Review
Up-Conversion Nanoparticles for Gastric Cancer Targeted Imaging and Therapy
Yuming Yang 1,2*, Yu Han 1,2, Caixia Yue 1,2
1 Department of Instrument Science and Engineering, School of Electronic Information and
Electrical Engineering, Shanghai Jiao Tong University, Shanghai 200240, China.
2 Shanghai Engineering Research Center for Intelligent Diagnosis and Treatment Instrument, Shanghai 200240, China.
* Corresponding author. E-mail: yumingyang@sjtu.edu.cn
Received: Sep. 18, 2016; Accepted: Sep. 20, 2016; Published: Sep. 26, 2016.
Citation: Yuming Yang, Yu Han and Caixia Yue, Up-Conversion Nanoparticles for Gastric Cancer Targeted Imaging and Therapy. Nano Biomed. Eng., 2016, 8(3): 161-171.
DOI: 10.5101/nbe.v8i3.p161-171.
Yuming Yang was born in 1981 in Changchun, China. In 2009, she received her Ph.D. degree from Jilin University, and went to Fudan University as postdoctoral fellow. In order to enhance research level, Dr. Yang further made research cooperation with the department of Regenerative Medicine and Cell Biology, Medical University of South Carolina, USA and took office in University of Louisville in USA as a postdoctoral researcher during the years of 2012-2014. Since January 2015, she joined the School of Electronic Information and Electrical Engineering, Shanghai Jiao Tong University. Her major research interest is design and synthesis of multifunctional materials for tumor imaging and therapy.
Abstract
As a new generation of fluorophores, up-conversion nanoparticles (UCNPs) exhibit high penetration depth in tissues, low photodamage to biological samples, large Stokes shifts, sharp emission bands, free auto-fluorescence background and high photostability, thus making them an ideal kind of fluorescent labels for a variety of assay formats ranging from bio-imaging to cancer therapy. In this article, the method for the synthesis of biocompatible UCNPs, the recent progress regarding the UCNPs for multimodal targeted tumor imaging, drug delivery, photodynamic therapy (PDT), and photothermal therapy (PTT) are discussed on emphasis. The challenges and opportunities in this emerging field are also presented.
Keywords: Upconversion; Nanoparticle; Cancer therapy; Imaging; Drug delivery; Detection
Introduction
One of the most important achievements for the research of gastric cancer is the proposal of early gastric cancer (EGC) concept in 1962. The proposal of early gastric cancer concept aimed to make the clinical cure and longer survival time possible. After half a century of research and diagnosis, the knowledge on early gastric cancer is also gradually deepend. The diagnosis of early gastric cancer can be gradually refined. Especially in recent years, the guiding ideology for early treatment of gastric cancer, the original pursuit of longer survival period has changed to more requirements in the minimally invasive surgery to ensure that patients have a good quality of life, and so the treatment is also more personalized. At present, the diagnosis rate of early gastric cancer is still less than 10%, while in Japan it is as high as 70% ~ 50%. At present, the most important thing is the changing of diagnosis concept and the improvement of diagnosis rate of the early gastric cancer. To date, much effort has been put on the design of novel upconversion nanophosphors. Surface functionalized and multifunctional UCNPs have been synthesized. Great advances on the improvement of probe targeting, pharmacokinetics, biocompatibility, photophysics, and the maturation of multimodal techniques are also made. In this paper, we will introduce the synthesis, and the toxicity of the UCNPs. The progress of UCNPs applications on biological imaging, cancer, especially gastric cancer in recent years will also be introduced in detail.
Synthesis of Rare Earth Doped UCNPs
Due to the excellent properties and wide application prospect of UCNPs, the development of rare earth has attracted wide attention. At present, different methods have been developed to get upconversion nanophosphors, such as sol–gel [1-9], hydrothermal [10-15], co-precipitation [16-19], and thermal decomposition methods [20-33] etc. Nanoparticles synthesized by sol-gel method are usually not suitable for biological applications due to the particle agglomeration in the process of high temperature treatment. In contrast, hydrothermal method is a good method for the preparation of UCNPs with high crystallinity, homogeneous size, and good dispersion, but the disadvantage is that the process of crystal growth can not be observed. The thermal decomposition method and co precipitation method are developed in recent years, which can synthesize UCNPs with small and homogeneous size. At present, many papers have been reported based on both mehtods. Such as Daxiang Cui’s team of Shanghai Jiaotong University synthesized NaGdF4: 20%Yb, 2%Er based on the thermal decomposition method. The synthesized nanoparticles have good water solubility; the CT imaging and near infrared fluorescence imaging are successfully achieved [34] (Fig. 1). In order to obtain the UCNPs with good biocompatibility, "one step" method was developed in recent years with the help of hydrophilic or binary cooperative ligands, such as polyols [10, 35, 36], EDTA [37-40], citrate [37, 41-45], sodium dodecyl sulfate (SDS) [46], PVP [47], small-molecule binary acid [48, 49], polyethylene glycol (PEG) [49], PVP [50], PAA [51, 52], PEI[51], 3-mercaptopropionic acid (3MA) and 6-aminocaproic acid ( 6 A A ) [53] etc. This "one step" method has the advantages of high efficiency and could be operated at mild reaction conditions [54, 55]. Daxiang Cui‘s group reported the UCNPs with different mophorlogy in the presence of n-octanol and 1-butyl-3-methylimidazolium tetrafluoroborate [56]. The results show that the UCNPs synthesized by this method have good water solubility, and that the 1-butyl-3-methylimidazolium tetrafluoroborate palys very important role in controlling the size and morphology of the UCNPs (Fig. 2).
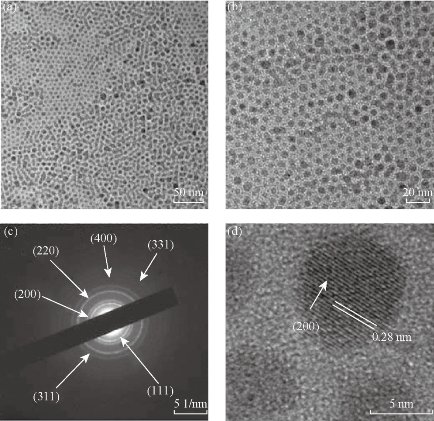
Fig. 1 (a) and (b) TEM images of NaGdF4 : 20%Yb, 2%Er; (c) SAED of UCNPs; d) HR-TEM of NaGdF4 : 20%Yb, 2%Er. Reprinted with permission of Ref 34.
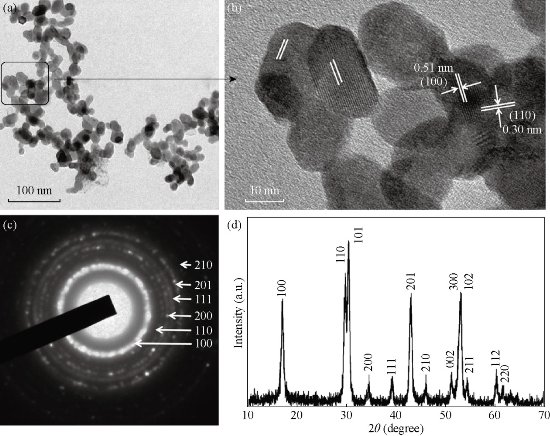
Fig. 2 (a) TEM image of NaGdF4:Yb,Er (n-octanol (10 mL)/BmimPF6 (15 mL)); (b) Magnification of selected area; (c) The corresponding SAED pattern; (d) The corresponding XRD pattern. Reprinted with permission of Ref 56.
UCNPs Used for Gastric Cancer Imaging
As a new imaging technique, UCNPs imaging offers a unique approach for visualizing morphological details in tissue with sub cellular resolution, and has come to be a powerful noninvasive tool for visualizing the full range of bio-species from living cells to animals. Exploitation in general internalization studies was performed using bare or modified UCNPs. They have been widely used for in vitro or in vivo imaging to demonstrate their promise in biological in vivo applications [57-60]. Kobayashi et al. demonstrated in vivo multiple color lymphatic imaging using upconverting nanocrystals in 2009 [61]. Multicolor in vivo lymph node mapping UCL imaging was further demonstrated by Liu’s group in the following year, and found the in vivo detection limit of UCNPs to be at least one order of magnitude lower than that of QDs [62]. Li’s group reported a series of articles related the UCNPs for lymphatic imaging [63-65]. Niagara et al. applied silica/NaYF4:Yb,Er to dynamically track live myoblast cells in vitro and in a living mouse model of cryo-injured hind limb [66]. In vivo confocal imaging of nanoparticle-loaded cells intravenously injected into a mouse tail vein showed them flowing in the ear blood vessels. Nanoparticle-loaded cells were also unambiguously identified with superior contrast against a negligible background at least 1,300 μm deep in a fully vascularized living tissue upon intramuscular injection. Tumor targeted molecular imaging plays a very important role in the diagnosis and prognosis of gastric cancer [67]. It has absorbed a wide range of attention in the use of UNCPs for tumor imaging in vivo. Different kinds of antibodies, aptamers, small molecules and peptides were conjugated on the surface of the UCNPs for targeted imaging of specific kinds of cells or biological molecules. Zako originally reported the tumor cell-targeted upconversion imaging using UCNPs modified with cyclic RGD peptide (RGD-Y2O3) [68]. FA is also widely chosen as targeting ligands for specific targeting and imaging of cancer cells. Cui’s group reported the NaYbF4: 25%Gd, 2%Tm/SiO2 modified by FA, which could be applied on the gastric cancer imaging in vivo [69] (Fig. 3). In addition to the optical imaging technique, molecular imaging technique also contains positron emission tomography (PET), magnetic resonance imaging (MRI), Single-Photon Emission Computed Tomography (SPECT) and X-ray computer tomography (CT). These techniques have already become routine clinical examination techniques in hospitals. However, each imaging modality has its own merits and disadvantages, and a single technique does not possess all the required capabilities for comprehensive imaging. Optical imaging as an inexpensive, robust and portable method provides the highest sensitivity and spatial resolution for in vitro imaging, but still lacks the full capability to obtain anatomical and physiological details in vivo. MRI and CT techniques have excellent spatial resolution, good depth for in vivo imaging and exceptional anatomic information, but suffer from limited sensitivity and lack resolution for imaging at the cellular level. Hence, multimodal UCNPs for bioimaging are quickly becoming important tools for biomedical research and clinical diagnostics. The most commonly used MRI contrast agent is Gd3+ chelate complex. The contrast of MRI imaging is improved by changing the exchange rate of the internal and external layers of the water to shorten the longitudinal relaxation time T1. Prasad group is one of the pioneers who originally developed Gd3+ and Er3+/Yb3+/Eu3+ codoped NaYF4 for the dual modality of optical and MR imaging [70]. For UCNPs used as CT contrast agents, there are two approaches to improve the X-ray attenuation coefficient. The first method is to increase the lanthanide elements content in a single particle. Liu et al. prepared the PEGylated Yb2O3:Er nanoparticles with high Yb content in a single particle suitable for both X-ray CT imaging and UCL imaging [71]. The second way is to choose higher atomic number elements among RE elements for the preparation of UCNPs. For the REF3 and NaREF4 fluorides, the La content in LaF3 is 70.9 %, much higher than other RE elements content in NaREF4. Therefore, REF3-based UCNPs can serve as excellent CT contrast agents and ideal building blocks for multimodal imaging agents. Based on this mechanism, FA conjugated silica modified LaF3:Yb,Tm UCNPs (UCNPs@SiO2-FA) with high La content in a single particle were strategically designed by Cui’s group for simultaneously targeted dual-modality imaging of UCL and CT [72]. Fig. 4 shows the real-time in vivo UCL images after intravenous injection of UCNPs@SiO2-FA in nude mice at 2 h. NaGdF4 is also a good contrast agent for UCL/CT dual mode imaging [73-77]. Cui’s team synthesized rare earth ion doped NaGdF4 micron crystal using one step synthesis method. This material displays good conversion luminescence, magnetism and biocompatibility, and were successfully used for gastric cancer CT imaging [78], as shown in Fig. 5. Compared with CT scan, an MRI is suited for examining soft tissue, such as ligament and tendon injury, spinal cord injury, brain tumors etc. While a CT scan is better suited for bone injuries, lung and chest imaging, and detecting cancers. Making a combination of UCL, CT and MRI imaging is no doubt a good way to realize better tissue scans [79-82]. PET is a nuclear medical imaging technique that produces a three-dimensional image or picture of functional processes in the body. PET scans are increasingly read alongside CT or MRI scans to provide excellent spatial resolution and high sensitivity. CT provides exceptional anatomical information, but suffers from limited sensitivity. PET provides a visualization method with high sensitivity, but a low (∼mm) spatial resolution. However, CT, PET, and MRI are all unsuitable for visualizing living cells because of low planar resolution, but this can be remedied by combining with UCNPs. The combination provides the highest spatial resolution and is suitable for imaging living cells.
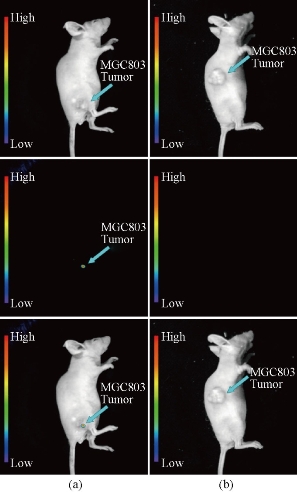
Fig. 3 Targeted in vivo NIR luminescence imaging of subcutaneous MGC803 tumor (indicated by short arrows) after intravenous injection of (a) NaYbF4: 25%Gd, 2%Tm-FA; and (b) NaYbF4: 25%Gd, 2%Tm @SiO2-NH2 nanoparticles for 4 h. Reprinted with permission of Ref 69.
Fig. 4 Real-time in vivo UCL images after intravenous injection of UCNPs@SiO2-FA in nude mice at 2 h. Reprinted with permission of Ref 72.
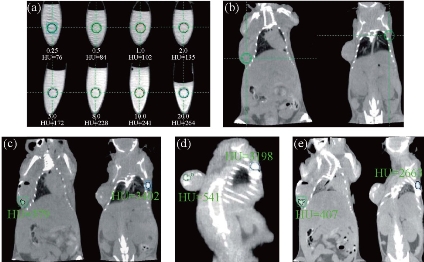
Fig. 5 In vitro CT imaging of (a) NaGdF4:Yb3+/Er3+ submicrocrystals suspended in PBS solution; (b) in vivo CT imaging of mouse before injection; (c) after injection; (d) after 2 h injection; and (e) after 4 h injection. As for the investigated nude mice, tumor sites are located in the left side of back, and normal tissues are located in the right side of back. Nude mice loaded with gastric cancer MGC803 cells was selected as the animal model. The tumor was implanted and grown in nude mice to an appropriate size (3–5 mm in diameter). Reprinted with permission of Ref 78.
UCNPs for Cancer Therapy
Apart from being used for cancer cell related bioimaging, UCNPs were selected as probes for disease related sensing [83-85] or therapy [86]. For example, mesenchymal stem cells have shown great potential in regenerative medicine. Sensitive and reliable methods for stem cell labeling and in vivo tracking are thus of great importance. Liu’s group reported the use of oligo-arginine conjugated UCNPs as an exogenous contrast agent to track mouse mesenchymal stem cells in vivo. As few as ~10 cells labeled with UCNPs are detected in vivo, which highlights the promise of using UCNPs as a new type of ultrasensitive probes for labeling and in vivo tracking of stem cells at nearly the single cell level [87]. In another case, Liu and coworkers developed MnO2 nanosheet modified UCNPs for rapid, selective detection of glutathione in aqueous solutions and living cells [88]. Up to now, most of the UCNPs drug delivery system were realized by coating PEG or mesoporous silica for probe attachment or drug loading [89-92]. Liu and coworkers functionalized UCNPs with a PEG grafted amphiphilic polymer. Then the PEGylated UCNPs were loaded with DOX molecules and conjugated with FA for targeted drug delivery and cell imaging. The loading and releasing of DOX from UCNPs are controlled by varying pH, with an increased drug dissociation rate in acidic environment, favorable for controlled drug release [89]. Cui and coworkers reported a multifunctional “nanorattle” hollow spheres that consist of RE-doped NaYF4 shells with a SiO2-coated Fe3O4 inner particle. The material emits visible luminescence upon NIR excitation and can be directed by an external magnetic field to a specific target, making it an attractive system for a variety of biological applications. In vivo experiments exhibit encouraging tumor shrinkage with the DOX loading and significantly enhanced tumor targeting in the presence of an applied magnetic field [93]. Although the SiO2 modified UCNPs have made a great progress, but the core/shell structure needs complicated procedure, and it is difficult to realize batch production. To solve this problem, Cui’s group proposed a one-step method for the synthesis of mesoporous structure of UCNPs. Drugs for the treatment of gastric cancer can be filled in the mesoporous of UCNPs and then delivered to the tumor site. SEM images of the synthesized mesoporous UNCPs are shown in Fig. 6. In view of Magneto-optical imaging features of NaGdF4, the nanoparticles can be successfully applied to CT imaging (Fig. 7) and the delivery of anti tumor drugs for treatment of gastric cancer [94]. Small interfering RNA (siRNA) has emerged as a gene-based therapy due to their highly desirable roles in RNA interference and gene silencing effects in biomedical research. The key requirements for an effective siRNA therapy is that sufficient siRNA needs to be introduced into cells or organs, and that the release of siRNA inside target cells in a highly spatial and temporal precision can be remotely controlled. Up to now, a few types of UCNPs have been developed for the purpose of effective siRNA delivery. Zhang and coworkers reported a UCNPs based system for targeted delivery of siRNA to cancer cells. The siRNA was attached to anti-Her2 antibody conjugated UCNPs, and the delivery of these nanoparticles to SK-BR-3 cells was studied, which certified the capability of using UCNPs as a fluorescent probe and delivery system for simultaneous imaging and delivery of biological molecules [95]. Very recently, Yang et al. reported a system of silica coated UCNPs, which were functionalized with cationic photocaged linkers through covalent bonding. Anionic siRNA could be effectively absorbed onto the linkers through electrostatic attractions and were easily internalized by living cells. Upon NIR light irradiation, the photocaged linker on the Si-UCNPs surface could be cleaved by the upconverted UV light and thus initiated the intracellular release of the siRNA [96]. PDT is a relatively new clinical therapeutic modality which involves killing of disease cells by excitation of photosensitizer chemicals with high-energy light to generate reactive oxygen species from surrounding dissolved oxygen [97-99]. However, poor tissue penetration of high-energy light and hydrophobic photosensitizers limits the effectiveness to superficial pathologies. UCNPs can be used to activates the photosensitizers in deep tissue because NIR light can penetrate a few centimeters into soft tissue due to weak absorption in the optical “transparent window”. Four main classes of photosensitizers have been approved by the U.S. Food and Drug Administration for clinical use against cancer cells, containing porphyrin derivatives, chlorins, phthalocyanines, and porphycenes [100, 101]. The second-generation photosensitizers of zinc (II)-phthalocyanine (ZnPc) have been proven highly selective for tumor targets and showed enhanced cytotoxic effects both in vitro and in vivo. PTT employs photo absorbers to generate heat from light absorption, leading to thermal ablation of cancer cells. Various nanomaterials with high NIR light absorbance such as gold and silver nanoshells, nanorods and nanocages etc. have been utilized for PTT treatment of cancer [102]. Song et al. reported the synthesis of core–shell structured hexagonal-phase NaYF4:Yb3+, Er3+@Ag nanoparticles and their unique biofunctional properties. HepG2 cells fromhuman hepatic cancer and BCap-37 cells from human breast cancer incubated with the composite UCNPs in vitro were found to undergo photothermally induced death on exposure to 980 nm [103]. Liu and coworkers synthesized a new class of multifunctional nanoparticles consisting a UCNP particle as the core, a layer of ultra-small iron oxide nanoparticles (IONPs) as the intermediate shell, and a thin layer of gold as the outer shell. The layer of IONPs between UCNPs and the Au shell not only affords the magnetic properties but also significantly reduces the luminescence quenching effects of the gold nanostructure to UCNPs. The UCNP–IONP–Au nanoparticles are then coated with PEG to improve its biocompatibility in physiological solutions. Those multifunctional nanoparticles are used for UCL/MR multimodal imaging as well as in vitro photothermal ablation of cancer cells [104]. In the next year of 2012, they achieved highly efficient in vivo magnetically targeted PTT by using the same multifunctional nanoparticles. In vivo dual modal optical/MR imaging of mice uncovers that by placing a magnet nearby the tumor, the UCNPs show high tumor accumulation after intravenous injection, which is ~8 folds higher than that of without magnetic nearby. An outstanding PTT therapeutic efficacy with 100 % of tumor elimination in a murine breast cancer model is realized [105]. Most recently, Hilderbrand et al. reported the design and synthesis of NaYF4:Er3+, Yb3+@SiO2 core-shell nanocomposites with highly absorbing NIR carbocyanine dyes in the outer silica shell for combined NIR imaging and photothermal therapy. Photo-thermal cell killing under 750 nm excitation light source demonstrated the capability of UCNPs for both diagnostic optical imaging and therapeutic thermal therapy [106]. With the development of Nano Science in the field of tumor diagnosis and treatment, the multi-modal treatment of cancers based on one kind of nanoparticles has been developed. Recently, Liu’s group reported a kind of double function UCNPs. Two kinds of material were selected for the surface modification of UCNPs to achieve the dual function of PTT and PDT. Bengal Rose was used to absorb the green fluorescence of UCNP to realize the PTT, and the infrared absorption dye of IR825 was used to realize PDT [107]. The Bu team reported a MRI/NIR dual- modal nanoparticle, which can treat tumor with chemo-/ radio-/ photodynamic modals [108].
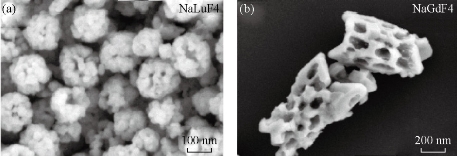
Fig. 6 SEM images of (a) NaLuF4; and (b) NaGdF4
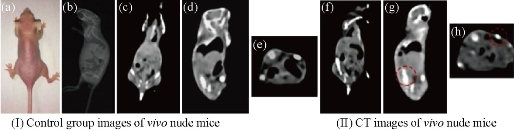
Fig. 7 (I) Images of control group before injection: (a) The photograph of the nude mouse; (b) X-ray image and (c), (d) and (e) CT images of nude mouse model as a control. (II) CT images of (f) coronal plane; (g) sagittal plane; and (h) transverse plane through subcutaneous injection with NaLuF4 upconversion nanoparticles. Reprinted with permission of Ref 94.
Conclusions
This article makes a review on recent progress in UCNPs for bioimaging and gastric cancer therapy. Despite that tremendous number of excellent results have been made in the past few years in this region, there are still many challenges which hinder potential applications of UCNPs as therapeutic and bioimaging agents. Firstly, the quantum yields of the most UCNPs is usually a little more than 0.005 % but no more than 0.3 %, which significantly limits the use of these UCNPs in optical bioimaging and PDT. Secondly, the potential long-term toxicity study of rare earth doped UCNPs is still in its infancy. Much more systematic investigations are still in need. Thirdly, for UCNPs to be successfully used as in vivo diagnostic agents, any small changes to UCNPs shape, size, surface charge and coating will have big impacts on its behaviors toward biological systems. A systematic research on these aspects is still in great need.
Acknowledgments
This work is supported by the National Key Basic Research Program (973 Project) (No.2015CB931802), the National Natural Scientific Foundation of China (Grant Nos. 81225010, 81028009 and 31170961), the 863 project of China (Project No. 2014AA020700) and the Shanghai Science and Technology Fund (13NM1401500).
References
Copyright© 2016 Yuming Yang, Yu Han and Caixia Yue. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.