Research Article
High Ionic Strength Enhances the Anti-Pepsin Activity of Titanium Dioxide Nanoparticles
Hussein Kadhem Al-Hakeim 1*, Khlowd Mohammed Jasem 2
1 Department of Chemistry, Faculty of Science, Kufa University, Kufa, Iraq.
2 Department of Chemistry, College of Science, Kerbala University, Kerbala, Iraq.
* Corresponding author. E-mail: headm2010@yahoo.com
Received: Jun. 28, 2016; Accepted: Aug. 9, 2016; Published: Aug. 19, 2016.
Citation: Hussein Kadhem Al-Hakeim, Khlowd Mohammed Jasem, High Ionic Strength Enhances the Anti-Pepsin Activity of Titanium Dioxide Nanoparticles. Nano Biomed. Eng., 2016, 8(3): 136-143.
DOI: 10.5101/nbe.v8i3.p136-143.
Abstract
Gastro-esophageal reflux disease (GERD) is a state that evolves when there is a return of acid and pepsin enzyme into the esophagus. Inhibition of pepsin enzyme is one of the strategies used successfully for the treatment of GERD. Titanium dioxide nanoparticles (TiO2 NPs) are among the safest nanoparticles (NPs) that can be used inside the human body. The aim of the present study is to use TiO2 NPs as an inhibitor for the pepsin enzyme as a new treatment for GERD. The activity of pepsin before and after the addition of certain amounts of the NPs to the reaction mixture was measured spectrophotometrically. These experiments were repeated at different temperatures and different ionic strengths. The Michaelis constant (Km) and maximum velocity (Vmax) of the pepsin catalyzed reactions were calculated from the Lineweaver-Burke plots. The results revealed a significant reduction in the pepsin activity by TiO2 NPs. Both Km and Vmax are changed after inhibition indicating a mixed inhibition of pepsin activity. The result also showed that the combination of high ionic strength and TiO2 NPs causes a complete inhibition of pepsin activity. It can be concluded that the best condition for inhibition of pepsin activity is by using a combination of TiO2 NPs and high concentration NaCl at 37°C.
Keywords
GERD; TiO2 NPs; Enzyme inhibition; Titanium dioxide nanoparticles; Pepsin
Introduction
Gastro-esophageal reflux disease (GERD) is a state that evolves when a reflux of stomach contents into the esophagus causes complications, bothersome symptoms or both [1]. GERD is a popular disease with a spread as 10%-20% in the western world [2]. GERD is one of the most common diseases affecting patients worldwide, but its risk factors and causes are not clearly known [3]. While the symptoms suggestive of gastroesophageal reflux (heartburn, reflux or chest pain) affect up to 30% of the population in Western countries and their prevalence continues to increase [4]. The disease can apparent in various symptoms which can be classed into typical, atypical and extra-esophageal symptoms [2]. The most-known symptoms of GERD in adults are heartburn and regurgitation [5]. Acidic reflux material can be reaching into the esophageal, oropharyngeal and tracheal mucosa. Gastric contents involve pepsin and acid in any reflux episode. Various studies showed that acid doesn't damage the mucosa by itself but pepsin plays the major role in mucosal injury. Current Western medicine treatment of GERD focuses mainly on symptom alleviation. Although proton-pump inhibitors are known to alleviate symptoms in most patients, a significant portion of patients continue to present GERD [6]. Pepsin plays the main role in the formation of GERD and associated diseases [7]. Pepsin is an important enzyme in the aspartic protease family which digests proteins with many unique characteristics. Peptide bonds can be easily cleaved letting to degradation of proteins in acidic conditions [8]. There is a wide range of specific inhibitors that can bind to the active site and electively remove the activity of pepsin. One of the best known ones is pepstatin, a specific pepsin inhibitor [9]. Pepstatin, at acidic pH, tightly binds to the catalytic site of both pepsin and its precursor pepsinogen [10]. Titanium dioxide nanoparticles (TiO2 NPs) is among the safest nanoparticles that can be used inside the human body [11]. TiO2 NPs occur in three main forms, i.e., anatase, rutile, and brookite each of which has unique properties and uses [12]. TiO2 NPs is considered as a safe and inert material and used in many applications for decade [13]. However, research on the health response to orally administrated TiO2 NPs is still limited [14]. Few research concerning about the enzyme toxic effect of TiO2 NPs on proteins and enzymes [15]. As having a high surface area, TiO2 NPs have the ability to adsorb and bind large molecules including proteins [16]. For example, it was reported that TiO2 NPs could be used in protein separation and purification [17]. The aim of the present study is to optimize the process of pepsin inhibition by TiO2 NPs as a possible new treatment for GERD.
Materials and Methods
Materials
Pepsin (EC 3.4.23.1), M.wt=36,450D, purity (99.5%) was purchased from BDH, England, TiO2 NPs purity(99.9%) from US Research nanomaterials, Inc, USA, lyophilized hemoglobin (human red blood cells), purity (96%) from Lee Biosolutions, Missouri, USA. Trichloroacetic acid (TCA), purity (98%) was purchased from Alpha Chemika, India, hydrochloric acid from Central Drug House, New Delhi, India.
Estimation of pepsin activity
The enzyme activity was determined by kinetic method [19]. The principle depend on the fact that pepsin cleaves peptides from hemoglobin which are soluble in trichloroacetic acid (TCA). The tyrosine and tryptophan content of these TCA-soluble peptides is determined by the measurement of the extinction at 280 nm. Briefly, pepsin solution dissolved in 0.01 N HCl to obtain a concentration of 0.5 mg/ml. Just prior to assay dilute further in 0.01 N HCl to a concentration of 5-20 μg/ml. The steps of the method are as follows: one milliliter of hemoglobin substrate was pipetted into test tubes containing 0.2 ml of the diluted pepsin at 37°C. After 10 minutes, the reaction was stopped by adding 2 ml of 5% TCA at timed intervals. The tubes containing reaction mixture were removed from water bath after 5 min, and clarified (filtrates should be clear). E280 nm was read of filtrate and subtract E280 nm of the appropriate blank using spectrophotometer set at 280 nm and 37°C. The method of estimation of pepsin activity was repeated at different temperatures 22, 27, 32 and 42°C. Unit of pepsin enzyme is defined as an amount of enzyme which renders TCA soluble 0.001 E280 nm per minute at 37°C, using a denatured hemoglobin substrate.
Inhibition of pepsin activity by TiO2 NPs
To study the inhibition of pepsin by TiO2 NPs, a weight of 15 mg of TiO2 NPs was dissolved in 10 ml of 0.01 N HCl, and agitated by ultrasonic water bath at 37°C for 20 minutes. Five milligrams of pepsin was added to the TiO2 NPs tubes and incubated at 37°C for 30 minutes. These amounts of pepsin and NPs is calculated to produce pepsin monolayer on the TiO2 NPs. TiO2 NPs and pepsin surface areas were calculated in order to estimate theoretically the number of pepsin that enough to cover the surface of one TiO2 NP in one layer manner. The calculations recruit the TiO2 NPs properties (radius=9*10-7 cm, density=3.9 g/cm3, mass of one NP=1.19*10-17 g, volume of TiO2 NP=3.06*10-18 cm3) and pepsin properties (radius=2.18*10-7 cm, mass of one pepsin=5.98*10-20 gm, volume of pepsin molecules=43.63*10-21 cm3). From these parameters, the calculated surface areas of pepsin and TiO2 NPs were 1.50*10-13 cm2 and 1.02*10-11 cm2, respectively. Number of pepsin molecules that can cover one TiO2 NP in a monolayer manner is obtained from division of one TiO2 NPs surface area on one pepsin surface area ≈ 68 molecules of pepsin per TiO2 NPs. This means that a 5 mg of pepsin required to cover 15 mg of TiO2 NPs to obtain a monolayer of the adsorbed pepsin. The method of estimation of pepsin activity was repeated and the activity of the immobilized pepsin was estimated. To study the effect of NPs weight on the pepsin activity, same method was repeated using different weights of TiO2 NPs (30, 45, 60 and 75 mg) in the mixture solution.
Effect of temperature on the inhibition of pepsin activity by TiO2 NPs
To study the effect of temperature in the presence of TiO2 NPs, a weight of 15 mg of TiO2 NPs was dissolved in 10 ml of 0.01 N HCl, and agitated by ultrasonic water bath at different temperatures (22, 27, 32, 37 and 42°C) for 20 minutes. Five milligrams of pepsin were added to the tubes in number 1 and incubated at 42°C for 30 minutes and the method of estimation of pepsin activity was repeated and the pepsin activity was estimated after adding the TiO2 NPs.
Effect of a combination of ionic strength and TiO2 NPs on the inhibition of pepsin activity
To study the effect of ionic strength in the presence of TiO2 NPs, a weight of 15 mg of TiO2 NPs and 1.3 gm of NaCl was dissolved in 10 ml of 0.01 N HCl, and agitated by ultrasonic water bath at 37°C for 20 minutes. Five milligrams of pepsin were added to the tubes and incubated at 37°C for 30 minutes. Then, the method of estimation of pepsin activity was repeated and the pepsin activity was calculated.
Results and Discussion
The properties of the purchased TiO2 NPs were further confirmed using TEM techniques. The results indicated that the TiO2 NPs are spherical with a diameter of 18 nm. These NPs were used in all the following experiments.
Effect of TiO2 NPs weight on the pepsin catalyzed reaction
In the present study, the activity of the enzyme was measured in the presence of seven different concentrations of hemoglobin and five different weight of added TiO2 NPs (15, 30, 45, 60, & 75 mg to the 10 ml of the 0.01 N HCl) to the TiO2 NPs suspension. These experiments were used to examine the effect of TiO2 NPs on the activity of pepsin catalyzed reaction. The Lineweaver-Burk plots of the five experiments, in addition to the experiment carried out without TiO2 NPs for comparison, were plotted in Fig.1. The results in Fig.1 revealed that the presence of TiO2 NPs causes reduction in the Vmax of pepsin catalyzed reaction. The values of Vmax and Km of the pepsin catalyzed reaction in the presence of TiO2 NPs are cited in Table 1. These weights are selected from a calculations for the amount of TiO2 NPs needed to be coated with a monolayer of pepsin molecules. The first weight (15 mg) of TiO2 NPs in the reaction mixture represents a NPs coated with a monolayer of pepsin molecules. The next weights used are the duplicates of the weight required to make a single layer of pepsin on the TiO2 NPs surface. The results showed that the reduction percentage in the Vmax of the immobilized enzyme is about 34.65% from the free enzyme Vmax. The finding indicated that the forces between the immobilized presence of TiO2 NPs causes inhibition of pepsin activity. When the amount of TiO2 NPs increases five times the reduction in the Vmax is duplicates as seen in Fig.2. These extra effects may be due to the fact that the extra NPs will adsorb the substrate molecules (hemoglobin) also and make them unavailable to the enzyme active site. Fig.2 demonstrates the relationship between the maximum velocity of pepsin with the weight of TiO2 NPs. The results showed that TiO2 NPs have significant inhibitory effect on pepsin activity where the Vmax of the immobilized enzyme was decreased in comparison with the free enzyme. A few studies were deals with the effect of TiO2 NPs on protein level. The results showed that the activity of pepsin decreased with the increase of the concentration of NPs. Many techniques were used to prove the binding effect of TiO2 NPs on pepsin, which indicated potential usage for oral drug delivery of TiO2 NPs [20]. When NPs enter a biological fluid, proteins and other biomolecules compete for the nanoparticle surface, thus leading to the formation of a protein corona that defines the biological identity of the particle [21]. From the results of Fig.2 and Table 2, it can be concluded that the TiO2 NPs causes a mixed inhibition of enzyme activity as both Vmax and Km are changed. In some studies, it is found the polyamidoamine, alginate, and phenylpropanoid causes a mixed inhibition of pepsin activity [22, 23]. The mixed inhibition system reveals that both the inhibitor and the substrate can be bound to the enzyme at any given point of time. When both the substrate and the inhibitor are bound, the enzyme-substrate-inhibitor complex cannot form a product but can only be converted back into the Enzyme-substrate complex or the enzyme-inhibitor complex [24]. In one study, it is found that TiO2 NPs had coordination interaction with pepsin besides physical binding effect. The secondary structure of pepsin was unfolded with the treatment of TiO2 NPs which might consequently affect the active center of pepsin, and then reduce the enzyme activity [25]. The results also found that pepsin molecules are only physically adsorbed on the TiO2 NPs surface. The effect on activity and on the secondary structure of pepsin were resulted from non-covalent reactions, including electrostatic force and hydrophobic binding [25]. The interaction between TiO2 NPs and pepsin has a different effects on the Km as the concentration of TiO2 NPs increases as seen in Fig. 3. As the weight of the added TiO2 NPs increases, the concentration of the substrate required to reach the half of Vmax increases due to the adsorption of some of the substrate on the surface of NPs. But when the velocity reduced significantly, the concentration required to reach the low Vmax is also reduced. The explanation based on the mathematical bases because the Km can be calculated from multiplying the Vmax by the slope. The changes of Km and Vmax values are probably caused by several factors. Some active sites of pepsin enzyme can be hidden and damaged when the enzyme become immobilized on the solid surfaces [26]. In one study, it is found that the Km of immobilized enzyme is increased, whereas the Vmax is decreased. Further, the Vmax/Km value for immobilized pepsin is about 50% of the value for free enzyme [27].
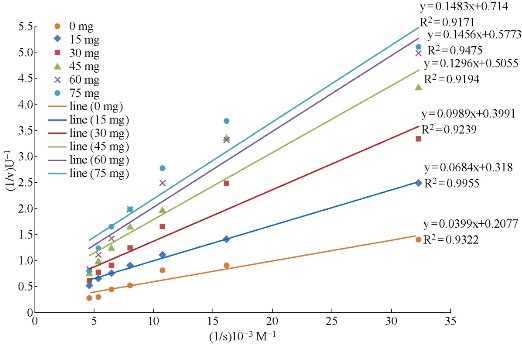
Fig.1 Lineweaver-Burke lines of pepsin catalyzed reaction after adding different amounts of TiO2 NPs at 37°C.
Table 1 Effect of weight of TiO2 at 37°C on the activity of pepsin immobilized on TiO2 NPs.
|
Wt of Tio2 NPs (mg) |
Vmax (U) |
Km*10-5 (M) |
Reduction in Vmax (%) |
Increase in Km (%) |
|
0 |
4.82 |
19.21 |
0 |
0 |
|
15 |
3.15 |
21.55 |
34.65 |
12.18 |
|
30 |
2.51 |
24.82 |
47.93 |
29.20 |
|
45 |
1.98 |
25.66 |
58.92 |
33.58 |
|
60 |
1.73 |
25.19 |
64.11 |
31.13 |
|
75 |
1.40 |
20.76 |
70.95 |
8.07 |
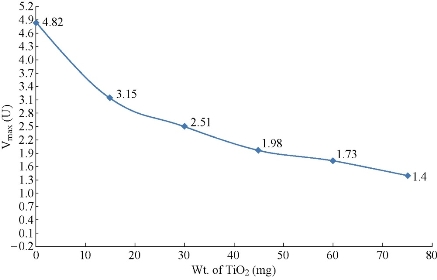
Fig.2 Effect of weight of TiO2 NPs on the maximum velocity of pepsin catalyzed reaction.
Table 2 Effect of temperatures on the activity of TiO2-coated pepsin.
|
Temp. (°C) |
Slope |
Intercept |
Vmax (U) |
Km*10-5 (M) |
|
22 |
0.277 |
0.507 |
1.97 |
54.74 |
|
27 |
0.155 |
0.485 |
2.06 |
31.98 |
|
32 |
0.123 |
0.421 |
2.38 |
29.20 |
|
37 |
0.068 |
0.318 |
3.15 |
21.51 |
|
42 |
0.128 |
0.304 |
3.29 |
42.17 |
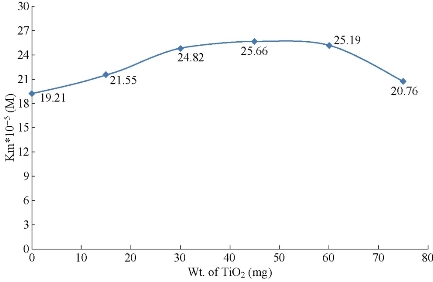
Fig. 3 Effect of weight of TiO2 NPs on the Michaelis constant of the pepsin catalyzed reaction.
Effect of temperature on the interaction of TiO2 NPs with pepsin
In the present experiments, the activity of the enzyme was measured at different temperatures (22, 27, 32, 37, & 42°C) by using 15 mg of TiO2 NPs and the same concentration of hemoglobin. These experiments were used to examine the effect of temperature on the activity of pepsin catalyzed reaction in the presence of TiO2 NPs, The graphical relationship of temperature effect on Vmax of pepsin activity is presented in Fig. 4. The values of Vmax and Km of the pepsin catalyzed reaction at different temperatures in the presence of TiO2 NPs are cited in Table (2). The results revealed that the Vmax at 37°C is 3.145 U and then reduced as temperature decreases. Whereas the Km at 37°C equal to 21.51x10-5 M then increases as temperature decreases. It means that the change in temperature may cause a change in the form of active site and thus will change Vmax. Changes of temperature of the environment can alter the intramolecular attractive forces (hydrogen bonding, dipole-dipole interaction, hydrophobic interaction etc.) of the protein (e.g. enzyme), This can alter the active site of the enzyme rendering it inactive [28]. The enzyme does not lose all activity and still works even in cases of immobilized pepsin. These results can be explained by the multiple effects of both TiO2 NPs and temperature. TiO2 NPs have the ability to adsorb protein molecules effectively as seen previously [29]. The adsorption of protein molecules on the solid surfaces caused a reduction in the enzyme activity. However, the adsorption capacity is temperature-dependent phenomenon [30]. Therefore, when the temperature increases at a certain degree, the pepsin molecules tend to desorbed from the surface of TiO2 NPs and became free again and hence restore some of their original activity and Vmax increases. These findings can be used in some medical and industrial applications when a long storage time and stability are required [30].
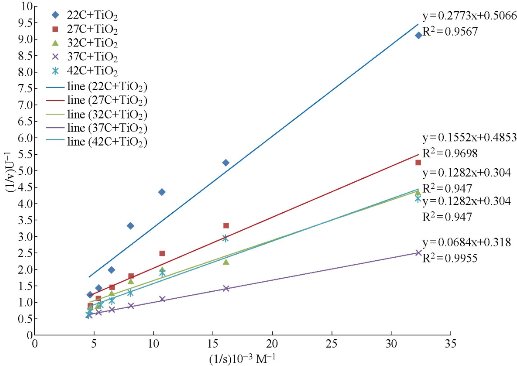
Fig. 4 Lineweaver-Burke lines of the TiO2-immobilized pepsin catalyzed reaction at (22, 27, 32, 37, & 42°C).
Effect of TiO2 NPs and ionic strength
In the present study, the immobilized pepsin on TiO2 NPs in the presence of 130 mg NaCl was completely inhibited. The activity of the enzyme is unmeasurable because the differences in the absorbance were zero. These results indicated that the effect of the combination of high ionic strength and TiO2 NPs causes a complete inhibition of pepsin activity. The loss of activity of pepsin may be explained by the negative action of the presence of TiO2 NPs and NaCl on the three dimensional structure of the active site of pepsin and the substrate (hemoglobin). These changes in the structures lead to prevention of binding of substrate with the active site due to the loss of fit between them. These findings are reinforced by the results of Zhu et al (2010) [25]. They found that the secondary structure of pepsin was unfolded after treatment with TiO2 NPs which might consequently affect the active center of pepsin, and then reduce the enzyme activity [25]. Other researchers studied the effect of surfactants salts on the activity of pepsin enzyme [31]. The proteolytic activity of pepsin is affected by the presence of the salts and a significant reduction in the activity of pepsin after adding different concentrations of surfactants salts was noticed [31]. Proteolysis of β-casein by pepsin was completely inhibited in the presence of 10% NaCl and was very significantly reduced by 5% NaCl [32]. Many researches focus on the positive effect of TiO2 NPs. For example, it was reported that TiO2 NPs could be used in protein separation and purification [18]. On the other hand, TiO2 NPs could easily enter into the human body via skin, gastrointestinal tract due to its ultrafine property [33]. The results of the previous researches and the findings of the present research encourages the use of TiO2 NPs as an inhibitor of pepsin for the treatment of GERD in vivo.
Conclusions
The results of the present study revealed that the TiO2 NPs has an ability to inhibit the pepsin activity. Both Km and Vmax values were changes after incubation of pepsin with these nanoparticles which suggested that the type of inhibition was mixed inhibition. The results showed that the best conditions of inhibition of pepsin enzyme is by using TiO2 NPs in the presence relatively high concentration of NaCl at 37°C.
Conflict of Interest
No potential conflicts of interest were disclosed.
References
[1] N. Vakil, S. van Zanten, P. Kahrilas, et al., The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol, 2006, 101: 1900-1920.
[2] R. Badillo, D. Francis, Diagnosis and treatment of gastroesophageal reflux disease. World J Gastrointest Pharmacol Ther, 2014, 5: 105-112.
[3] J. Kim, S. Oh, S. Myung, et al., Association between coffee intake and gastroesophageal reflux disease: a meta-analysis. Dis Esophagus, 2014, 27: 311-317.
[4] H. El-Serag, S. Sweet, C. Winchester, et al., Update on the epidemiology of gastro-oesophageal reflux disease: A systematic review. Gut, 2014, 63: 871-880.
[5] P. Zajac, A. Holbrook, M. Super, et al., An overview: Current clinical guidelines for the evaluation, diagnosis, treatment, and management of dyspepsia. Osteopathic Family Physician, 2013, 5: 79-85.
[6] C. Ho, Y. Goh, X. Zhao, et al., GERD: an alternative perspective. Psychosomatics, 2016, 57: 142-51.
[7] T. Samuels, N. Johnston, Pepsin as a causal agent of inflammation during nonacidic reflux. Otolaryngol Head Neck Surg, 2009, 141: 559-563.
[8] L. Zhao, S. Budge, A. Ghaly, et al.. Extraction, purification and characterization of fish pepsin: A critical review. J Food Process Technol, 2011, 2: 126-133.
[9] K. Nagahama, H. Nishio, M. Yamato, et al., Orally administered l-arginine and glycine are highly effective against acid reflux esophagitis in rats. Med Sci Monitor, 2012, 18(1): BR9-BR15.
[10] J. Sayer, J. Louis, Interactions of different inhibitors with active-site aspartyl residues of HIV-1 protease and possible relevance to pepsin. Proteins, 2009, 75: 556-568.
[11] National Institute for Occupational Safety and Health, NIOSH Pocket Guide to Chemical Hazards. Cincinnati, OH. June, 2002. Pub. No. 2002-140.
[12] D. Macwan, C. Shalini, A review on nano-TiO2 sol–gel type syntheses and its applications. J Mater Sci, 2015, 46: 3669-3686.
[13] M. Skocaj, M. Filipic, J. Petkovic, et al., Titanium dioxide in our everyday life; is it safe? Radiol Oncol, 2011, 45: 227-247.
[14] Z. Chen, Y. Wang, L. Zhuo, et al., Effect of titanium dioxide nanoparticles on the cardiovascular system after oral administration. Toxicol Lett, 2015, 239: 123-130.
[15] H. Shi, R. Magaye, V. Castranova, et al., Titanium dioxide nanoparticles: a review of current toxicological data. Part Fibre Toxicol, 2013, 10: 1-33.
[16] R. Tedja, M. Lim, R. Amal, et al., Effects of serum adsorption on cellular uptake profile and consequent impact of titanium dioxide nanoparticles on human lung cell lines. ACS Nano, 2012, 6: 4083-4093.
[17] S. Sousa, B. Manuela and P. Moradas-Ferreira, Dynamics of fibronectin adsorption on TiO2 surfaces. Langmuir, 2009, 32: 7046-7054.
[18] X. Chen, S. Mao, Titanium dioxide nanomaterials: synthesis, properties, modifications, and applications. Chem Rev, 2007, 107: 2891-2959.
[19] J. Knight, M. Lively, N. Johnston, et al., Sensitive pepsin immunoassay for detection of laryngopharyngeal reflux. Laryngoscope, 2005, 115(8): 1473-1478.
[20] Y. Wang, L. Yuan, C. Yao, et al., Cytotoxicity evaluation of pH-controlled antitumor drug release system of titanium dioxide nanotubes. J Nanosci Nanotechnol, 2015, 15: 4143-4148.
[21] C. Borgognoni, M. Mormann, Y. Qu, et al., Reaction of human macrophages on protein corona covered TiO₂ nanoparticles. Nanomedicine, 2015, 11: 275-282.
[22] X. Wu, W. Wang, T. Zhu, et al., Phenylpropanoid glycoside inhibition of pepsin, trypsin and α-chymotrypsin enzyme activity in Kudingcha leaves from Ligustrum purpurascens. Food Res Int, 2013, 54 : 1376-1382.
[23] M. Höldrich, A. Sievers-Engler and M. Lämmerhofer, Gold nanoparticle-conjugated pepsin for efficient solution-like heterogeneous biocatalysis in analytical sample preparation protocols. Anal Bioanal Chem, 2016, 408: 5415-5427.
[24] D. Si, Y. Wang, Y. Zhou, et al., Mechanism of CYP2C9 inhibition by flavones and flavonols. Drug Metab Dispos, 2009, 37: 629-634.
[25] R. Zhu, W. Wang, X. Sun, et al., Enzyme activity inhibition and secondary structure disruption of nano-TiO2 on pepsin. Toxicol In Vitro, 2010, 24: 1639-1647.
[26] C. Wu, J. Lee and W. Lee, Protein and enzyme immobilization on non-porous microspheres of polystyrene. Biotechnol Appl Biochem, 1998, 27: 225-230.
[27] J. Hu, S. Li and B. Liu, Properties of immobilized pepsin on Modified PMMA microspheres. Biotechnol J, 2006, 1: 75-79.
[28] A. Banga, Therapeutic peptides and proteins formulation, processing and delivery systems (2nd ed.). New York: Taylor & Francis, 2006.
[29] H. Wang, L. Du, Z. Song, et al., Progress in the characterization and safety evaluation of engineered inorganic nanomaterials in food. Nanomedicine (Lond), 2013, 8: 2007-2025.
[30] G. Altun, S. Cetinus, Immobilization of pepsin on chitosan beads. Food Chem, 2007, 100: 964-971.
[31] M. Guzman, M. Marques, M. Olivera, et al., Enzymatic activity in the presence of surfactants commonly used in dissolution media, Part 1: Pepsin. Pharma Sci, 2016, 2: 15-19.
[32] P. Foxa, B. Walleya, Influence of sodium chloride on the proteolysis of casein by rennet and by pepsin. J Dairy Res, 2009, 38: 165-170.
[33] H. Liu, L. Ma and J. Zhao, Biochemical toxicity of nanoanatase TiO2 particles in mice. Biol. Trace Elem Res, 2009, 126: 170-180.
Copyright© 2016 Hussein Kadhem Al-Hakeim, Khlowd Mohammed Jasem. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.