterization and pharmacological evaluation. J. Pharm. Res. 2013; 7: 219-223.
[8] Moorthi C., Kathiresan K., Application of Plackett- Burman factorial design in the development of curcumin loaded Eudragit E 100 nanoparticles. Nano Biomed. Eng. 2013; 5(1): 28-33.
[9] Moorthi C., Senthil kumar C., Mohan S., Kathiresan K., Anionic surfactant based topical curcumin n a n osu sp e n si o n: f a b r i c a t i o n, c h a ra c t e r i z a t i o n a nd evaluation. Nano Biomed. Eng. 2013, 5(2): 86-89.
[10] Cheng A.L., Hsu C.H., Lin J.K., Hsu M.M., Ho Y.F., Shen T.S., Ko J.Y., Lin J.T., Lin B.R., Ming-Shiang W., Yu H.S., Jee S.H., Chen G.S., Chen T.M., Chen C.A., Lai M.K., Pu Y.S., Pan M.H., Wang Y.J., Tsai C.C., Hsieh C.Y., Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001; 21(4B): 2895-2900.
[11] Moorthi C., Kiran K., Manavalan R., Kathiresan K., Preparation and characterization of curcumin-piperine dual drug loaded nanoparticles. Asian Pac. J. Trop. Biomed. 2012; 2(11): 841-848.
[12] Rachmawati H., Al Shaal L., Müller R.H., Keck C.M., Development of curcumin nanocrystal: physical aspects. J. Pharm. Sci. 2013; 102(1): 204-214.
[13] Anand P., Kunnumakkara A.B., Newman R.A., Aggarwal B.B., Bioavailability of curcumin: problems and promises. Mol. Pharm. 2007; 4(6): 807-818.
[14] Yallapu M.M., Ebeling M.C., Khan S., Sundram V., Chauhan N., Gupta B.K., Puumala S.E., Jaggi M., Chauhan S.C., Novel curcumin-loaded magnetic nanoparticles for pancreatic cancer treatment. Mol. Cancer Ther. 2013; 12: 1471-1480.
[15] Alam S., Panda J.J., Chauhan V.S., Novel dipeptide nanoparticles for effective curcumin delivery. Int. J. nanomedicine. 2012; 7: 4207-4222.
[16] Moorthi C., Kathiresan K., Curcumin-Piperine / Curcumin-Quercetin / Curcumin-Silibinin dual drug-loaded nanoparticulate combination therapy: A novel approach to target and treat multidrug-resistant cancers. J. Med. Hypotheses Ideas. 2013; 7: 15-20.
[17] Moorthi C., Kathiresan K., Nanoparticulate drug delivery system to overcome the limitations of conventional curcumin in the treatment of various cancers: A review. Drug Deliv. Lett. 2014; DOI:10.2174/2210303103999131 211110708.
[18] Liu CX. Research and Application of Targeting Nano-Drugs. Nano Biomed. Eng. 2011, 3(2): 73-83.
[19] Fessi H., Puisieux F., Devissaguet J.Ph., Ammoury N., Benita S., Nanocapsule formation by interfacial polymer deposition following solvent displacement. Int. J. Pharm. 1989; 55(1): R1-R4.
[20] Moorthi C., Kathiresan K., Fabrication of dual drug l oa de d pol ym e ri c na nosuspe nsi on : Inc orpora t i ng analytical hierarchy process and data envelopment analysis in the selection of a suitable method. Int. J. Pharm. Pharm. Sci. 2013; 5(2): 499-504.
[21] Moorthi C., Kathiresan K., A Step-by-step optimization process to fabricate narrow sized dual drug loaded polymeric nanoparticles using modified nanoprecipitation technique. Nano Biomed. Eng. 2013; 5(3): 107-115.
[22] Mo o rt h i C . , Ka t h i r e sa n K., Mo d i f i c a t i o ns t o t h e conventional nanoprecipitation technique: an approach to fabricate narrow sized polymeric nanoparticles. Adv. Pharm. Bull. 2014; 4(2): 205-208.
[23] Moorthi C., Senthil kumar C., Mohan S., Kiran K., Kathiresan K., Application of validated RP-HPLC-PDA method for the simultaneous estimation of curcumin and piperine in Eudragit E 100 nanoparticles. J. Pharm. Res. 2013; 7: 224-229.
[24] Moorthi C., Kathiresan K., Reve rse d pha se hi gh performance liquid chromatographic method for simultaneous estimation of curcumin and quercetin in pharmaceutical nanoformulation. Int. J. Pharm. Pharm. Sci. 2013; 5(3): 622-625.
[25] Moorthi C., Kathiresan K., Simultaneous estimation of curcumin and silibinin using validated RP-HPLC- PDA method and its application in pharmaceutical nanoformulation. Int. J. Pharm. Pharm. Sci. 2013; 5(3): 475-478.
[26] Francis D., Rita L., Rapid colorometric assay for cell growth and survival modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J. Immunological Methods. 1986; 89: 271-277.
[27] Moorthi C., Kathiresan K., Nanotoxicology: Toxicity of engineered nanoparticles and approaches to produce safer nanotherapeutics. Int. J. Pharm. Sci. 2012; 2(4): 117-124.
[28] Moorthi C., Kathiresan K., Kiran K., Manavalan R., In-vitro cell based assay: A preferred anticancer drug screening techniques for the academic researchers. J. Pharm. Res. 2011; 4(3): 671-675.
[29] Houghton P., Fang R., Techatanawat I., Steventon G., Hylands P.J., Lee C.C., The sulphorhodamine (SRB) assay and other approaches to testing plant extracts and derived compounds for activities related to reputed anticancer activity. Methods, 2007; 42(4): 377-387.
Copyright© 2014 Moorthi Chidambaram and Kathiresan Krishnasamy. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.