Editorial
Gastric Cancer Nanotheranostic Engineering: Gastric Cancer Prewarning and Early Theranostic System
Daxiang Cui *
Institute of Nano Biomedicine and Engineering, Shanghai Engineering Research Center for Intelligent Diagnosis and Treatment Instrument, National Center for Translational Medicine, Collaborative Innovational Center for System Biology, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai 200240, P. R. China.
* Corresponding author. E-mail: dxcui@sjtu.edu.cn
Published: Sep. 20, 2016
Citation: Daxiang Cui, Gastric Cancer Nanotheranostic Engineering: Gastric Cancer Prewarning and Early Theranostic System. Nano Biomed. Eng., 2016, 8(3): 109-111.
DOI: 10.5101/nbe.v8i3.p109-111.

Daxiang Cui is Professor at the Institute of Nano Biomedicine and Engineering, Shanghai JiaoTong University, and Editor-in-chief of Nano Biomedicine and Engineering. His research interests focus on nano theranostic technologies and their clinical translations.
Gastric cancer is the fourth commonest cancer and the second leading cause of cancer-related death worldwide [1]. Its incidence ranks no. 2 and its mortality rate ranks no. 3 among all malignant tumors in China according to the latest cancer disease spectrum [2]. The gastric cancer prognosis is very poor with 5-year survivals below 24% [3]. Early gastric cancer can be cured with surgery; advanced gastric cancer often needs multidisciplinary combined therapy. Gastric cancer seems insensitive to current chemotherapy agents, which may be closely related to stomach cancer stem cells [4]. Early gastric cancer refers to the in situ gastric cancer which locates only in stomach mucous membraneand does not infiltrate into thin submucosa. In China, early gastric cancer diagnosis rate is less than 20%, while the diagnosis rate of early gastric cancer in Japan and South Korea has reached 30% - 50% [5,6]. It is definite to classify H. pylori as the class I carcinogen by the World Health Organization. Approximately 75% of the global gastric cancer are attributable to H. pylori-induced inflammation and injury [7]. To date, the precise mechanism of H. pylori-associated gastric carcinogenesis has not been well clarified. To develop prewarning and early theranostic system of gastric cancer can save a lot of patients with gastric cancer and can also reduce the cost of treatment and even cure or significantly prolong the lifespan of patients. Tumor biology studies show that formation process of gastric cancer includes three stages such as normal gastric mucous stage, precancerous lesion stage and gastric cancer stage. For every stage, there exists a lot of differentially expressed genes and proteins. Understanding the biological processes of cancer initiation at the gene expression level is very important for early cancer detection. In the course of normal gastric mucous to precancerous lesion and precancerous lesion to gastric cancer, there exist characteristic differentially expressed genes and proteins. Using these changes of transcript levels and translation levels from differentially expressed genes and proteins as theory basis, through real-time examination of gene and protein expression level changes, gastric cancer pre-warning and early diagnosis system can be established. How to find key differentially expressed genes and proteins involved in initiation and progression of gastric cancer has become key scientific problem. Our team firstly proposed the concept of gastric cancer pre-warning and early diagnosis system concept in 1998[8]. The primary concept of gastric cancer prewarning and early diagnosis system is that the information processing system and bio-chip technology platform were used as support tool, gastric biopsy specimens or blood samples were checked; the expression level changes of differentially expressed genes or proteins associated with normal gastric mucosa, precancerous lesions and gastric cancer were examined; and bioinformation analysis tool was used to compare examined results with prewarning database, and to determine whether the patient is in the stage of gastric cancer, or precancerous lesions or normal gastric tissues. As shown in Scheme 1, the system consists of three parts, that is, gastric cancer prewarning chip, gastric cancer warning database and information processing system. In order to establish gastric cancer prewarning and early diagnosis system, we finished screening of differentially expressed genes associated with normal gastric mucous, precancerous lesion and gastric cancer status, designed and fabricated the gastric cancer gene expression profile chip, developed the gastric cancer information processing software, and established primary gastric cancer prewarning data library[9]. Primary gastric cancer prewarning and early diagnosis system can be used for advanced gastric cancer diagnosis, prognosis analysis and the therapeutic effective evaluation. However, this gastric cancer pre-warning and early diagnosis system cannot solve all the problems associated with gastric cancer diagnosis. For example, gastric cancer prewarning and gastric cancer genotyping. The prewarning database lacked serological examination database of gastric cancer and imaging detection database as well as breath biomarker detection database. In order to make this system more suitable for clinical application, it is very pressing to improve the gastric cancer prewarning and early diagnosis system. In order to make the gastric cancer prewarning and early diagnosis system perfect, three key scientific problems must be solved, that is, gastric cancer early biomarker problem, biomarker ultrasensitive simultaneous detection problem, and quantitative and qualitative visual problem of gastric cancer biomarkers in vivo. With the rapid development of nanotechnology, the emerging nanotheranostic engineering brings new opportunities to solving the three key scientific problems associated with prewarning and early diagnosis system of gastric cancer. It also broadens the concept, that is, the concept of gastric cancer prewarning and early theranostic system. Nanotechnology realizes simultaneous diagnosis and therapy[10]. Nanoparticles own unique nano-effects, and especial sound, light, electricity, heat, and magnetic properties; the use of these features can not only enhance the marker detection sensitivity and specificity, but can also realize qualitative and quantitative visualization of gastric cancer biomarkers. More importantly, multifunctional nanoprobes make nanoparticles combined with molecular imaging and photothermal, chemo-photodynamic therapy together, and realize multimodal imaging-directed simultaneous therapy [11-15]. In recent years, great advances have achieved in gastric cancer biomarker screening and identification, nanoparticles controlled synthesis and characterization, established novel methods to enhance sensitivity and specificity of biomarker detection, prepared multifunctional nanoprobes for multi-modality imaging, in vivo genotyping and gastric cancer boundary identification, and biosafety evaluation of nanoprobes and their clinical trials. In this special issue, we focus on gastric cancer nanotheranostic engineering, summarize some advances of nanotechnology-based gastric prewarning and early theranostic system in recent years. We aim to accelerate the development of gastric cancer pre-warning and early diagnosis system based on nanotechnology, enrich gastric cancer prewarning big data, actively promote the clinical validation verification of gastric cancer prewarning and early diagnosis system, and promote the commercial development and clinical translation of this system.
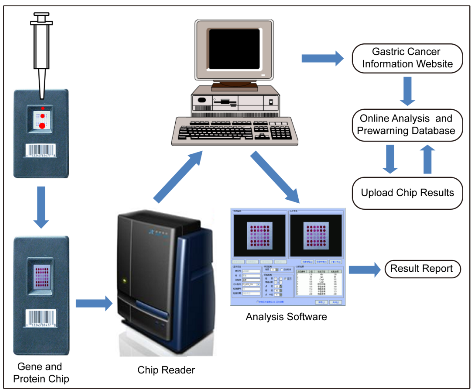
Scheme 1 Schematic of gastric cancer prewarning and early diagnosis system
References
Copyright© 2016 Daxiang Cui. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.