Research Article
Arta (Calligonum Comosum, L'Her.) Shoot Extracts: Bio-Mediator in Silver Nanoparticles Formation and Antimycotic Potential
Afrah E. Mohammed *
Department of Biology, Faculty of Science, Princess Nourah bint Abdulrahman University, Riyadh 11671, Saudi Arabia.
* Corresponding author. E-mail: farhati@hotmail.com
Received: Jun. 26, 2016; Accepted: Jul. 25, 2016; Published: Aug. 1, 2016.
Citation: Afrah E. Mohammed, Arta (Calligonum Comosum, L'Her.) Shoot Extracts: Bio-Mediator in Silver Nanoparticles Formation and Antimycotic Potential. Nano Biomed. Eng., 2016, 8(3): 128-135.
DOI: 10.5101/nbe.v8i3.p128-135.
Abstract
Environmentally friendly green synthesis of nanomaterial has a very significant part in nanotechnology. In the present research, the synthesis of silver nanoparticles (AgNPs) was established by treating silver ions with the aqueous extract of Calligonum comosum green shoots at room temperature. AgNPs formation was firstly detected by the colour change of mixed extract (plant extract and AgNO3). Further characterization was done by ultraviolet, (UV)-Vis spectrophotometer, transmission electron microscopy (TEM), scanning electron microscopy (SEM), zeta potential and fourier transform infrared spectroscopy (FTIR). The peak values for UV-VIS- spectroscopy were in the range of 440 nm, TEM micrograph showed spherical shape for the particles and zeta potential showed formation of negative charged nanoparticles with an average size of about105.8 nm. 1635.41 and 3249.83 cm−1 are the peaks detected from the FTIR analysis. In this study, biosynthesized silver nanoparticles mediated by C. comosum were tested for their antimycotic activity using a well diffusion method against fungal species; Aspergillus flavus, Penicillium sp, Fusarium oxysporum. Our findings indicated that biosynthesized AgNPs showed an efficient antimycotic activity against tested species. The antimycotic action of AgNPs varied according to different fungal species. Results confirmed the ability of C. comosum green shoot extract to act as an reducing and stabilizing agent during the synthesis of AgNPs.
Keywords: AgNPs; Antimycotic; Calligonum comosum; TEM; SEM; Zeta potential; FTIR
Introduction
Applications of nanoparticles (NPs) in the field of nanomedicine have been well documented recently. Therapeutic application to target definite sites, such as lung tissue, vaccinations and cancer therapy were recently employed [1]. Currently, the increasing use of NPs in medicine is closely associated with its potential to overcome microbial resistance. Furthermore, to circumvent the microbial resistance, NPs were used in a form of metallic NPs, chitosan NPs and nitric oxide-releasing NPs [2]. Among the metallic NPs, AgNPs possess excellent antibacterial, antifungal and anti-viral potentials [3]. Several hypotheses have been suggested for the antibacterial mechanisms of AgNPs. (1) The surface-area-to-volume ratio increases in the smaller size material compared with the bulk material, which might provide opportunities for interactions with bacteria cell [4]. (2) AgNPs can cause cell lysis, inhibit cell transduction, changes in the cell membrane permeability and destruction of microbial genome and DNA fragmentation [5,6]. (3) AgNPs attach the surface of the cell membrane, penetrating in bacteria and disturb the cell function [7]. (4) AgNPs might modulate the phosphor tyrosine profile of treated bacterial peptides which affect cellular signaling that leads to inhibition of bacterial growth [8]. (5) It was observed that silver nanoparticles inhibit the formation of microbial biofilm which is an efficient barrier against the host immune system and antimicrobial agents [9]. (6) Through destruction of membrane integrity AgNPs exerted potent antifungal effects on fungi [10]. The author further postulated that, AgNPs is able to saturate and cohere to fungal hyphal cell which leads to deactivation of plant pathogenic fungi. Exact mechanism for the antimicrobial of NPs is not clearly known; nevertheless, it was suggested that electrostatic attraction between a negatively charged cell membrane and the positively charged NP, could be a good evidence for the significant antimicrobial action of NPs. Conversation of Ag ions to AgNPs by sodium borohydride as chemical reducing agent is widely applied [11]. Furthermore, hazardous chemical residues in the final product may cause potential environmental [12]. Compared to the use of hazardous chemicals as a tradition for this process, green synthesis is arising as an environmentally-friendly technique for biosynthesis of AgNPs. Recently, bacteria, fungi or plant parts such as leaves, fruits, and flowers beside pure compounds from plants, algae, carbohydrates, and microorganisms utilized for the biosynthesis of NPs [4,13]. In phytonanosynthesis, biochemical diversity of plant extract, non-pathogenicity, low cost and flexibility in reaction parameters are accounted for high rate of AgNPs production with different shape, size and applications [14]. Phytochemicals such as terpenoids, flavones, ketones, aldehydes, amides and carboxylic acids in addition to plant-derived polysaccharides play dual roles as reducing and stabilizing agents [15]. Polysaccharides have hydroxyl groups, a hemiacetal reducing end, which is oxidized to carbonyl group beside other functionalities can play important roles in both the reduction and the stabilization of metallic NPs [16]. Using plant extracts bacteria, fungi, actinomycetes, yeast, algae for the biosynthesis of nanoparticles was reported [17]. A recent review revealed that using plant extract as abio-mediator for silver nanoparticles is faster than using microbes [5]. Plants and natural resources such as Aloe vera, Ziziphus spina-christ and Eucalyptus camaldulensis, Moringa oleifera, tea, Catharanthus roseus and Chrysanthemum morifolium Ramat have been used for the synthesis of silver nanoparticles in addition to the application of the bio-prepared material on different types of microorganisms confirming the antimicrobial ability for this NPs [18-24]. In the present investigation Calligonum comosum was used as biomediater for AgNPs formation. Arta, Calligonum comosum L`Her. belongs to the family of the Polygonaceae. Woody based ascending shrub, glabrous much branched, straight up to 250 cm high, older branches rigid with whitish gray bark and swollen knotty nodes, young shoots green flexible 1-2 mm in diameter, branching and angled at nodes. Leaves minute, soon deciduous and usually absent. It is a plant of tropical and subtropical regions. Frequent in deeper sand, dunes, plains and valleys; cultivated around desert plantations as wind breaks; tolerates saline conditions. <http://www.haad.ae/HAAD/LinkClick.aspx?fileticket=Sr3tjeg5xvo=&tabid=791>. It is a plant with a wide spread in Saudi Arabia. Anthraquinones and flavonoids are the major antioxidant constituents in Arta which had cytotoxic effect [25,26]. A recent studies demonstrated the antibacterial and antifungal ability of different plant parts of C. comosum [27-29]. Lack of information regarding C. comosum as a bio material for silver nanoparticles formation encourages us to use this plant types for the present investigation. In the present work C. comosum green shoots extract was used to mediate reduction of the Ag+ present in the form of aqueous solution of silver nitrate to AgNPs. The main objective was to evaluate its antimycotic impact on some fungal species. To realize that goal inhibition zone was assessed.
Materials and Methods
Materials
Arta, Calligonum comosum green shoots were collected from Riyadh, Saudi Arabia. Silver nitrate (AgNO3) and Sabouraud Dextrose Agar (SDA) were purchased from (Wateenalhyaa company, Riyadh, Saudi Arabia) for the antimycotic assays.
Biosynthesis of silver nanoparticles (AgNPs)
The aqueous extract of C. comosum prepared by mixing 10 g of the dry sample with 100 ml of highly purified water. The mixture was heated for 10 mins at 80 °C to denature the enzymes of the extracts. The solution was filtered through a Whatman filter paper No. 1 (pore size 125 μm). The supernatant (filtrate) was further filtered through a Whatman filter paper No. 1 (pore size 25 μm) to remove the residues. For synthesis of the AgNPs, 12 ml of aqueous C. comosum extracts as reducing agents was mixed with 88 ml of 1 mM AgNO3 solution in an Erlenmeyer flask and allowed to react at room temperature. Ultra-high purity water was used as a reaction medium to avoid the presence of chloride ions and also to prevent precipitation of silver chloride. The AgNPs extract was stored at 4 °C until further analysis [19].
Characterization of AgNPs
The reduction of silver ions to AgNPs in the solution was monitored by measuring the ultraviolet-visible spectrum of the solution using a UV 2450 double- beam spectrophotometer (Shimadzu, Tokyo, Japan) operated at a resolution of 2 nm in the range from 400–500 nm [19].
Transmission electron microscopy (TEM)
JEOL microscope (JEM-1011) was applied to determine AgNPs size, shape and morphology. Images were obtained at a 80 kV voltage. Ag NPs' drop on the carbon-coated copper of the TEM grids after drying under vacuum and then loaded on the sample holder.
Transmission electron microscopy (TEM) and scanning electron microscopy (SEM)
SEM technique (Quanta 250, FEI) was applied to confirm AgNPs size, shape and morphology. Images were obtained at 10 kV.
Dynamic light scattering (DLS) and zeta potential measurements
Dynamic light scattering (DLS) and zeta potential measurements for the biogenic AgNPs were assessed using Zetasizer, Nano series, HT laser, ZEN 3600 (Malvern Instruments Ltd., Malvern, UK). A range between 0.1 and 10,000 nm was applied.
Fourier transform infrared spectroscopy (FTIR)
A range of 500 to 5000 cm−1 FTIR (Shimadzu FT-IR Prestiage 21) was used for the biogenic AgNps. The biomolecules that act as reducing, capping, and stabilizing agent of the AgNps were recorded using diffuse reflectance mode.
Evaluation of antimycotic activity of AgNPs
The antimycotic activity of the synthesized AgNPs was measured using the well diffusion methods. Three fungal species were obtained from the biology laboratory, Faculty of Science, PNU. Selected fungal pathogens are commercially important and cause various diseases on vegetables, fruits, and crop plants. Stock cultures of Aspergillus flavus, Penicillium sp, Fusarium oxysporum were prepared and sub cultured in Sabouraud Dextrose Agar (SDA) slants at 4 ºC. Each strain was swabbed uniformly onto individual agar plates using sterile swabs. Subsequently, four adequately spaced wells (holes) of 4 mm diameter reach were made per plate at the culture agar surface using sterile metal cup borer. In each hole, 0.2 ml of each biosynthesized AgNPs extract and AgNO3 were put under aseptic conditions, kept at room temperature for one hour to allow the extracts to diffuse into agar medium and incubated accordingly. Sterile distilled water was used as the reference negative control. All the plates were incubated at 25 °C for 48 hours. The plates were examined for sign of zone of inhibition, which appear as a clear space around the wells. The diameter of inhibition zones was measured using a ruler. Experiments performing in four replicates. Mean value was calculated.
Statistical analysis
All results were computed and expressed as mean ± standard deviation (SD) from four replicates. Statistical analysis was performed using JMP software (version18.0) with analysis of variance (One-Way ANOVA).
Results
Using C. comosum as a reducing and a stabilizing bio-agent for silver nanoparticles formation was the principle aim of the current investigation besides, antifungal effect against some fungal species (Aspergillus flavus, Penicillium sp, Fusarium oxysporum) was tested for the bio-prepared AgNPs. Amount of 12ml of plant extract with the concentration of 1mg/ml showed an ability to convert the colour of silver nitrate 1M from yellow to a dark brown colour (Figure 1). The intensity of the brown color increased with an increasing in the incubation period of the mixture. Further confirmation of silver nanoparticles formation is the using of UV-Vis spectroscopy. The plasmon absorption bands of silver nanoparticles formed had an absorbance peak around 440 nm. TEM image confirmed the development of Ag nanostructures with different sizes by using C. comosum and well indicated that the bio-prepared AgNPs are spherical in shape with a smooth surface morphology (Figure 2). SEM image shows the morphologies of the biogenic synthesized nanoparticles at two magnifications (Figure 3) confirming the spherical shape of the particles. Furthermore, dynamic light scattering of AgNPs biosynthesized by C. comosum showed an average size of 105.2 nm (Figure 4). Zeta potential graph showed the potential stability of the particles in the solution. Negative charge of the nanoparticles -6.62 mV was well confirmed in this study for the plant-synthesized AgNPs (Figure 5). FTIR spectra of the biogenic AgNPs showed two peaks located at 1635.41 and 3249.83 cm−1 (Figure 6). Color change, spectrophotometer, TEM and SEM images, zeta potential and FTIR results revealed that, stable AgNPs can be easily produced when C. comosum aqueous extracts used as reluctant an stabilizing agent. Treatment of fungi with silver nitrate solution showed (10.5 – 14.0 mm) range of inhibitory zone confirming the ability of silver nitrate to suppress the fungal growth (Table 1). Moreover, Silver nanoparticles (AgNPs) synthesized by C. comosum showed an antifungal ability indicated by an inhibition zone against Aspergillus flavus (19.5 ± 0.9 mm) as the highest zone compared with other tested species, Penicillium sp (18 ± 1.2mm) and Fusarium oxysporum (16± 0.7 mm) Table 1. AgNPs had higher ability to suppress the fungal growth compared with silver nitrate solution.

Fig. 1 Synthesis of AgNPs by C. comosum shoot extract. The figure shows (a) C. comosum extract and silver nitrate; and (b) C. comosum extract and silver nitrate after 2 hours. It was observed that the color of the solution turned to brown dark, indicating the formation of AgNPs.
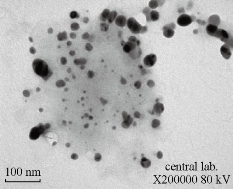
Fig. 2 Transmission electron microscopy (TEM) images indicating shape, morphology and size for AgNPs synthesized from C. comosum shoot extract. Scale bar = 100 nm.
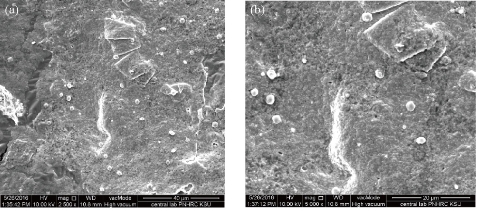
Fig. 3 Scanning electron microscope (SEM) image showing spherical shape of silver nanoparticles synthesized from C. comosum shoot extract at different magnifications: (a) 2500 μm; (b) 5000 μm.
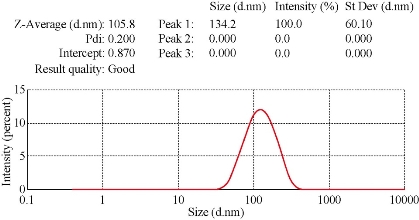
Fig. 4 Zitasizer for AgNPs synthesized from C. comosum shoot extract.
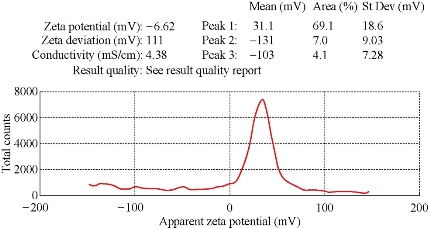
Figure 5: Zeta potential graph of AgNPs prepared from the C. comosum shoot extract.
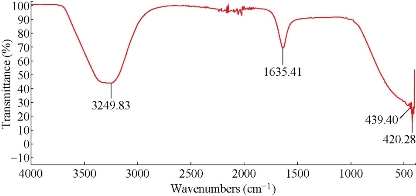
Figure 6: FTIR spectrum recorded for biogenically synthesized AgNPs prepared from the C. comosum shoot extract.
Table 1: Inhibition zone (mm) of fungi treated with AgNPs synthesized by C. comosum shoot extract. Data are expressed as mean ± SD of four replicates. Different letters within a raw indicate significant differences in the Tukey test; α = 5%.
|
Treatment |
Aspergillus flavus |
Penicillium sp. |
Fusarium oxysporum |
|
AgNPs (Silver nanopartices mediated C. comosum) |
19.5±0.9 a |
17 ± 1.2 ab |
15± 0.7 b |
|
AgNO3 |
14±1 a |
12.5±0.5 ab |
10.5±0.5 b |
Discussion
Recently, development of a new antimicrobial compounds has proved to be efficient in suppressing microorganisms and thus widely employed in medical field as a reliable alternative for antibiotics to which certain microbes have developed a relative resistance. Silver and Klasen [30,31]. demonstrated that, there is a serious medical need for antibiotics with novel antimicrobial mechanisms because fungi are eukaryotic organisms with a metabolism and construction that are similar to those of eukaryotic hosts (plant, animal and human). Silver nitrate is known for its antimicrobial ability against bacteria and fungi. Interestingly, silver nanoparticles is found to be more efficient in mitigating the effect of harmful microbes compared with other forms of silver compounds. The present study was firstly aimed to evaluate the capacity of C. comosum as a reducing and a stabilizing bio-agent for AgNPs formation. A further goal was to study the ability of the biosynthesized silver nanoparticles with respect to their antifungal effect against some fungal species (Aspergillus flavus, Penicillium sp, Fusarium oxysporum). C. comosum extract showed an ability to convert the extract of silver nitrate to dark colour in 2 hours which is an indication for the conversion of silver nitrate to silver nanoparticles. The colour changes are acquired due to the excitation of SPR in the synthesized NPs [32]. Time needed for the conversion of Ag ions to NPs was differ according to the type of plant extract used since Ocimum sanctum extract have taken 1 h, Azadirachta indica and P.nigrum leaf and stem extracts show the formation of brown color in 10 mins [32,33,34]. UV-Vis spectroscopy in this study confirmed silver nanoparticles formation since abroad absorption peak ranging from 400-450 nm is a characteristic band for the Ag, arising from the excitation of longitudinal plasmon vibrations of AgNP in the solution [35]. On the other hand, TEM images results were well documented by the observations recorded by Singh et al; Heydari and Rashidipour [36,37]. when AgNPs prepared by Oak fruit hull. Using biogenic material for AgNPs formation is an uncontrolled process provides wide range of particle size [38] this might indicate why AgNPs are variable in size in this study (Figure 2). AgNPs were enclosed to a thin layer of organic material (carbon) which is providing its stability by acting as capping agent [39]. Relatively spherical shaped silver nanoparticles were also detected by Krithiga et al and Banerjee et al. [40,41], where Clitoria ternatea and banana extracts were used as bio-mediator in silver nanoparticle formation respectively, and SEM images were recorded. A range between 50 to 300 nm particle size of the synthesized silver nanoparticles using red apple fruit extract was obtained [42], which is in line with our results of dynamic light scattering AgNPs biosynthesized by C. comosum. Zeta potential graph showed the potential stability of the nanoparticles in the solution since negative charge was detected [43].Similar trend of observations was also recently reported by Singh et al. [36], where silver nanoparticles were synthesized from P. amarus and T. cordifolia. On the other hand, color change, spectrophotometer, zeta potential, TEM and SEM results revealed that stable AgNPs with variability in size can be easily produced by C. comosum aqueous extracts. Such methods might be a feasible, low-cost and environmentally friendly technique. C. comosum produce a unique secondary metabolites such as Anthraquinones, flanovonids and dehydrodicatechin which had cytotoxic effect and antioxidant activity [25,26]. These compounds might be the main reason for the reduction process of silver nitrate to silver nanoparticles since the plant secondary metabolites can act for reduction/oxidation reactions in the bio-synthesis of silver nanoparticles [5]. Reducing and stabilizing agents for silver nanoparticles were detected by the FTIR spectra. Peaks located at 1635.41 cm−1 which might be due to the presence of a primary amine NH band. Absorbance between 1600 and 1700 cm-1 can indicate the amide I vibration for proteins which might be due to the C=O stretching vibration [45]. The absorption peak at 1635.41 cm-1 is near to that reported for natural proteins [46]. This evidence suggest that proteins are interacting with biosynthesized nanoparticles were not affected during reaction with Ag+ ions or after binding with Ag nanoparticles [47]. Furthermore, peak at 3249.83 cm−1 could be due to O-H groups of phenolic compounds. These results confirm the presence of proteins and phenolic compounds which may act as reducing and stabilizing agents for the biogenic AgNPs. Furthermore, AgNPs had higher ability to suppress the fungal growth compared with silver nitrate, this might be due to the larger surface areas of the nanoparticles which increased the interaction zone with biological targets. Similar observations regarding a potent antifungal activity of biosynthesized AgNPs were also demonstrated against C. albicans and Saccharomyces cerevisiae [48] and against Aspergillus niger, Candida albicans and Candida parasilopsis [49]. Furthermore, inhibitory effects of phytosynthesized AgNPs aginst Aspergillus sp and Fusarium sp. was well detected [10, 50]. Different antifungal ability regarding the biogenic AgNPs might be associated with the characteristics of certain species which was also documented by many different studies [48,49]. It has been hypothesized that silver nanoparticles can cause cell lysis, inhibit cell transduction, changes in the cell membrane permeability and probably destruction of fungal membrane integrity besides, AgNPs penetrate in the fungi cell that lead to cell death through stop cell division [5,10,48,51]. Furthermore, AgNPs is able to saturate and cohere to fungal hyphal cell which leads to deactivation of plant pathogenic fungi beside Complexes of the bases contained in DNA with Ag+ is a powerful inhibitor of fungal DNAases [10,52]. The antimicrobial action of AgNPs is well correlated with its decreased size and shape having increased surface area that lead to superior antimicrobial action. Furthermore, the biosynthesis rate of AgNPs from plant origin is an ecofriendly method that is costless with no toxic chemicals.
Conclusions
In summary, AgNPs exerted potent antimycotic effects on fungi tested in vitro, probably through destruction of membrane integrity. Further study is needed to maintain the adequately required concentrations for use, with careful handling of this nanotechnology; AgNPs can be a good friend for the betterment of human life and the environment at large.
Acknowledgements
The author would like to thank the Prof. Jehan Albrahim, Department of Biology, Faculty of Science, Princess Nourah bint Abdulrahman University for the nice comments throughout the experimental period of this study.
Conflict of Interest
The authors declare that they have no conflict of interest.
References
Copyright© 2016 Afrah E. Mohammed. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.