Research Article
The Determination of Biomass / Selenium Ratio and the Impact of Glucose and Piperitone upon Selenium Ion Reduction Using Bacillus sp. MSh-1 Whole Cells
Mojtaba Shakibaie 1, Erfan Kheradmand 2, Mohammad Hossein Yazdi 2, Faranak Mavandadnejad 2, Ruhollah Mirjani 3, Ahmad Reza Shahverdi 2*
1 Herbal and Traditional Medicines Research Center, Kerman University of Medical Sciences, Kerman, Iran.
2 Department of Pharmaceutical Biotechnology and Biotechnology Research Center, Faculty of Pharmacy, Tehran University of Medical Sciences, Tehran, Iran.
3 Department of Advanced Technologies, Faculty of Medicine, AJA University of Medical Science, Tehran, Iran.
* Corresponding author. E-mail: shahverd@sina.tums.ac.ir; Tel/Fax: +9864122303.
Received: Mar. 6, 2017; Accepted: Mar. 15, 2017; Published: Mar. 20, 2017.
Citation: Mojtaba Shakibaie, Erfan Kheradmand, Mohammad Hossein Yazdi, Faranak Mavandadnejad, Ruhollah Mirjani, and Ahmad Reza Shahverdi, The Determination of Biomass / Selenium Ratio and the Impact of Glucose and Piperitone upon Selenium Ion Reduction Using Bacillus sp. MSh-1 Whole Cells. Nano Biomed. Eng., 2017, 9(1): 15-20.
DOI: 10.5101/nbe.v9i1.p15-20.
Abstract
Many bacteria are potential to reduce the metal ions to elemental nanoparticles and are able to grow in the presence of these inorganic compounds. Whole bacterial cells suspended in aqueous media can also convert some soluble metal ions into elemental nanomaterials. In this study, the reduction of different concentrations of selenium ions was investigated using various amounts of Bacillus sp. MSh-1 whole cell biomass, with the aim of increasing the yield of selenium nanoparticles. The MSh-1 test strain used in this study was previously isolated from the Caspian Sea (north of Iran) which can produce selenium nanoparticles. Reduction of selenium ions by whole Bacillus sp. MSh-1 cells in normal saline had a close relation between the ratio of applied biomass and the selenium ion concentration, as no considerable reduction was detected in the ratios lower than 1 gram of biomass for each 100 mg of selenium. Neither compounds of piperitone nor glucose had positive effects on selenium reduction by Bacillus sp. MSh-1. It also should be mentioned that Se NPs were not efficiently produced even after 20 hours and 32 hours, respectively.
Keywords: Bacillus; Selenium nanoparticles; Glucose; Piperitone; Reduction
Introduction
Toxic metals or metalloid ions are reduced to their elemental forms by living organisms as a strategy for decreasing the toxicity of these ions [1, 2]. Metal or metalloid ions are usually secreted as amorphous or crystalline nanomaterials within the intercellular spaces of the living cells [3]. These secreted nanomaterials can be released and separated for experimental use following cell disruption by various physical and chemical methods [4]. Selenium, a vital trace element for both eukaryotes and prokaryotes has broad applications [5]. It is also a micronutrient metalloid with extensive applications [6]. Dietary selenium compounds play an important role in human health. They show various benefits including antioxidant effects, cancer prevention, and antiviral activities [7, 8]. A number of different microorganisms are known to reduce selenium ions to elemental selenium nanoparticles (Se NPs) [9-11]. Recently, Bacillus sp. MSh-1 strain was isolated from the Caspian Sea in the northern part of Iran which reduced Se4+ ions to elemental Se NPs under aerobic conditions. This isolation is not only useful for bioremediation purposes but can also be used for the preparation of intercellular Se NPs [12]. In addition, we reported another biological method for preparation of Se NPs using Klebsiella pneumoniae [13]. Both of these biological methods allowed reduction of selenium ions to be performed in culture media containing the test strains. The Bacillus sp. MSh-1 cultures had a negligible residual concentration of Se4+ after 16 hours of incubation and the rate of biological reduction of selenium ions was more remarkable than the rate obtained from K. pneumoniae. The viability and potency of selenium reduction by Bacillus sp Msh-1 cells closely depend on the initial concentrations of Se4+ ions used for the production of Se NPs [12]. In the present study, this limitation was addressed by separating the biomass of Bacillus sp. MSh-1 and investigating its capability to reduce the toxic amount of Se4+ ions to SeNPs. On the other hand, membrane-bound nitrate reductase and periplasmic nitrate reductase in different bacteria have been reported to utilize selenate and tellurite ions as electron acceptors and reduce these ions to elemental particles [14, 15]. Some monosaccharides have also been reported to interfere with metal biotransformation [16]. Thereupon, the focus of the present study is to obtain a better understanding of the mechanisms involved in selenium bioreduction by Bacillus sp MSh-1 by studying the interaction of piperitone, a reported inhibitor of nitroreductase enzymes [17] and glucose, which interferes with transport of selenium ions into bacterial cells.
Materials and Methods
Compounds and microorganism
Ferrous sulfate, glucose, nutrient broth (NB), potassium thiocyanate, and selenium dioxide (SeO2) were purchased from Merck Chemicals (Germany). Piperitone (3-methyl-6-(1-methylethyl)-2-cyclohexen-1-one) was kindly provided by Charabot (06332 Grasse, France). All other chemicals and solvents were of analytical grade and obtained from Merck Company. The Bacillus sp. MSh-1 strain used in this study had been previously isolated and confirmed [12]. This microorganism was grown on nutrient agar (NA) plates and contained 1.26 mM SeO2 and sub-cultured every 14 days.
Determination of the Se4+ ion concentration
Se4+ ion concentration was determined by an indirect spectrophotometric method, following the procedure of Shakibaie et al., with some modifications [12]. The method was based on oxidation of Fe2+ ions to Fe3+ ions by Se4+ ions under acidic conditions. Subsequent addition of SCN− ions causes the Fe3+ ions to produce a blood-red complex with a maximum absorption at 480 nm [18]. Different concentrations of Se4+ ions (1–100 mg l−1) in deionized water were prepared with SeO2. 1 ml of Se4+ solution was separately transferred to a test tube. Moreover, 1 ml of 0.16 M FeSO4, freshly prepared with 5 M HCl was added to the mentioned tube. The mixtures were allowed to stand at 25 °C for 20 min. Then 1 ml of 5 M potassium thiocyanate solution was added to it. After the reagents were mixed, 100 μl of the mixture was diluted with 900 μl of deionized water, and the absorbance was measured at 480 nm with a UV–visible spectrophotometer (UVD-2950; Labomed). A calibration curve was prepared by plotting the measured absorbance at 480 nm against the known Se4+ ion concentrations. All absorption measurements incorporated a blank consisting of 1 ml of deionized water subjected to the same reaction steps. These procedures were repeated three times on different days, and the mean of the absorbance values was adopted to draw a suitable standard curve. Some samples that contained only trace concentrations of selenium ions (< 1 mg l−1) were analyzed by atomic absorption spectroscopy (Varian Techtron).
The effect of glucose on selenium ion reduction
A set of growth experiments was performed to study the effect of glucose on the ability of Bacillus sp. MSh-1 to convert Se4+ ions to SeNPs. The growth medium consisted of nutrient broth media (NB) (100 ml) (pH 7) and supplemented with Se4+ ions (100 mg l−1; which is equal to 1.26 mM SeO2). This was further supplemented with a filter-sterilized stock solution of glucose to obtain the final concentrations of 0.5, 1, 2 and 4 mg ml−1. A medium without glucose supplementation was used as a control. All culture media were inoculated with 1 ml of the fresh Bacillus sp. MSh-1 inocula (OD600, 0.1). The flasks were plugged with cotton and incubated at 30 °C for 32 hours in a shaker incubator (150 rpm). Residual selenium ion concentrations were then determined by sampling at different time intervals during the incubation period, using the indirect spectrophotometric method described above. All experiments were performed in triplicate.
The effect of piperitone on selenium ion reduction
The role of nitroreductase enzymes in the reduction of selenium ions to SeNPs was examined by adding piperitone at a sub-inhibitory concentration (200 µg ml-1) to NB media containing Se4+ ions (100 µg ml-1). Dimethyl sulfoxide (DMSO) (1% v/v) was used as a co-solvent. In a similar trial, NB media containing Se4+ ions were prepared without piperitone and used as a control. All culture media were inoculated with fresh Bacillus sp. MSh-1 inocula (1 ml; OD600 = 0.1). The flasks were plugged with cotton and incubated at 30 ◦C for 24 hours in a shaker incubator (150 rpm). Residual selenium concentrations were determined by withdrawing samples at different intervals, centrifuging the samples (6000 ×g, 30 min), and measuring the Se ion concentrations in the supernatants with the above mentioned method. All experiments were performed in triplicate.
Selenium ion reduction by biomass
A fresh inoculum of Bacillus sp. MSh-1 was added (5% v/v) to NB culture medium (2000 ml) and incubated in a shaker incubator (200 rpm) at 30 °C for 24 hours. In the next step, the biomass was separated by centrifugation (6000 ×g, 30 min) and washed with a sterile normal saline solution. Subsequently, different amounts of collected biomass (1, 2, 4 and 8 g) were suspended in sterile normal saline solution supplemented with sub-toxic (200 mg l-1) and toxic (400, 800 and 1600 mg l-1) concentrations of Se4+ ions. A series of sterile normal saline solutions with no inoculation were also supplemented with Se4+ ions and used as control samples. All reaction vessels were incubated in a shaker incubator (200 rpm) at 30 °C for 48 hours. The residual Se4+ ions in the samples and control vessel were determined at different incubation times (24 and 48 hours). No reduction was observed in the control flasks, and so the yield of Se4+ reduction (%) was calculated simply by the following equation:
Se4+ reduction (%) = (remaining Se4+) / (initial Se4+) ×100.
Results and Discussion
Selenium ion reduction by whole cells of Bacillus sp. MSh-1
Bacillus sp. MSh-1 has previously been shown not to grow aerobically on the media that contained SeO2 concentrations greater than 3.16 mM (250 mg l−1 Se4+ions). Consequently, the bacterium requires Se ions at sub toxic levels to produce Se NPs in the culture media [12]. In the present study, whole cells of Bacillus sp. MSh-1 were first cultured in the absence of selenium ions. In the next step, the whole cells were mixed with toxic concentrations of Se4+ions and evaluated with the aim of increasing the yield of Se NPs. The Se4+ reduction (%) at different ratios of Bacillus biomass and Se4+ ions (200, 400, 800 and 1600 mg l-1) after 24 hours was shown in Fig. 1. No appreciable Se4+ion reduction occurred at any selenium concentration when the bacterial concentration was only 1 g l-1. The addition of larger amount of biomass (2, 4, and 8 g l-1) resulted in a complete reduction (> 99%) of all Se4+ ions of all tested selenium concentrations. These results indicated that the ratio of biomass to Se4+ion concentration is a critical factor in the initiation of the reduction process by whole cells, and this ratio should not be less than 1 g of biomass per 100 mg l-1 Se4+ ions. Also, no considerable changes in Se4+ reduction rates were detected after longer incubation (48 hours) (Data not shown).
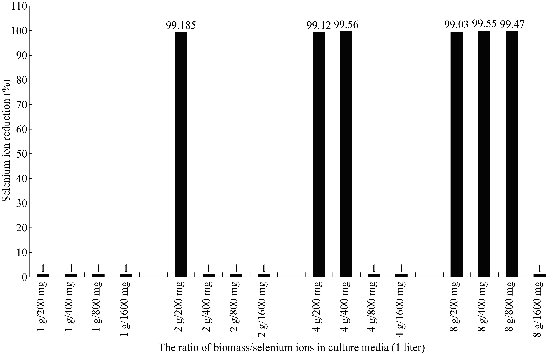
Fig. 1 The reduction of Se4+ ions by Bacillus sp. MSh-1 at different ratios of biomass/selenium ion concentration after 24-hour incubation.
The retardation of Se4+ion reduction by glucose
It was shown in Fig. 2 that different Se4+ion reduction patterns can be observed when cells cultured in NB culture medium were supplemented with different concentrations of glucose. The Se4+ ions were completely reduced in NB medium with no supplemented glucose, and no remaining Se4+ ions were detected in culture supernatants collected after 14 hours. In contrast, Se4+ ions were detected even after 32 hours when cells were cultured in the presence of glucose. Higher Se4+ concentrations were detected at higher concentrations of glucose (Fig. 2). In Fig. 3, it is shown that the increase in optical density (OD 600) of culture media supplemented with or without glucose was due to cell growth as well as the accumulation of the red Se NPs that were secreted by the bacterial cells during the Se4+ ion reduction process. This result shows that increasing the glucose concentration significantly decreased the rate of Se4+ ion reduction by Bacillus sp. MSh-1 (or Se NPs production). Some organic acids have been reported to compete with metal ions in translocation into the bacterial cells [19]. Glucose can be fermented by Bacillus sp. MSh-1 and results in a decrease in the pH of the culture medium [12]. The lactic acid produced from glucose by this bacterium may be indirectly involved in decreasing the rate of Se4+ ion reduction. In other words, the Se4+ ion uptake by bacterial cells can be limited in the presence of fermentation metabolites such as lactic acid; therefore, the concentrations of Se4+ ions increase in the culture medium.
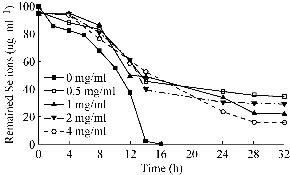
Fig. 2 The effect of different concentrations of glucose on Se4+ ion reduction profile by Bacillus sp. MSh-1. (The standard deviations were lower than 5% in three independent experiments, so all error bars were omitted for clarity of the picture.)
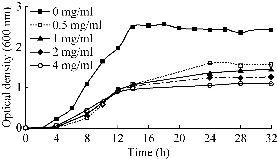
Fig. 3 Growth profile of Bacillus sp. MSh-1 in the presence of Se4+ ions (100 mg l-1) supplemented with different amounts of glucose. (The standard deviations were lower than 5% in three independent experiments, so all error bars were omitted for clarity of the picture.)
The effect of piperitone on Se4+ ion reduction
The effect of piperitone on the reduction of Se4+ ions by Bacillus sp. MSh-1 was shown in Fig 4. The major oxygen-insensitive nitroreductase of Enterobacteriacae, NfsA, is a flavoprotein that is able to reduce nitro groups in many different nitroaromatic compounds under aerobic conditions [20]. This enzyme is also responsible for the reduction of chromate to the less soluble and less toxic Cr (III) form [21]. As mentioned in the introduction, piperitone has been shown to have inhibitory effects on nitroreduction activity of Enterobacteriacae [22, 23]. This natural compound has also been reported to cause a partial inhibition of the reduction of Ag+ to metallic silver nanoparticles in K. pneumoniae [17]. Our recent docking simulation study demonstrated that piperitone can directly interact with the nitroreductase enzyme of Enterobacteria and reduce enzyme activity [24]. In the present study, piperitone did not completely inhibit Se4+ ion reduction by Bacillus sp. MSh-1; however, this monoterpene partially decreased the reduction rate of Se4+ ions when added to the culture broth, in contrast to the control flask (Fig. 4). The longer time needed for complete reduction of Se4+ ions may be related to the effect of piperitone on bacterial growth. The growth curves for Bacillus sp. MSh-1 when cultured in NB medium containing Se4+ ions in the presence and absence of piperitone were shown in Fig. 5. Although it was indicated in Fig. 5 this monoterpene did not absolutely inhibit the growth of Bacillus sp. MSh-1, the growth rate of this bacterium has delayed in the presence of piperitone in compared with its absence.
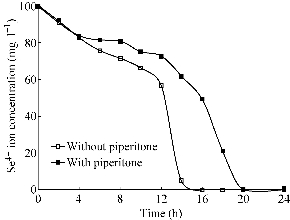
Fig. 4 Se4+ ion reduction pattern in the absence (0 µg ml-1) and presence (200 µg ml-1) of piperitone. (The standard deviations were lower than 5% in three independent experiments.)
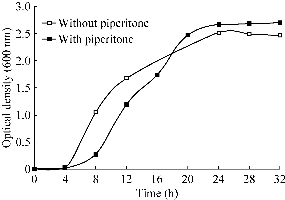
Fig. 5 Growth profile of Bacillus sp. MSh-1 in the presence of Se4+ ions (100 mg l-1) and piperitone (0 and 200 µg ml-1). (The standard deviations were lower than 5% in three independent experiments.)
Conclusions
To sum up, the inhibitory effect of piperitone on the bacterial growth may have indirectly prolonged the duration of Se4+ ion reduction by Bacillus sp. MSh-1 (Fig. 4). Nevertheless, piperitone can inhibit the reduction of silver ions by inhibition of the nitrate reductase in K. pneumoniae [17], no similar effect of this compound was observed for the reduction of Se4+ions by Bacillus sp. MSh-1.
Acknowledgments
The first part of this investigation was funded by the Herbal and Traditional Medicines Research Center, Kerman University of Medical Sciences (Kerman, Iran), and the second part was supported by the Faculty of Pharmacy, Tehran University of Medical Sciences, Tehran, Iran (grant No 11756). The authors express their thanks to these academies.
References
Copyright© 2017 Mojtaba Shakibaie, Erfan Kheradmand, Mohammad Hossein Yazdi, Faranak Mavandadnejad, Ruhollah Mirjani, and Ahmad Reza Shahverdi. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.