Research Article
New Cyclic Voltammetry of 3-(4-N-Pyridine-2-yl Benzene Sulfonamide Azo)-1-Nitroso Naphthol and the Use of it for Enhancement of Cobalt Oxide Nano Particles
Hussein Jasem Mohammed *
Department of Chemistry, Faculty of Science, Kufa University, P.O. Box (21), Iraq.
* Corresponding author. E-mail: Hussein.alshujairi@uokufa.edu.iq
Received: Apr. 24, 2017; Accepted: May 27, 2017; Published: Jun. 19, 2017
Citation: Hussein Jasem Mohammed, New Cyclic Voltammetry of 3-(4-N-Pyridine-2-yl Benzene Sulfonamide Azo)-1-Nitroso Naphthol and the Use of it for Enhancement of Cobalt Oxide Nano Particles. Nano Biomed. Eng., 2017, 9(2): 135-142.
DOI: 10.5101/nbe.v9i2.p135-142.
Abstract
The electrochemical behavior of the 3-(4-N-pyridine-2-yl benzene sulfonamide azo)-1-nitroso naphthol reagent and its complex with Co (II) has been studied at glassy carbon disk electrode in different supporting electrolyte at concentration 1 mol and scan rate (100 mV/s). 3-(4-N-pyridine-2-yl benzene sulfonamide azo)-1-nitroso naphthol was used for enhance the properties of nano cobalt oxide by ligand exchange reaction on nanoparticles.
Keywords: Cyclic voltammetry; Cobalt oxide nano particles; 3-(4-N-pyridine-2-yl benzene sulfonamide azo)-1-nitroso naphthol
Introduction
Azo dyes belong to the one of the largest class of analytical reagents. Their important feature is electro activity which makes them an important reagent for the voltammetric determination of metal ions [1-3]. Recently, many researchers developed a sensitive method for the determination of metal ions with heterocyclic azo compounds as complexemetric agents by polarographic and voltammetric techniques. In addition, sulfa drugs alone have the ability to coordinate with different metal ions [4]. Heterocyclic azo compound reagents have received a great deal of attention as they are sensitive and selective chromogenic reagents. To continue improving the sensitivity, the selectivity and the metal complexes of this kind of regeants, the electrochemical characteristics of the reagents and their metal complexes have been studied [5-10]. 3-(4-N-pyridine-2-yl benzene sulfonamide azo)-1-nitroso naphthol (PBSAN) is bidentate via azo group and hydroxyl group, which has the following structure:
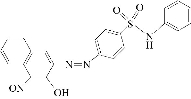
Structure of reagent PBSAN
Nano particles are microscopic particles with at least one dimension on the nanometer scale (1-100 nm). The transition from micro particles to nano particles can lead to a number of changes in physical properties. The two main factors in this area are the increase in the surface-to-volume-ratio and the size of the particles moving into the system, where quantum effects dominate [11]. The particle size gets smaller as the surface-area-to-volume ratio increases and, thus, leads to dominance of the actions of atoms on the surface than those in the bulk interior of the particles [12]. Ligand exchange reaction is a well-known method to exchange bound ligands on nano particles by simply mixing the free ligands with nano particles, which results in a replacement of the bound ligands by anew ligands (Scheme 1) [13]. After the exchange of ligands, they may be used as biosensors, catalysts, or for optoelectronics applications. The particle size can also be adjusted by exchanging the ligands bound on particles [14].
Scheme 1 Ligand exchange reaction on nano particles.
Experimental
All chemicals used in this work were of analytical grade. Voltammetric experiments were carried out using a computer – controlled electro analysis system using an EZ-State by NuVant system. A three-electrode combination system was an Ag / AgCl reference electrode, Pt wire auxiliary electrode and glassy carbon electrode as working electrode. The potential range selected was in the range of 1-1.25 mV. Atomic force microscopy (AFM) was carried out by AA2000 atomic force microscope of Angstrom Advanced Inc..
Reagents
Cobalt stock solution ( 200 µg/mL)
The solution was prepared by dissolving (0.2018 g) of cobalt chloride hexahydrate in (250 mL) of deionized water.
3-(4-N-pyridine-2-yl benzene sulfonamide azo)-1-nitroso naphthol (1*10-3 mol)
This solution was prepared by using 0.0321 g reagent in 100 mL dimethyl sulfoxide.
Results and Discussion
Electrochemical behavior of azo dye and redox mechanism in aqueous solution
The cyclic voltammogrames of the investigated azo dyes showed 1-3 irreversible cathodic peaks [15,16]. The number of peaks depends on the pH and the nature of the compounds. The peaks observed were due to the reduction of azo -N=N- center. The reduction mechanism includes the formation of hydrazo derivatives followed by the cleavage of the -N=N- bond and the final formation of amines [17,18], according to the following formulas (Scheme 2).
-N=N- +2H+ + 2e- ----------- -NH=NH- (1)
-NH=NH- +2H+ + 2e- -----------2(-NH2-) (2)
Different supporting electrolytes were used with the reagent at glassy carbon electrode (GCE) with the scan rate at 0.1V/s for all cyclic voltammogrames (Fig. 1-8). All voltammogrames presented a reduction peak of azo group (-N=N-) at the potential range of -500 – 750 mV). The choice of the better supporting electrolyte depends on the higher current for the oxidation peak and the clarity of the peak (Table 1). For the reagent PBSAN, it was proposed that the best supporting electrolyte was Na2HPO4 (Fig 6), and the current was the highest among the other electrolytes. Cyclic voltammogram revealed an irreversible electrochemical system in which the electron transfer rates were significantly lowere than that of mass transport and reduction in two steps. The first reduction attributed to the azo group giving a hydrazo derivative, and the second reduction peak broke the N-N linkage to form two primary amine molecules. All current peak ratios showed an irreversibility of electrochemical system at different electrolytes, due to Ipc/Ipa ≠ 1. The deviation from number one was due to the chemical reaction that arised subsequent transmission electron. Such interactions can be complex, involving dissociation and isomerization [19] (Table 2). The enhancement in the current of peak follows the following sequence: Na2 HPO4 ˃ NaCL = KClO3 ˃ K2HPO4 ˃ KCl ˃ NaH2PO4 ˃ K2SO4 ˃ KNO3.
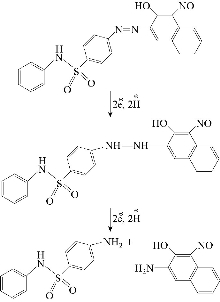
Scheme 2 Proposed mechanism of voltammetric reduction of reagent.
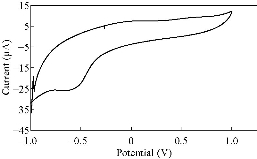
Fig. 1 CV of PBSAN in the supporting electrolyte solution of KCl (1 mol).
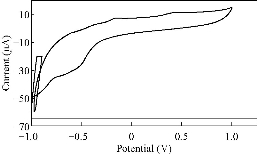
Fig. 2 CV of PBSAN in the supporting electrolyte solution of KClO3 (1 mol).
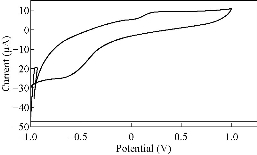
Fig. 3 CV of PBSAN in the supporting electrolyte solution of K2HPO4 (1 mol).
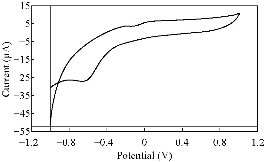
Fig. 4 CV of PBSAN in the supporting electrolyte solution of KNO3 (1 mol).
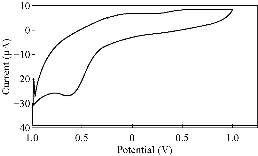
Fig. 5 CV of PBSAN in the supporting electrolyte solution of K2SO4 (1 mol).
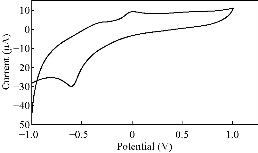
Fig. 6 CV of PBSAN in the supporting electrolyte solution of Na2 HPO4 (1 mol).
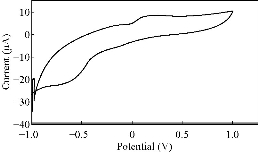
Fig. 7 CV of PBSAN in the supporting electrolyte solution of NaH2PO4 (1 mol).
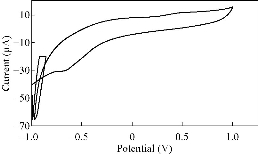
Fig. 8 CV of PBSAN in supporting electrolyte solution of NaCl (1 mol).
Table 1 Current – Potential data of PBSAN in 1 mol of different supporting electrolyte solutions with the scan rate of 0.1V/s
|
Ec3 (mV) |
Ic3 (µA) |
Ec2 (mV) |
Ic2 (µA) |
Ec1 (mV) |
Ic1 (µA)
|
Ea3 (mV) |
Ia3 (µA)
|
Ea2 (mV) |
Ia2 (µA) |
Ea1 (mv) |
Ia1 (µA) |
Supporting electrolyte |
No. |
|
-- |
-- |
-- |
-- |
610 |
25.9 |
293 |
4.72 |
50.6 |
7.24 |
500 |
8.92 |
KCl |
1 |
|
-- |
-- |
-- |
-- |
519 |
27.5 |
406 |
2.06 |
142 |
7.41 |
409 |
11.5 |
KClO3 |
2 |
|
-- |
-- |
-- |
-- |
601 |
23.4 |
--- |
--- |
185 |
3.86 |
205 |
9.09 |
K2HPO4 |
3 |
|
-- |
-- |
-- |
-- |
636 |
27.2 |
--- |
--- |
294 |
2.76 |
76.3 |
6.43 |
KNO3 |
4 |
|
-- |
-- |
-- |
-- |
623 |
26.7 |
341 |
3.88 |
76.6 |
6.85 |
587 |
8.43 |
K2SO4 |
5 |
|
-- |
-- |
-- |
-- |
649 |
30.3 |
--- |
--- |
116 |
7.41 |
474 |
11.5 |
NaCl |
6 |
|
-- |
-- |
-- |
-- |
645 |
21.7 |
--- |
--- |
202 |
3.46 |
171 |
8.46 |
NaH2PO4 |
7 |
|
-- |
-- |
-- |
-- |
645 |
29.8 |
--- |
--- |
137 |
7.41 |
470 |
11.6 |
Na2HPO4 |
8 |
|
|
|
|
|
|
|
|
|
|
|
|
No res. |
TBABr |
9 |
|
|
|
|
|
|
|
|
|
|
|
|
No res. |
BR |
10 |
TBABr: Tetra-n-butylammonium bromide; BR: Britton – Robinson buffer
Table 2 Current – Potential data for PBSAN at 1 mol of different supporting electrolyte solutions with the scan rate of 0.1 V/s
|
No |
Supporting electrolyte |
Ea1 (mV) |
Ec1 (mV) |
Ia1 (µA) |
Ic1 (µA) |
∆E1 (mV)
|
E1/2 (mV) |
Ia1/Ic1 (µA) |
|
1 |
Na2HPO4 |
470 |
645 |
11.6 |
29.8 |
1115 |
-87.5 |
0.3892 |
|
2 |
NaH2PO4 |
171 |
645 |
8.46 |
21.7 |
816 |
-237 |
0.389 |
|
3 |
NaCl |
474 |
649 |
11.5 |
30.3 |
1123 |
-87.5 |
0.3795 |
|
4 |
KCl |
500 |
610 |
8.92 |
25.9 |
1110 |
-55 |
0.3444 |
|
5 |
KClO3 |
409 |
519 |
11.5 |
27.5 |
928 |
-55 |
0.4181 |
|
6 |
KNO3 |
76.3 |
636 |
6.43 |
27.1 |
712.3 |
-279.85 |
0.237 |
|
7 |
K2HPO4 |
205 |
601 |
9.09 |
23.4 |
806 |
-198 |
0.388 |
Redox behavior of PBSAN-Co complex
Different supporting electrolytes were used with the reagent PBSAN at GCE with the scan rate at 0.1 V/s for all cyclic voltammogrames (Fig. 9-16) (Table 3). The cyclic voltammogram of cobalt (II) complex showed two redox couple peaks in 1 mol Na2HPO4 as revealed in Fig. 10. The first redox couple was at Epc1 = -148 mV vs. Ag/AgCl (Ipc1 = -2.73 µA), and Epa1 = -90.9 mV vs. Ag/AgCl (Ipa1 = 5.50 µA). The second redox couple was at Epc2 = -531 mV (Ipc2 = 9.36 µA ) and Epa2 = -398 mV (Ipa2 = 1.64 µA) (Table 4). The first reduction peak was attributed to Co2+ + e- ----- Co1+, which was followed by the second reduction of cobalt (I) to cobalt (0) (Co+ + e- ---- Co0), when the scan was reversed. The first oxidation was attributed to the oxidation of Co0 to Co1+, while the second oxidation peak was attributed to the oxidation of cobalt (I) to cobalt (II) (Co1+ + e- ------ Co2+). We observed a difference between the redox couple of azo dye and it was complex with cobalt. It was observed that ∆Ep1 = 57.1 mV, ∆Ep2 = 133 mV, and the ratio of the first anodic to cathodic peak currents (Ipa/Ipc ≠ 1) corresponded to more than one electron transfer process, which was also true with the second peak (Table 4). The difference in the value of Epc - Epa was ∆Ep1, which was close to the value required for a reversible process, i.e. 59 mV, indicating that the reduction of cobalt (II) to cobalt (I) complex at silver electrode was reversible, while the reduction of cobalt (I) to cobalt (0) was an aquasi-reversible character [20-23]. The enhancement in the current of peak follows the following sequence: K2HPO4 ˃ K2SO4 ˃ KNO3 ˃ KClO3 > Na2HPO4 ˃ NaH2PO4
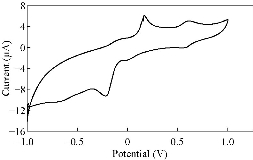
Fig. 9 CV of PBSAN-Co in NaH2PO4 (1 mol).
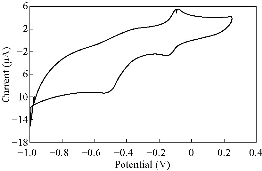
Fig. 10 CV for PBSAN-Co in Na2HPO4 (1 mol).
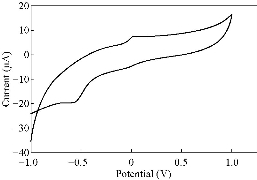
Fig. 11 CV for PBSAN-Co in KNO3 (1 mol).
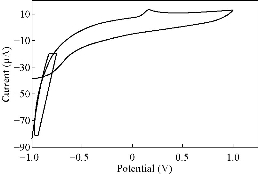
Fig. 12 CV for PBSAN-Co in K2HPO4 (1 mol).
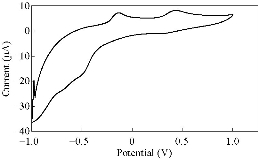
Fig. 13 CV for PBSAN-Co in K2SO4 (1 mol).
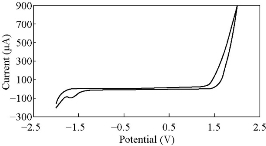
Fig. 14 CV for PBSAN-Co in KCl (1 mol).
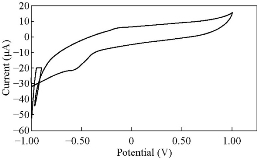
Fig. 15 CV for PBSAN-Co in KClO3 (1 mol).
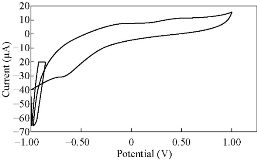
Fig. 16 CV for PBSAN-Co in NaCl (1 mol).
Table 3 Current – Potential data for PBSAN-Co complex at 1 mol of different supporting electrolyte solutions at the scan rate of 0.1 V/s.
|
Ec3 (mV) |
Ic3 (µA) |
Ec2 (mV) |
Ic2 (µA) |
Ec1 (mV) |
Ic1 (µA)
|
Ea3 (mV) |
Ia3 (µA)
|
Ea2 (mV) |
Ia2 (µA) |
Ea1 (mV) |
Ia1 (µA) |
Supporting electrolyte |
No. |
|
-- |
-- |
531 |
9.36 |
148 |
2.73 |
-- |
-- |
-398 |
1.64 |
-90.9 |
5.50 |
Na2HPO4 |
1 |
|
-- |
-- |
-- |
-- |
584 |
21.1 |
-- |
-- |
-- |
-- |
-111 |
6.01 |
KClO3 |
2 |
|
-- |
-- |
-- |
-- |
146 |
7.31 |
-- |
-- |
-- |
-- |
166 |
13.1 |
K2HPO4 |
3 |
|
-- |
-- |
579 |
19.4 |
33.2 |
4.97 |
-- |
-- |
-384 |
1.12 |
40.5 |
7.62 |
KNO3 |
4 |
|
-- |
-- |
-488 |
16.9 |
305 |
1.36 |
-- |
-- |
-137 |
7.20 |
452 |
8.25 |
K2SO4 |
5 |
|
224 |
9.45 |
10.1 |
2.41 |
574 |
55.9 |
-93.9 |
1.37 |
171 |
5.09 |
621 |
4.98 |
NaH 2PO 4 |
6 |
|
-- |
-- |
-- |
-- |
1.67 |
94.4 |
-- |
-- |
-- |
-- |
-- |
-- |
KCl |
7 |
|
|
|
|
|
|
|
|
|
|
|
|
No res. |
NaCl |
8 |
Table 4 Current – Potential data for PBSAN-Co in 1 mol Na2HPO4 as the supporting electrolyte solution at the scan rate of 0.1 V/s.
|
Com-pound |
Ea1 (mV) |
Ec1 (mV) |
Ia1 (µA) |
Ic1 (µA) |
∆Ep1 (mV) |
(Ipa1/Ipc1)1 (µA) |
[E1/2]1 (mV) |
Ea2 (mV) |
Ec2 (mV) |
Ia2 (µA) |
Ic2 (µA) |
∆Ep2 (mV) |
(Ipa2/Ipc2)2 (µA) |
[E1/2]2 (mV) |
|
PBSAN-Co |
-90.9 |
-148 |
5.50 |
-2.73 |
57.1 |
2.01 |
-119.4 |
-398 |
-531 |
1.64 |
-9.36 |
133 |
0.175 |
-464.5 |
Enhancement of Nano CoO Particales
Morphology and structure
Nano particles are characterized by their small size, active function, large specific area, and strong interfacial interaction with the organic matrix of polymer [24]. Therefore, they can improve the physical and optical properties of the organic polymer, as well as provide impedance to environmental stress-caused cracking and aging [25]. AFM (atomic force microscopy) observations showed that the products were very aggregate and were converted to spherical particles (Fig. 17 & 18) after ligand exchange [26]. This morphological change might be partially due to the surface of Co ions which was bound to the original ligand at the highly reactive sites, such as tips or corners of the nano pyramids. They were stripped in the ligand exchange process. Etching of the surface of colloidal nano crystals capped by azo ligands or short chain alcohols has also been reported by other groups. On the other hand, the AFM results of the CoO nano crystals showed that the average diameter as determined by the AFM was 96.54 nm for nano particles before ligands exchange and 89.03 for PBSAN after ligand exchange (Table 5 & 6). Fig. 17 exhibits that the G.S. for CoO was 96.54 nm, while Fig. 18 exhibits that the G.S. reduced to 89.03 nm for PBSAN after ligand exchange, which occurred due to the fact that R1 correlated with the metal oxide, so that the Ron. and R.M.S. in Table.6 decreased after the dye was added.
Table 5 Diameters of Co before ligand exchange
|
Status |
Grain size (G.S.) |
Roughness average (Ron.) |
Root mean square (R.M.S) |
|
CoO |
96.54 nm |
0.621 nm |
0.730 nm |
Table 6 Diameters of CoO after ligands exchange
|
Status |
Grain size (G.S.) |
Roughness average (Ron.) |
Root mean square (R.M.S) |
|
R1-CoO |
89.03 nm |
0.424 nm |
0.503 nm |
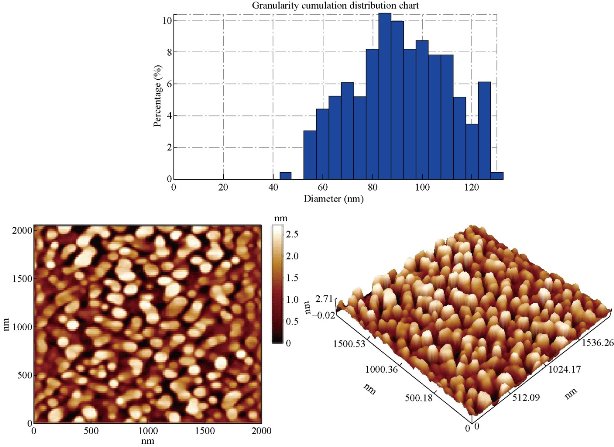
Fig. 17 The average diameter as determined by the AFM for CoO was 96.54 nm before ligand exchange.
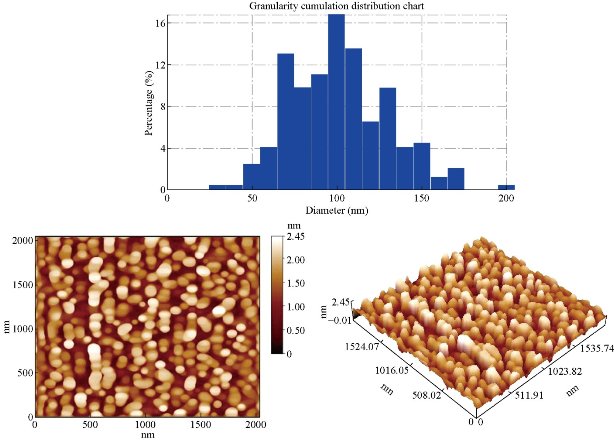
Fig.18 The average diameter as determined by the AFM for Nano R1-CoO was 89.03 nm after ligand exchange.
Conclusions
In this work, the cyclic voltammetric system of PBSAN led to the formation of the same hydrazine derivative which remained at the electrode surface according to the following equation:
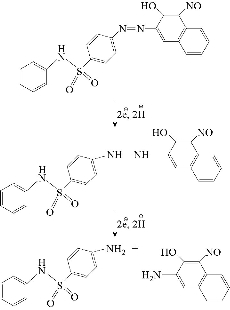
The enhancement of nano particles of cobalt oxide was also investigated by ligand exchange mechanism with the azo dye of PBSAN.
References
Copyright© 2017 Hussein Jasem Mohammed. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.