Research Article
Electrochemical Effect of Ascorbic Acid on Redox Current Peaks of CoCl2 in Blood Medium
Muhammed Mizher Radhi 1*, Hanaa Naji Abdullah 1, Majid Sakhi Jabir 2, Emad Abbas Jaffar Al-Mulla 3**
1Department of Radiological Techniques, Health and Medical Technology College, Middle Technical University, Baghdad, Iraq.
2 Analytical Technical Lab, Health and Medical Technology College, Middle Technical University, Baghdad, Iraq.
3 Department of Applied Science, University of Technology, Baghdad, Iraq.
4 Department of Chemistry, Faculty of Science, University of Kufa, P.O. Box 21, An-Najaf 54001, Iraq.
* Corresponding authors. E-mail: mmrradhi@yahoo.com; imad.almulla@uokufa.edu.iq
Received: Apr. 11, 2017; Accepted: Apr. 26, 2017; Published: May 3, 2017
Citation: Muhammed Mizher Radhi, Hanaa Naji Abdullah, Majid Sakhi Jabir, and Emad Abbas Jaffar Al-Mulla, Electrochemical Effect of Ascorbic Acid on Redox Current Peaks of CoCl2 in Blood Medium. Nano Biomed. Eng., 2017, 9(2): 103-106.
DOI: 10.5101/nbe.v9i2.p103-106.
Abstract
Cyclic voltammetric technique using glassy carbon electrode of cobalt chloride Co(II) was studied in blood medium for the effect of ascorbic acid as an electrocatalyst reagent on the oxidation reduction current peaks of Co(II). It was found that the two oxidation reduction current peaks for Co(II) in 0.1M KCl as electrolyte were at 900 and 250 mV respectively. In the study of Co(II) in blood medium the result was different, in that the reduction current peak disappeared and the oxidation current peak shifted to a higher potential at 1.4 V. The other study for the effects of different concentrations of ascorbic acid on the anodic current peak of Co(II) in blood medium showed an enhancement of the oxidation current peak about three times and the ascorbic acid acted as an electrocatalyst in blood components which caused damage to blood cells.
Keywords: Co(II); Cyclic voltammetry; Blood medium; GCE
Introduction
Human beings need cobalt compounds in small amounts, but cobalt is toxic in large quantities. It can cause skin irritation when touched. The human body needs small amounts of cobalt for certain vitamins. Cobalt compounds are used to stop cyanide from poisoning the body [1-3]. Many scientists in the early time studied the electrochemical properties of pollutants in blood medium by the cyclic voltammetric techique [4-8]. The study of the kinetics of cobalt electro winning and the mechanism of the involved reactions have been carried out. The obtained results confirmed the mechanism of cobalt precipitation by depletion of hydroxides. The effects of temperature and scan rate parameters were studied on electro-winning of cobalt by cyclic voltammetry technique. The diffusion coefficient and the rate constant of the reactions were measured and calculated by performed experiments [9]. The cyclic voltammetric (CV) behavior of tris(1, O-phenanthroline)cobalt(III), Cohen) in an aqueous medium which upon the addition of DNA can be used to probe the interaction between these species. Coordination complexes of 1,IO-phenanthroline and 4,7-diphenyl-I,IO- phenanthroline with Ru(I1) and Co(III) and other metal chelates3 that intercalate between the stacked base pairs of native DNA have been actively investigated as probes of DNA structure in solution and as stereo selective or conformation-specific agents for the photo activated cleavage of DNA [10]. Pencil-based renewable carbon paste electrode modified with Zn/Al-2(3-chlorophenoxy)propionate (Zn/Al-CPPA) nanocomposite was developed for the voltammetric determination of cobalt(II). The best voltammetric response was achieved for an electrode composition of 7.5% Zn/Al-CPPA nanocomposite in the paste, 0.1 M sodium acetate of pH 8.0 and the scan rate at 100 mVs-1 in the voltammetric measurement. Under optimized conditions, a linear response to cobalt(II) was found in the ranges of 1x10-3 M - 1x10-8 M and detection limit of 1.26 x 10-8 M. High sensitivity and reproducibility were found for the voltammetric determination of cobalt(II) [11]. Cobalt hydroxides were studied on parent substrates with different structural properties: nano-fibre structure, magnetron-sputtered and mechanically treated. The nano-fibred structure was formed electrochemically from an alkaline plating solution. Hydroxide layers were formed on the substrates by anodic polarization. The charge capacity for the nano-fibre sample was found to be up to 5 times higher as compared to a conventional cobalt surface (mechanically abraded). Cyclic voltammetry showed that the electrochemical charge transfer reactions Co(II) ↔ Co(III) ↔ Co(IV) were not associated with a remarkable electrode mass change [12]. The reaction of divalent cobalt(II) and trivalent ruthenium(III) salts (NO3, SCN and SO4) with macrocyclic ligands L1, L2 and L3 with N2S2, N4 and N5 cores, have been designed and carried out. All these three macrocyclic ligands and their complexes were obtained in the pure form. Electrochemical studies showed that the macrocyclic ligand L1 was more effective electron donors to ruthenium than L2 and L3. Electronic spectral properties also showed that the sulphur donor atom of L1 weakened the ligand field with respect to ligand-to-metal charge-transfer band. [13-17]. In this work, Co(II) ion was studied by electrochemical analysis using voltammetric technique to find the properties of redox current peaks of cobalt compounds in blood medium in the presence of AA (ascorbic acid).
Experimental
Reagents and chemicals
Blood samples were extracted from healthy humans. Cobalt chloride CoCl2.6H2O was bought from Seelze Hannover, Germany; AA was bought from Technicon chemicals Co. (Oreq.Tournai Belgique); KCl was bought from SCRC, China. Other chemicals and solvents were of annular grade and used as received from the manufacturers. Double distilled water was used for the preparation of aqueous solutions. All solutions were deaerated with oxygen free nitrogen gas for 15 min prior to performing the measurement.
Apparatus and procedures
Instruments
EZstat series (potentiostat / glvanostat) of NuVant Systems Inc., USA were used. Electrochemical workstations of bioanalytical system with potetiostate driven by electroanalytical measuring softwares were connected to ersonal computer to perform Cyclic Voltammetry (CV). An Ag/AgCl (3 M NaCl) and platinum wire (1 mm diameter) was used as a reference and a counter electrode respectively. The glassy carbon working electrode (GCE) was used in this study.
Results and Discussion
Identification of redox current peaks of Co(II) in aqueous solution
Fig. 1 shows the cyclic voltammograme of CoCl2 in electrolyte solution (0.1 M KCl). The electrochemical properties of cobalt ions in the aqueous solution had one oxidation current peak at 1 V and one reduction current peak at 0.3 V. Cobalt ions acted as an oxidative reagent in an electrolyte.
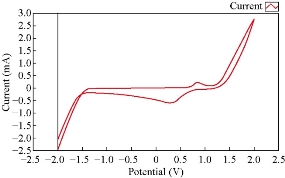
Fig. 1 The cyclic voltammograme of Co(II) in 0.1 M KCl using GCE, at 100 mVsec-1 versus Ag/AgCl.
Calibration graph of Co(II) for anodic current peak in aqueous solution
It was found that with increasing the concentration of Co(II) in KCl as an electrolyte, the anodic current peak of Co(II) increased as well. as shown in Fig. 2. The figure shows a good linearity of the relationship between the current and the concentration of cobalt ions as in Y = 52.45 X – 10.842 with good sensitivity, R2 = 0.9536. The increasing of the concentration of active ions caused the enhancement of current values. The behavior of the cobalt ion in the electrolyte was among the expected good results.
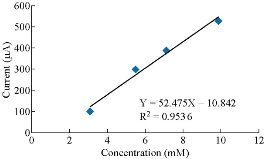
Fig. 2 The plot anodic current peak of Co(II) against different concentrations of Co(II) in 0.1 M KCl solution.
Effect of blood medium on redox current peaks of Co(II)
Fig. 3 & 4 show the effect of normal human blood medium as an electrolyte on the redox current peaks of cobalt ions. It was found that the reduction current peak of Co(II) in blood medium totally disappeared and the oxidation current peak enhanced about three times. Cobalt ions acted as an oxidative reagent in blood medium and the high concentration caused the decomposition of blood components. The trace of cobalt ion as a tonic medicine for human body was of enough amount; higher amounts would cause the bad state in blood decomposition by oxidation in the blood.
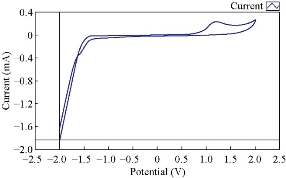
Fig. 3 The cyclic voltammograme of Co(II) in blood medium using GCE, at 100 mVsec-1 versus Ag/AgCl.
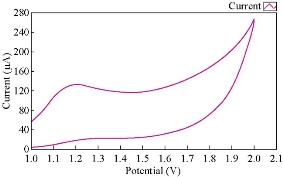
Fig. 4 The voltammograme of the anodic current peak of Co(II) in blood medium using GCE, at 100 mVsec-1 versus Ag/AgCl.
Effect of AA on the redox current peaks of Co(II) in blood medium
In blood medium cobalt ions with the presence of AA as an antioxidative reagent enhanced the oxidation current peak of Co(II) and shifted to a higher potential as shown in Fig. 5. But the new phenomena appeared when increasing the concentration of AA which decreased the oxidation current peak of Co(II) as shown in Fig. 6. When polotting the oxidation current peak of Co(II) agansit the concentration of ascorbic acid, the linear relationship was Y= 62.585 X – 70.814 with a good sensetivity, R2 = 0.982. AA acted as a good inhibiter for the effect of cobalt ions in blood medium.
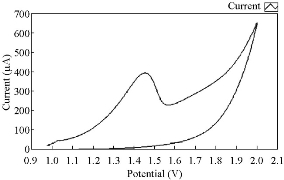
Fig. 5 The voltammograme of anodic current peak of Co(II) in blood medium with AA using GCE, at 100 mVsec-1 versus Ag/AgCl.
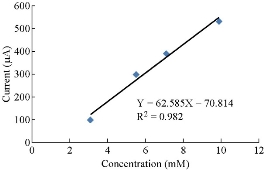
Fig. 6 The plot oxidation current peak of CoCl2 against the concentration of AA.
Conclusions
The electrochemical study of cobalt ions in the presence of important interference with AA using GCE in blood medium was reported by cyclic voltammetric technique. It was found that the redox current peaks of Co(II) in aqueous electrolyte (0.1 M KCl) as an oxidative and antioxidative metal in aqueous electrolyte. But in blood medium, the Co(II) ions had an oxidative behavior by reducing the reduction current peak. Also, it was found that AA acted as an electrocatalyst by enhancing the oxidative current peak in blood medium. Hence, the cobalt ion in blood medium acted as an oxidative agent and with AA acted as an inhibiter factor in blood medium.
References
Copyright© 2017 Muhammed Mizher Radhi, Hanaa Naji Abdullah, Majid Sakhi Jabir, and Emad Abbas Jaffar Al-Mulla. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.