Research Article
Electrochemical Study of Pb(II) in Present of Each Ascorbic Acid, Glucose, Urea and Uric Acid Using Blood Medium as an Electrolyte
Muhammed Mizher Radhi 1*, Ali Abdulabbas Abdullah Albakry 2, Amani Mohammad Jassim 1, Sura Ali Alassady 1, Emad A. Jaffar Al-Mulla 3*
1 Health and Medical Technology College-Baghdad, Middle Technical University.
2 Electrical Department, Al-Furat Al-Awsat Technical University, An-Najaf Engineering Technical College, An-Najaf, Iraq.
3 Department of Chemistry, Faculty of Science, University of Kufa, P.O. Box 21, An-Najaf 54001, Iraq.
* Corresponding authors. E-mail: mmradhi@yahoo.com; imad.almulla@uokufa.edu.iq
Received: Oct. 3, 2015; Accepted: Jan. 30, 2016; Published: Feb. 24, 2016.
Citation: Muhammed Mizher Radhi, Ali Abdulabbas Abdullah Albakry, Amani Mohammad Jassim, Sura Ali Alassady and Emad A. Jaffar Al-Mulla. Electrochemical Study of Pb(II) in Present of Each Ascorbic Acid, Glucose, Urea and Uric Acid Using Blood Medium as an Electrolyte. Nano Biomed. Eng. 2016 8(1), 9-15.
DOI: 10.5101/nbe.v8i1.p9-15.
Abstract
Electrochemical of redox current peaks of lead sulphate PbSO4 was studied in blood medium in present of different reagents such as ascorbic acid (AA), glucose, urea, and uric acid using cyclic voltammetric technique at glassy carbon electrode (GCE). It was found that Pb(II) ions in aqueous electrolyte (0.1 M KCl) have oxidation current peak at 540 mV and reduction current peak at 600 mV. But, it was different electrochemical properties of the redox current peaks of Pb(II) ions in blood medium, the reduction current peak was disappearing and the oxidation current peak was enhanced. Also, in the different reagents (glucose, AA, urea and uric acid) causes an enhancement of the oxidation current peak and reducing of the reduction current peak or disappearing. It means that the reagents (glucose, AA, urea and uric acid) were oxidative effective in the blood component for the lead ions in the damage the blood cells.
Keywords: Pb(II); Cyclic voltammetry; Blood medium; GCE; Redox process
Introduction
The scientific researches of all pollutants were studied in different aqueous electrolytes. The important studies in electrochemical properties of the different ions in blood medium were done with biosensors using cyclic voltammetric technique [1-4]. Square-wave anodic stripping voltammetry was used to determine the lead ions at different concentrations in blood samples [5]. Cyclic voltammetry and electrochemical detectors for HPLC were used to characterize antioxidants as reducing agents at inert electrodes. Results are presented for cyclic voltammetry at a glassy carbon electrode in 50% methanol, 50% 0.1 M HClO4, a solution typically used in HPLC separations of natural antioxidants. Cyclic voltammetry of complex mixtures such as blood serum and wine produces a measure of the total antioxidant status due to antioxidants with a low oxidation potential. The results of cyclic voltammetry studies are relevant for interpreting the performance of carbon electrodes in electrochemical detectors for HPLC [6, 7]. Heavy metals, particularly Lead (Pb) and Cadmium (Cd), had been long identified as hazardous pollutants in the environment. The current work describes the utilization of two different electro-analytical techniques, cyclic voltammetry (CV) and square wave anodic stripping voltammetry (SWASV), in conjunction with the novel Titanium Dioxide/ Zirconium Dioxide/ Tween 80 (TiO2/ZrO2/Tween 80) carbon paste composite electrode to qualitatively and quantitatively analyze Pb and Cd. It was calculated two kinetic parameters, electron transfer coefficient (α) and heterogeneous electron transfer rate constant (k0), for the oxidation-reduction process of Pb and Cd at the electrode surface [8]. The effects of possible interferences present in serum samples on response of enzyme electrode were examined using cyclic voltammetry technique. The determination of total cholesterol in serum samples was performed by using proposed enzyme electrode and results were in good agreement with those obtained by spectrophotometric method [9]. A new enzyme electrode concept featuring glucose oxidase (GOD) immobilized on fine carbon powder in a fluid state and a cross-linked GOD enzyme layer has been developed. This enzyme electrode has been tested in vitro at 37 °C and has a lifetime of 3 months after which it can be recharged with fresh enzyme. In vitro interference tests carried out on this sensor used with cellulose acetate membranes are described. The sensors showed a stable and linear response to glucose concentrations >300 mg/dL, in the presence of glucose alone in the phosphate buffer medium and in the presence of interferences. The effects of ascorbic acid, bilirubin, creatinine, L-cysdne, glycine, uric acid, and urea on the amperometric signal of the sensor were studied [10]. Zinc oxide nanoflakes (ZnO-NF) structures with a wall thickness around 50 to 100 nm were synthesized on a gold coated glass substrate using a low temperature hydrothermal method. The enzyme uricase was electrostatically immobilized in conjunction with Nafion membrane on the surface of well oriented ZnO- NFs, resulting in a sensitive, selective, stable and reproducible uric acid sensor. The electrochemical response of the ZnO-NF-based sensor vs. a Ag/AgCl reference electrode was found to be linear over a relatively wide logarithmic concentration range (500 nM to 1.5 mM). The sensor response was unaffected by normal concentrations of common interferents such as ascorbic acid, glucose, and urea [11]. A novel modified electrode MnO2 was fabricated and used for the selective detection of uric acid (UA) in the mass of ascorbic acid (AA). The modified electrode shows a good electron transfer ability and displays a novel performance for the detection of UA. More interestingly, this electrode can effectively eliminate the interference of AA. A linear dependence on the concentration of UA was measured by differential pulse voltammetry. The good sensitivity and repeatability of the proposed electrode enabled its successful application for the selective determination of UA in human urine sample analysis. These results will enhance our understanding of the applicability of MnO2 in electrochemical analysis [12]. The blood glucose monitoring devices (BGMDs) are an integral part of diabetes management now-a- days. They have evolved tremendously within the last four decades in terms of miniaturization, rapid response, greater specificity, simplicity, minute sample requirement, painless sample uptake, sophisticated software and data management. This article aims to review the developments in the technologies behind commercial BGMD, especially those in the areas of chemistries, mediators and other components. The technology concerns, on-going developments and future trends in blood glucose monitoring (BGM) are also discussed [13-22]. The reduction of Pb(II) has been investigated in presence of different potassium salts as supporting electrolytes at a Hanging Mercury Drop Electrode (HMDE). The correlation of cathodic peak current and anodic peak current, and the difference in cathodic peak potentials and anodic peak potentials with varying voltage scan rates indicates that the reduction of Pb(II) is reversible [13, 23-33]. In this work cyclic voltammetric technique was used to studying Pb(II) ions in blood medium by glassy carbon electrode. The redox current peaks of Pb(II) in blood medium with different oxidative and antioxidative reagents were studied the effective on these peaks.
Materials and Methods
Reagents and chemicals
Blood samples were used from healthy human. Lead sulphate PbSO4 from Fluka (Germany), Ascorbic acid (AA) from Technicon chemicals Co. (Oreq. Tournai Belgique), KCl from SCRC (China) and other chemicals and solvents were of annular grade and used as received from the manufacturer. Double distilled water was used for the preparation of aqueous solutions. All solutions were deaerated with oxygen free nitrogen gas for 15 min prior to making the measurement.
Instruments and procedures
EZstat series (potentiostat/glvanostat) NuVant Systems Inc. pioneering electrochemical technologies USA. Electrochemical workstations of Bioanalytical system with potetiostate driven by electroanalytical measuring softwares was connected to personal computer to perform cyclic voltammetry (CV), an Ag/ AgCl (3M NaCl) and Platinum wire (1 mm diameter) was used as a reference and counter electrode respectively. The glassy carbon working electrode (GCE) was used in this study and cleaning with alumina grand as shown in Fig. 1.
Results and Discussion
Redox current peaks of Pb(II) in aqueous solution
Fig. 2 shows the oxidation current peak of lead ions in 1M KCl solution as aqueous electrolyte at -540 mV and reduction current peak at -600 mV.
Fig. 1 Glassy carbon working electrode (GCE).
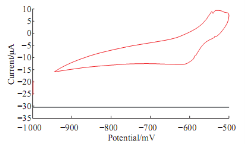
Fig. 2 Cyclic voltammograme of 10 mM Pb(II) in 1M KCl using GCE at 100 mV sec-1 verses Ag/AgCl as reference electrode.
Redox current peaks of Pb(II) in blood medium
It was found that redox current peaks of Lead ions in blood medium different than in aqueous solution (KCl), the reduction current peak was disappeared from the voltammograme as shown in Fig. 3. The oxidation current peak of Lead ions at -600 mV was enhanced by the oxidative of lead ions to the blood component. Fig. 4 shows the oxidative effect of the lead ions on the diluted blood with distill water. It means that lead ionsas pollutant reagent in the blood medium.
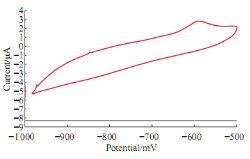
Fig. 3 Cyclic voltammograme of 10 mM Pb(II) in blood medium using GCE at 100 mV sec-1 verses Ag/AgCl.
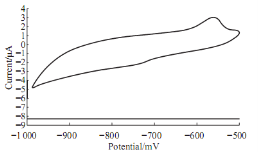
Fig. 4 Cyclic voltammograme of 10 mM Pb(II) in blood: water (1:1) using GCE at 100mV sec-1verses Ag/AgCl.
Enhancement of redox current peaks for lead ions in blood medium
Effected of AA
Fig. 5 shows the effect of ascorbic acid on the redox current peaks of Pb (II) in blood medium, the oxidation current peak was enhanced and shifted to 575 mV. The reduction current peak of Pb (II) in blood medium was appeared at 740 mV. So, the ascorbic acid acts as electro-catalyst for enhancement the redox process in blood medium which causes as anti-oxidative reagent for toxicity in lead compounds.
Effected of urea
The high value of urea (abnormal value) in blood causes some diseases in human body especially for kidney failure disease, it was found in the voltammetric study of Pb(II) in blood medium in present of urea causes the enhancement of the oxidation current peak of Pb(II) and disappearing of reduction current peak as shown in Fig. 6. So, the increasing of percentage of urea in blood medium causes as an oxidative complex in the component of blood and damage the structure of the blood cells.
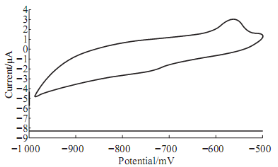
Fig. 5 Cyclic voltammograme of 10 mM Pb(II) in blood medium in present 10 mM AA using GCE at 100 mV sec-1 verses Ag/AgCl.
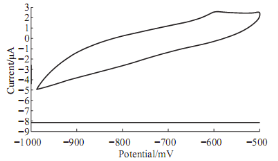
Fig. 6 Cyclic voltammograme of 10 mM Pb(II) in blood medium in present of urea using GCE at 100 mV sec-1 verses Ag/AgCl.
Effected of uric acid
The high value of uric acid in human blood means goat disease; it can be study the effect of uric acid on the redox current peaks of Pb(II) in blood medium by voltammetric technique. Uric acid conceders an oxidative reagent because that enhancement of the oxidation current peak of Pb(II) and disappearing of the reduction current peak as shown in Fig. 7.The enhancement of oxidation current peak of Pb(II) by the uric acid was gradually increasing by increasing the concentration of uric acid as shown in Fig. 8. The relationship has good sensitivity with R2 = 0.9383 and equation Y = 0.017X+3.3.
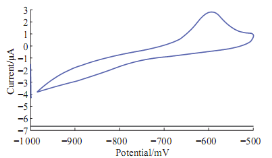
Fig. 7 Cyclic voltammograme of 10 mM Pb(II) in blood medium in present of uric acid using GCE at 100 mV sec1verses Ag/AgCl.
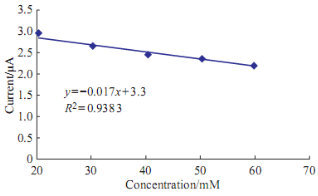
Fig. 8 Plot anodic current peak of Pb(II) versus the different concentration of uric acid in blood medium.
Effected of glucose
The high value of glucose in human blood means diabetic disease; Fig. 9 shows the effective of glucose on the redox current peaks of Pb(II) in blood medium. The anodic current peak of Pb(II) was enhanced by glucose solution and gradually increasing as shown in Fig. 10 with relationship in equation Y = 0.017X + 1.9467 and with good sensitivity R2 = 0.9753. The glucose acts as an oxidative reagent. Also, it causes as anti-oxidative reagent by appearing the cathodic current peak of Pb(II) at -780 mV as shown in Fig. 10.
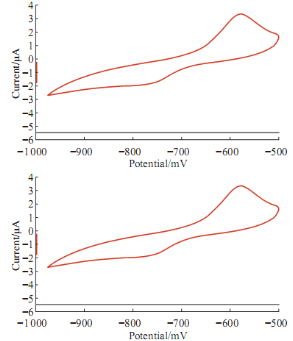
Fig. 9 Cyclic voltammograme of 10 mM Pb(II) in blood medium in present of glucose using GCE at 100 mV sec-1 verses Ag/AgCl.
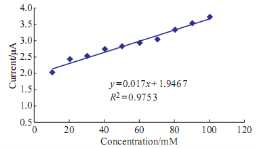
Fig. 10 Plot anodic current peak of Pb(II) versus the different concentration of glucose in blood medium.
Analysis of Pb(II) in blood medium
The determination of AA concentration in blood samples with Pb2+ using GCE. Recoveries experiment were evaluated using direct calibration of 99.6 ± 2.09% was obtained after the addition of 0.02 mM AA in to blood sample with Pb2+ as in Table 1 while recovery of 99.03 ± 2.1% was obtained after the addition of 0.03 Mm AA into blood sample with Pb2+ as in Table 2.
|
Table 1 Recovery rate of 0.02 mM of AA added in to the blood sample with Pb(II) |
||||
|
No. of sample |
Concentration of AA (mM) |
Recovery rate (%) |
Mean Recovery (%) |
Relative standard deviation (%) |
|
1 |
0.0205 |
102.5 |
99.6 |
2.09 |
|
2 |
0.0199 |
99.6 |
|
|
|
3 |
0.0198 |
99.0 |
|
|
|
4 |
0.0195 |
97.5 |
|
|
|
Table 2 Recovery rate of 0.03 mM of AA added in to the blood sample with Pb(II) |
||||
|
No. of sample |
Concentration of AA (mM) |
Recovery rate (%) |
Mean recovery (%) |
Relative standard deviation (%) |
|
1 |
0.0305 |
101.6 |
99.03 |
2.1 |
|
2 |
0.0290 |
96.6 |
|
|
|
3 |
0.0295 |
98.3 |
|
|
|
4 |
0.0299 |
99.6 |
|
|
Conclusions
Cyclic voltammetry for lead ions in blood medium was studied in present of different reagents oxidative and anti-oxidative such as glucose, AA, urea and uric acid:
1. It was found that blood medium enhance the oxidation current peak of lead ion and disappearing of reduction current peak, it means that Pb(II) ions act as an oxidative metal to the blood component and causes the damage of blood cells.
2. Different reagents such as glucose, AA, urea and reagents on the electrochemical properties (oxidative or anti-oxidative) in blood medium as in the following conclusion:
a. The effective of lead ions in present of AA in blood medium causes the enhancement of oxidation current peak of Pb(II) and causes a weak effect on the reduction current peak of lead ions on the blood component.
b. The present of urea in blood medium was highly effective that enhancement of oxidation current peak of Pb(II) and totally disappearing of reduction current peak. It means that patient with kidney failure has bad effective of the pollution with lead ions.
c. The diabetes patients with present of high value of glucose in blood that glucose causes the enhancement of oxidation current peak of Pb(II) and in the same time a little effective as anti- oxidative by appearing cathodic current peak.
d. Finally the goat patients with high value of uric acid that causes the enhancement of oxidation current peak of Pb(II) in blood and totally disappearing of reduction current peak.
Competing Interests
The authors declare that they have no competing interests.
Acknowledgments
All technical staff in Health and Medical Technology College, Middle Technical University are greatly acknowledged for their assistance.
References
Copyright© 2016 Muhammed Mizher Radhi, Ali Abdulabbas Abdullah Albakry, Amani Mohammad Jassim, Sura Ali Alassady and Emad A. Jaffar Al-Mulla. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.