Research Article
Plant Mediated Green Synthesis of Silver Nanoparticles Using Tecomella undulata Leaf Extract and Their Characterization
Sadhan Kumar Chaudhuri 1*, Shivani Chandela 2, Lalit Malodia 1
1 Visual Camouflage Group, Defence Laboratory, Defence Research & Development Organization, Ratanada Palace, Jodhpur, Rajasthan, India.
2 Lachoo Memorial College of Science and Technology, Sector-A, Shastri Nagar, Jodhpur, Rajasthan, India.
* Corresponding author. E-mail: skcdrdo@gmail.com
Received: Dec. 9, 2015; Accepted: Jan. 28, 2016; Published: Feb. 24, 2016.
Citation: Sadhan Kumar Chaudhuri, Shivani Chandela and Lalit Malodia. Plant Mediated Green Synthesis of Silver Nanoparticles Using Tecomella undulata Leaf Extract and Their Characterization. Nano Biomed. Eng. 2016 8(1), 1-8.
DOI: 10.5101/nbe.v8i1.p1-8.
Abstract
Green synthesis of silver nanoparticles (AgNPs) was carried out using aqueous extract of Roheda (Tecomella undulata) at 60 °C in orbital shaking incubator. The variation in pH values and colour changes were observed within few minutes during reduction of silver nitrate (0.1 to 1 mM final concentration) in presence of plant extract and the capping of synthesized stable silver nanoparticles. The UV-Vis spectra of reaction mixture at different time intervals were recorded to monitor the formation of nanoparticles. The maximum absorption peak was observed near 430 nm indicating the formation of AgNPs. The particle size distribution was analysed through dynamic light scattering (DLS) and it was found that the peaks at 5.85 nm and 77.48 nm. Polydisperse Index (PDI) of synthesized AgNPs is 0.378 indicating that nanoparticles are stable even after nine months storage and uniform size range. The stability of biogenic silver nanoparticles was monititored through zeta potential measurement. The surface properties and size of the nanoparticles were studied through scanning electron microscopy and atomic force microscopy. The purity of the synthesized nanoparticle was confirmed by electron dispersive X-ray spectroscopy (EDS). Atomic force microscopy images indicated that height of the particle ranges from 3-18 nm. The results of various techniques indicated that these are silver nanoparticles synthesized using leaf extract of Tecomella undulata.
Keywords: Biosynthesis; Silver nanoparticles; Tecomella undulata; UV-Vis spectra; DLS; SEM; EDS; AFM
Introduction
Over the past decade nanoscience and nanotechnology is a sprouting interdisciplinary field of research interspersing material science, bionanosciences and biotechnology. The synthesis of metal nanoparticles is an important topic of research in modern material science due to their distinctive potential in the fields of electronic, magnetic [1], optoelectronic [2], information storage [3] and drug delivery [4]. Nanocrystalline silver nanoparticles have found tremendous applications in the fields of solar energy [5], water treatment [6], medicine [7] and also as catalyst [8]. The increasing demand of eco-friendly approach must be accompanied by green synthesis procedure. Nanoparicles can be synthesized by chemical and physical methods, which are expensive, hazardous to environment and required high energy consumption. Biological methods using microorganisms [9], enzymes [10], fungus [11], plant extracts [12] have been suggested as possible eco-friendly alternative methods over chemical and physical methods. Nanoparticles synthesis using plant parts are advantageous over other biological process by eliminating the process of maintaining time consuming aseptic microbial culture. Nanoparticles produced by plant parts are more stable, the rate of synthesis is faster than that in the case of other organisms, low cost, eco-friendly and a single step method of biosynthesis process [13]. Many reports are available on plant mediated synthesis of silver nanoparticles, particularly leaf extracts of Azadiracta indica [14], Oscimum sanctum [15], Magnolia kobus [16], Mangifera indica [17], Calotropis gigantean [18], Annona squamosa [19] and seed extracts of Coriandrum sativum [20] pomegranate [21]. Biogenic synthesis of silver nanoparticles using fruit extract of Ficus carica and its antimicrobial activity also reported [22]. Recent investigation of cytotoxic assays indicated that silver nanoparticles synthesized from Scoparia dulcis exhibit a significant cytototoxic in A549 cells compared to PA-1 cell line in a dose dependent manner [23]. Tecomella undulata (family: Bignoniaceae) is a tree species that produces quality timber is also known as roheda, honey tree, desert teak, marwar teak (English), roheda (Hindi). Distribution of Tecomella undulata is restricted to the drier parts of the Arabian countries, southern Pakistan and Northwest India up to an elevation of 1200 meters. In India, it occurs naturally in Maharashtra, Gujarat, Rajasthan, Punjab and Haryana. The methanolic extracts of Tecomella undulata are known for their analgesic and anti- inflammatory potential, disorders, epilepsy, cholera, pain, urinary problems, malaria, heart problems, and sexually transmitted diseases. It was traditionally used in medicinal ailments like cancer, snake bite, skin disorders, gastrointestinal disorders, respiratory tract disorders, gynecological disorders, hepatic transmitted diseases. The plant is also used for treating liver and spleen diseases, tumours, conjunctivitis, hepatosplenomegaly, syphilis, gonorrhea, hepatitis, as a blood purifier and in wound healing. Compounds such as naphthaquinone derivative, iridoid glucoside, phytosterol, fatty alcohol, flavonols, flavonoid glucoside and triterpenoids have been reported. Anti HIV, anti bacterial, anti microbial, immune modulator, analgesic and hepatoprotective activities have been reported from its various aerial parts [24]. The phytochemical analysis revealed the presence of five compounds i.e. alkaloids, flavonoids, saponins, steroids, tannins [25]. Iron oxide and zinc oxide nanoparticles have potential for increased seedling growth in various agricultural crops like peanut [26], mung and gram [27]. Silver nanoparticles also showed faster and increased seed germination and plant height in an endemic and endanger medicinal tree [28]. Biogenic silver nanoparticles enhanced seed germination and seedling growth in Vigna radiata and Cicer arietinum [29], Pennisetum glaucum [30] and other crop (corn, watermelon and zucchini) plants [31]. There is not a single report of silver nanoparticles effects on tree seedlings growth in nursery stage. Therefore the present investigation was made to synthesize silver nanoparticles by using hot desert plant leaf extract of Tecomella undulata. First time we are reporting that like other plant leaf extracts, Tecomella leaf extract has a potential to synthesize silver nanoparticles.
Experimental Materials and Methods
Collection of plant material
The fresh leaves of Roheda (Tecomella undulata) plants available in Defence Laboratory campus, Jodhpur, India were collected in polythene packet.
Preparation of leaf extract
20 g of fresh leaves thoroughly washed in tap water followed by Milli-Q water to remove any dust particles, dried with water absorbent paper and chopped into small pieces with scissor. Then chopped leaves were blended in mixer grinder for few second with 10 ml of sterile Milli-Q water and take the paste with the help spatula. 90 ml of Milli-Q water was added to leaf extract paste in a 500 ml Erlenmeyer flask and placed in orbital shaker incubator at 60 °C with constant shaking at 120 rpm for 10 min. After cooling at room temperature and filtering through Whatman No.1 paper the leaf extract was stored in refrigerator (4-7 °C) for further experiments. All the glass goods used were cleaned in chromic acid and autoclaved.
Synthesis of silver nanoparticles
The AR grade silver nitrate (AgNO3,) was procured from Emerck Germany and plant extract used for the reduction of Ag+ ions to Ag0 at 60 °C in a constant shaking at 120 rpm in orbital shaking incubator. In a typical experiment, leaf extracts (3 ml and 6 ml) were added to 300 ml of final concentration of 0.1 mM and mM AgNO3 dissolved in Milli-Q-water.
Instrumentation pH analysis
Silver nitrate aqueous solutions (0.1 mM and 1 mM) show pH 6.00 and 5.91 respectively. Leaf extract pH was 5.49 (Table 2). The changed pH of reaction mixtures were determined using digital pH meter (Eutech Cybersacn pH 300), during synthesis of silver nanoparticles.
UV-Vis spectral analysis
The reduction in silver ion was monitored by measuring the UV-Vis spectroscopy of the reaction medium after diluting small aliquot of the reaction mixture 10 times diluted with Milli-Q water to avoid the error due to high optical density of reaction mixture at different intervals after 15 min. UV-Vis spectral analysis was done by using UV-Vis-NIR spectrophotometer (Ocean Optics).
Dynamic light scattering analysis
Particle size distribution and average size of silver nanoparticles was obtained through particle size analyzer (Malvem zetasizer, Nano Z500, UK). The sample holder temperature was maintained at 25°C. The measurements depend on the size particle core, the size of surface structure, particle concentration and the type of ion in the mixture. The zeta potential of the synthesized silver nanoparticles was determined in water as dispersant [32].
Scanning electron microscopy analysis
Scanning electron microscopy (SEM) analysis was carried out using ZISS model machine. Thin film of the sample was prepared on carbon coated tape by adhering small amount of dried fine powder of the sample on the grid, the extra sample was removed with the help of blotting paper and the film on the SEM grid were allowed to dry by putting it under a mercury lamp for 5 min. The SEM analysis was used to determine the structure of the reaction products during biosynthesis of silver nanoparticles.
Energy dispersive X-ray spectroscopic analysis
EDS analysis was carried out to determine the chemical purity, elemental composition and stoichiometry of the synthesized silver nanoparticles.
Atomic force microscopy analysis
The atomic force microscopy (AFM) is one of the foremost tools for imaging, measuring and manipulating material at nanoscale. It offers a capability of three dimensional visualization and both qualitative and quantitative information on many physical properties including size, morphology, surface texture and roughness. The liquid sample having silver nanoparticle was spread on mica sheet, dried at 35 °C incubator and scanned with semi contact mode with close loop 3×3 µm scanner (Solver Model, NTMDT, Russia).
Results and Discussion
Colour change
Incubation of reaction mixture in orbital shaker incubator at 60 °C, a change of colour from pale yellow to reddish brown was observed (Fig. 1) within few minutes. The change of colour indicates that the formation of silver nanoparticles. The intensity of colour gradually increased (Table 1) with increasing time and concentration of AgNO3.
Variation in pH during synthesis of silver nanoparticles
The pH of the reaction mixture decreased from 6.5 to 5.5 in presence of Tecomella leaf extract indicating that reduction of 1.0 mM AgNO3 during formation of silver nanoparticles (Table 2). Reduction of pH during biogenic synthesis of silver nanoparticles was also reported in presence of Jamun (Syzygium cumini) leaf extract [33].
UV-Vis spectral analysis
The UV-Vis spectroscopy of the synthesized nanoparticles were in the range of 410-450 nm. Tecomella leaf extract was able to synthesize the silver nanoparticles by the indication of suitable surface Plasmon resonance (SPR) with peaks near visible spectrum at 430 nm (Fig. 2). The peaks remain near 430 nm in different time intervals indicting the stability and uniformity of silver nanoparticles synthesis. The absorbance peak near 430 nm, can be attributed to the plasmonic peak of silver nanoparticles, formed in the reaction mixture. There is no shift of peak position in different time intervals, the variation of maximum absorbance with increasing time indicated that the rate of formation of Ag nanoparticles.



(a) (b)(c)



(d)(e)(f)
Fig. 1 Colour change of the reaction mixture indicating the formation silver nanopaticles in presence of Tecomella leaf extract. (a) & (d): 0.1 mM &1 mM AgNO3 solution (White colour). (b): 0.1 mM AgNO3 solution +3 ml leaf extract (Pale Yellow colour). (c): After four hours colour changed to light brownish colloidal solution indicating that formation silver nanoparticles. (e): 1.0 mM AgNO3 solution +3 ml leaf extract (Deep Yellow colour). (f): After four hours colour changed to deep brownish colloidal solution indicating that formation silver nanoparticles.
Table 1 Colour change of reaction mixture during formation of silver nanoparticles in presence of Tecomella leaf extract
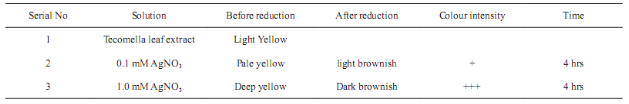
Table 2 Reduction of pH in reaction mixture during formation of silver nanoparticles in presence of Tecomella leaf extract

Dynamic light scattering analysis
Fig. 3 shows the DLS pattern of suspension of silver nanoparticles synthesized using Tecomella leaf extract. The size distribution profile indicates that the size of these silver nanoparticles show two peaks at 5.8 nm (5%) and 77.48 nm (95%). Polydisperse index (PDI) silver nanoparticles suspension is 0.378 indicating that synthesized particles are good and uniform sizes. Fig. 4 shows that the measured zeta potential value of biosynthesized silver nanoparticles in the colloidal solution. The nanoparticles possess a negative zeta potential value of -16.0 mV indicated that even after the storage of nine months in room temperature these are highly stable due to electrostatic repulsive force. The high negative value confrms the repulsion among the particles and the negative indicates that nanoparticles are stable. Measurement of zeta potential is depends on the movement of nanoparticles under influence of an applied electric field. This movement depends upon surface charge and the local environment of the particle.
Scanning electron microscopy analysis
SEM analyses show the images of high density Ag NPs synthesized by Tecomella leaf extract (Fig. 5). From the given SEM images, it is concluded that the green synthesized silver nanoparticles are relatively spherical shape with diameter 32-46 nm. Formation of silver nanoparticles was due to interactions of hydrogen bond and electrostatic interaction between the biomolecules capping with Ag0. The nanoparticles were not in direct contact even in the aggregated condition, indicating stabilization of nanoparticles bycapping agent.
Energy dispersive spectroscopic
Energy dispersive X-ray spectroscopic (EDS) analysis was carried out to determine the chemical purity, elemental composition and stoichiometry of he synthesized silver nanoparticles which is shown in Fig. 6. The strong signal of silver has been detected in EDS indicating the purity of synthesized silver nanoparticles. The other signals available in EDS may be coming from the bioactive molecules in the Tecomella leaves extract. The strong peak (signal) of silver indicating that the biosynthesized nanoparticles are indeed made up of only silver (Fig. 6). The small peaks of oxygen (O) and nitrogen (N) might be coming from the chamber of EDS [23].
Atomic force microscopy analysis
To validate the surface morphology powder coated AFM, three dimensional images were taken in non contact mode (Fig. 7). Results showed variability in morphological features of Tecomella leaf extract mediated silver nanoparticles. Height of the particles ranges from 3 to 18 nm and width ranges from 1.7 to 0.049 nm. It is evident from the AFM images that particles are uniform in size range and highly monodisperse nature. Silver has long been recgnized as having inhibitory effect on microbes present in medical and industrial process [34]. The most important application of silver and silver nanoparticles is in medical industry such as tropical oinments to prevent infection against burn and open woonds. Furether these biologically synthesized nanoparticles were found highly toxic against different multidrug resistant human pathogens. The efficacy of of silver nanoparticles in the killing of cancer cells was also tested. The results indicated they are more effective in overian cancer cell line [23].
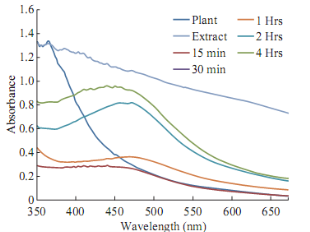
Fig. 2 UV-Visible spectra of silver nanoparticles at 1 mM silver nitrate in different time course of reaction with Tecomella leaf extract.
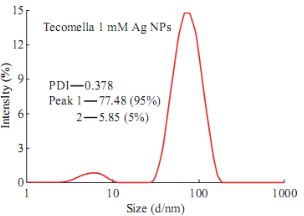
Fig. 3 DLS pattern of biogenic silver nanoparticles.
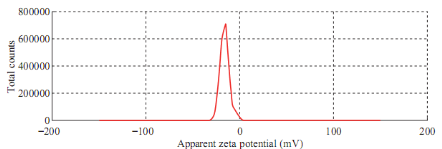
Fig. 4 Zeta potential measurement.
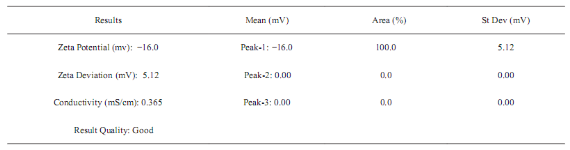
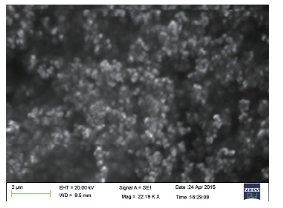
Fig. 5 SEM images of silver nanoparticles.
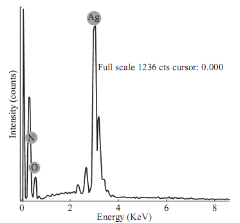
Fig. 6 Energy dispersive X-ray spectrum of synthesized silver nanoparticles.
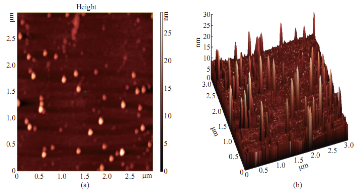
Fig. 7 (a) AFM 2D image; (b) Same feld in 3D image.
Conclusions
Synthesis of silver nanoparticles was quite stable without using any toxic chemicals as capping agent. Colour change visible due to the surface plasmon resonance during reaction bioactive compounds present in leaf extract of Tecomella undulata resulting in the synthesis of silver nanoparticles. The bio-molecules present in the Tecomella leaf extract are able to reduce silver nitrate and capping the silver nanoparticles at 60 °C. Biosynthesis of silver nanoparticles using plant parts extract may be influenced directly or indirectly by phytochemicals in the leaf extracts such as phenols, flavonoids and antioxidants as well as the physicochemical factors governing the kinetics of the reaction. The route is preferably docile as it is ecofriendly, require less energy intensive process and it is cost effective. Moreover, it is an efficient way of waste biomass utilization for the biosynthesis of silver nanoparticles. Biosynthesized silver nanoparticles are stable in liquid suspension even after nine months, as the particle size and distribution remain unchanged. The stability of fungal extracellular protein mediated biosynthesized ZnO nanoparticles and enhanced growth effect in clusterbean is also reported [35]. The green synthesis approach towards the synthesis of silver nanoparticles has many advantages. So far in literature survey, its applications are restricted in antimicrobial activity [20, 22], cytotoxicity assays [23, 36], and enhanced seedling growth in crop plants [31] but there is not a single report on its effect on tree seedling growth in nursery stage. Therefore, plant mediated biosynthesis of nanoparticles could be used for the study of faster tree seedling growth and development for arboriculture camouflage applications. With the huge plant diversity much more plant species are in way to be exploited and reported in future era towards rapid and single step protocol for the synthesis various types of metal nanoparticles with green principle. Biological synthesis of nanoparticles has upsurge in the field of nano-biotechnology to create novel materials that are eco-friendly, cost effective, stable nanoparticles with a great importance for wider applications in the areas of electronics, medicines, agriculture and forestry.
Acknowledgements
We acknowledge to Director, Defence Laboratory, Jodhpur, for providing infrastructure facilities and his keen interest on biosynthesis of nanoparicles for the development of fast growing stress tolerant tree saplings for military applications.
References
Copyright© 2016 Sadhan Kumar Chaudhuri, Shivani Chandela and Lalit Malodia. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.