Access to Optimal Calcination Temperature for Nanoparticle Synthesis from Hydroxyapatite Bovine Femur Bone Waste
Ali Sabea Hammood 1*, Sora Salem Hassan 1, Mohammed Talib Alkhafagy 2
1 Department of Material Engineering, Faculty of Engineering, University of Kufa, Iraq.
2 Faculty of Dentistry, University of Kufa, Iraq.
Corresponding author. E-mail: alis.altameemi@uokufa.edu.iq
Received: Jun. 30, 2017; Accepted: Sep. 13, 2017; Published: Sep. 27, 2017
Citation: Ali Sabea Hammood, Sora Salem Hassan, and Mohammed Talib Alkhafagy, Access of the Optimal Calcination Temperature to Nanoparticles Synthesis from Hydroxyapatite Bovine Femur Bone Waste. Nano Biomed. Eng., 2017, 9(3): 228-235.
DOI: 10.5101/nbe.v9i3.p228-235.
Abstract
Hydroxyapatite (HAp) is one of the vital and bioactive materials that are commonly used in biomedical field and concentrated in clinical area. It is a bio-ceramic powder synthesized by using different bio-waste materials such as bovine femur bone. In this study, the bovine femur bone powder was prepared to obtain nano powder. The purpose of this study was to reach the optimal temperature to obtain nanoparticles HAp. The resulted powder was calcinated in a furnace at different temperatures (900, 950, 1000, 1050 and 1100 °C) for 2 h at the heating rate of 10 °C/min and cooled slowly in a furnace. Results showed, the formation of pure HAp by the presence of peaks corresponding to (PO4)3- at 632/cm and OH- at 3,572/cm in fourier transformation infrared spectroscopy (FTIR). For the calcined samples, there were three main peaks of 211, 112 and 300 planes at 2θ near 31.8, 32.2 and 32.9 respectively. The amorphous raw bones were transformed into crystalline phase and the lattice parameters for HAp c and a were calculated in X-ray diffraction (XRD), Raman analysis showed that the calcination process removed the organic compound from the bovine femur bones matrix. Scanning electron microscopy (SEM) and energy dispersive X-ray analysis (EDX) showed that the shape of the granules was irregular, containing quantities of oxygen, magnesium, sodium and carbon; the ratio of calcium to phosphate was calculated, Atomic force microscopy (AFM) showed that the particles sizes ranged from nanometers to microns. EDX result found that the calcium to phosphate ratio reached 1.7058 after calcination at 950 °C which was close to stoichiometric hydroxyapatite (1.67). This result implied the formation of pure HAp phase at 950 °C.
Keywords: Hydroxyapatite; Bovine femur bone; Calcination temperature
Introduction
Hydroxyapatite (HAp, Ca10(PO4)6(OH)2) is among the few materials that are classified as bioactive, meaning that it will support bone in growth and osseointegration [1]. The chemical and structural similarity of HAp with bone minerals has proven to be hydroxyapatite, an attractive biomaterial for bone and tooth implantation. Hydroxyapatite is highly biocompatible with human organs. It has acknowledged a great consideration in the field of biomedical science due to its ability to form chemical bonds between hard tissues [2]. Bones consist of organic (30%) and inorganic compounds (70%). Mineral parts of bones provide their stiffness and proper mechanical properties. The model compound corresponding to a mineral phase of bones is a nonstoichiometric hydroxyapatite (HAp), i.e. HAp whose molar ratio of calcium to phosphorus is different to 1.67. Biological apatite are the components of bones and also pathological tissue (urolith, tooth scale and mineralized soft tissue) [3]. There are many applications of the HAp such as to bone and tooth defect filling, tooth pastes and bone cement, biocompatible and bioactive coatings on metal implants for dental implants, hip joint prosthesis and others [4]. Because of the attractive properties of HAp powders, various techniques have been and are being developed to produce hydroxyapatite. There are two main ways of obtaining HAp; one is through inorganic synthesis and the other way is to obtain HAp from natural sources [5]. Several methods which have been developed to synthesis HAp powders can be classified as either wet chemistry methods or solid-state reactions [6]. The main component of synthetic HAp is calcium. Calcium needed for the preparation of HAp from living bodies reduces the chance of impurities and the cost of production. Various natural sources like bovine bone, corals, fish bone and egg shells have been recognized so far due to their advantage of biological origin as well as a method to recycle inorganic waste [7]. In recent years, the use of bio-HAp has increased. Biomaterial also has other elements that are present in the human HAp. Although ions are found in the bone in small amounts, these ions are very important to biological reactions related with bone metabolism [8, 9]. The aim of this work is to approach the optimal temperature to obtain nanoparticles hydroxyapatite from the bovine femur bone. Recently, Lü et al., showed that the calcining method of high temperature was effective and feasible for the preparation of natural HAp from pig bones and pig teeth [10]. Commercial Bio-HAp has been studied by Figuereido et al., fourteen different samples were used in dentistry, three of them based on HAp and the others based on calcium carbonate [11]. Kupiec et al. studied the effect of calcination parameters on bone hydroxyapatite in artificial saliva and its bio-safety. The X-ray diffraction (XRD) analysis of HAp confirmed the stability of the material [12]. Chenab et al. found that the performances of calcium phosphates in application depended greatly on their morphologies, structures and chemical compositions. These nanostructured calcium phosphates are promising biomaterials for applications in tissue engineering scaffolds, drug/gene delivery and other medical areas [13]. Nosoudi et al. found the hydroxyapatite containing 35 μg etidronate showed low early strength, but high strength after soaking in simulated body fluid (SBF) [14]. Jaber et al., utilized the camelus bone to synthesize HAp powders, and the results showed that powders obtained at calcination temperature (1000 °C) was a pure HAp with the Ca/P ratio of 1.6557 [15]. The aim at this work is to approach the optimal temperature to obtain hydroxyapatite nanoparticles from the bovine femur bone.
Experimental
Materials and methods
Bovine femur bone preparation
Bovine femur bone was collected from the local butcher. The femur bone were extracted from the bovine bone, boiled in distilled water for 1 h and washed using a fast flow water jet to eliminate the organic and proteins materials. The raw bovine femur bone was cut by grinding, mortar pestle into powder, and left to be sun-dried for 2 weaks. It was crushed in a planetary mill (SFM-1(QM-3SP2)) which was equipped with alumina balls of different sizes at 4,000 rpm for 8 h. Particle size analysis for the powder before and after crushing was established by laser particle size analyzer.
Calcination process
In the calcination process, 10 g of bovine femur bone powder sample was placed in an open alumina crucible. All samples were heated at various temperatures of 900 °C, 950 °C, 1000 °C, 1050 °C and 1100 °C for 2 h at the heating rate of 10 °C/min and afterward cooled slowly in a furnace to room temperature.
Characterization of the synthesized bovine femur bone
Fourier transform infrared spectroscopy (FTIR)
FTIR-SHIMADSO (Japan) is a powerful tool for identifying the types of chemical bonds in a molecule and functional groups obtained at different temperatures by producing an infrared absorption spectrum. The synthesized powder was mixed with spectroscopic grate potassium bromide (KBr). 1 - 2 mg of HAp powder sample was mixed with 200 mg of KBr powder. The spectra were registered on the 400 – 4,000/cm regions using 20 scans and a spectral resolution of 4/cm.
X-ray diffraction (XRD)
The crystalline structure and the phase composition of the raw powder before and after calcination were determined by using XRD (XRD-6000 SHIMADZU, Japan, ICDD00-024-0033). CuKα radiation (λ = 0.154056 nm) was employed, with voltage = 40 kV and current = 30 mA; data were collected over the 2Ѳ from 10º to 50º (Bragg angle). From XRD the (hkl) planes and the values of lattice parameters c and a could be calculated.
Raman spectroscopy
In order to explore any structural changes in substitutions in the apatite lattice, Raman spectroscopy (Raman-Bruker Senterra model, Germany) was used with the wave lengths of 532 nm and 758 nm.
Scanning electron microscopy (SEM)
The morphology or microstructure of bovine femur bone before and after calcination process was observed under a scanning electron microscope (SEM-FEI Quanta model, Holland) at an acceleration of 12.5 kV. The local chemical compositions of raw powder before and after calcination process were analyzed by using energy dispersive spectroscopy (EDS), which was regarded as an auxiliary for SEM.
Atomic force microscopy (AFM)
The surface morphology, particle shape, particle size distribution, 2D and 3D images of the bovine femur bone before and after calcination process is observed under the atomic force microscope (AFM) (NT-MDT Ntegra model, Russian Federation), by recording the interaction forces between the surface and a sharp tip mounted on a cantilever. Samples were exposed to the ultrasonic device before exposure to AFM so that they would not be agglomerated.
Results and Discussion
Effect of calcination temperature on characterization of bovine femur bone
Fourier transform infrared spectroscopy (FTIR)
To analyze the changes that occur to the calcination process of bovine femur bone to extract natural hydroxyapatite, FTIR analysis was done at different stages of the process: the washed and dried bone powder (B0) and the bone powder calcined at different temperatures: 900 °C (B900), 950 °C (B950), 1000 °C (B1000), 1050 °C (B1050) and 1100 °C (B1100) were presented in Fig. 1. The B0 spectrum was present amid groups of proteins, namely at 1,639/cm [16]. The bands specific to CO32- group were present in B0 spectrum at 1,546/cm and 1,446/cm. The bands corresponding to the protein group disappeared in the calcination process. The color of bone particles changed from yellowhite to white after the calcination, which implied the decomposition of protein, collagen and other organic substances [16, 17] The peaks of bands of the HAp were shown to be at 3,572/cm and 632/cm and were attributed to the hydroxyl group (OH-); the peaks located at 1,094/cm, 960/cm, 601/cm and 474/cm corresponded to the phosphate group (PO43-) [18]. The FTIR band of 1,639/cm coinciding with the amide of collagen present in the raw bovine femur bone was completely eliminated from the calcined sample.
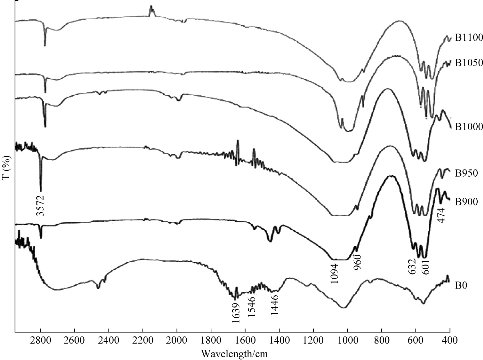
Fig. 1 FTIR for raw bovine femur bone (B0) and calcination at different temperatures: 900 °C (B900), 950 °C (B950), 1000 °C (B1000), 1050 °C (B1050) and 1100 °C (B1100).
X-ray diffraction (XRD)
Fig. 2 demonstrates the XRD patterns of the raw bovine femur bone (B0) and calcined bovine femur bone at 900 °C (B900), 950 °C (B950), 1000 °C (B1000), 1050 °C (B1050) and 1100 °C(B1100). For the calcined samples, there were three main peaks, namely 211, 112 and 300 planes at 2θ near 31.8°, 32.2° and 32.9°, respectively [19, 20]. It was evident that the intensity of diffraction peaks increased with the calcination temperature [21]. Heating at a temperature of 900°C resulted in broad diffraction peaks which coincided with a poor crystalline apatite. When increasing the calcination temperature from 900 °C to 1100 °C, the intensity of diffraction peaks increased and resulted in more intense, sharper and narrower diffraction peaks which denoted to increase the crystallinity and crystal size. It revealed that the amorphous raw bovine femur bone was transformed into crystalline phase with the organic phase and carbonates increasing, as the temperature increased from 900 °C to 1100 °C. The lattice parameters c and a could be calculated from the XRD and compared with the standard values of c and a for hydroxyapatite (Table 1).
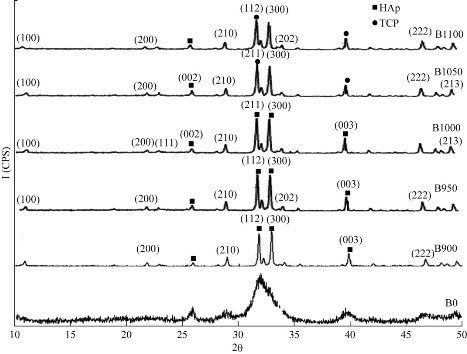
Fig. 2 XRD for raw bovine femur bone (B0) and calcination at different temperatures: 900 °C (B900), 950 °C (B950), 1000 °C (B1000), 1050 °C (B1050) and 1100 °C (B1100).
Table 1 Values of c and a for raw bovine femur bone and at different calcination temperatures
|
c (nm) |
a (nm) |
Sample condition |
|
0.67819 |
0.97614 |
Raw |
|
0.67856 |
0.94063 |
900 °C |
|
0.69087 |
0.94090 |
950 °C |
|
0.68501 |
0.93190 |
1000 °C |
|
0.69035 |
0.93047 |
1050 °C |
|
0.67496 |
0.92566 |
1100 °C |
Raman spectroscopy
Fig. 3 demonstrates the Raman spectra of the raw bovine femur bone (B0) and calcined bovine femur bone at 900 °C (B900), 950 °C (B950), 1000 °C (B1000), 1050 °C (B1050) and 1100 °C(B1100). These samples were dominated by the peaks at 962, 447 and 591/cm and related to the PO3-4 [18]. There was a very strong beak at 962/cm at 950 °C; the medium intensity peaks were at 447 and 591/cm, while CO23- was present at the peak of 1,073/cm [22]. The spectrum of the powder before calcination showed a weak intensity peak at 1,114/cm which was not observed in the spectrum of the powder after the calcination process. This was related to the presence of organic materials (C-H) after calcination.
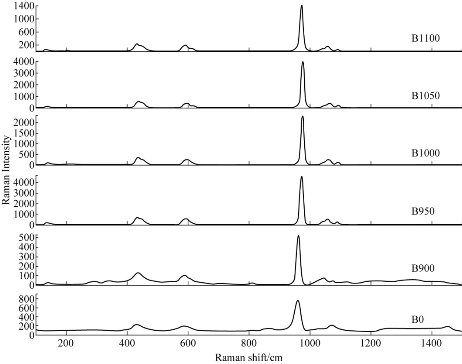
Fig. 3 Raman spectra for raw bovine femur bone (B0) and calcination at different temperatures: 900 °C (B900), 950 °C (B950), 1000 °C (B1000), 1050 °C (B1050) and 1100 °C (B1100).
Scanning electron microscopy (SEM)
Fig. 4 shows the SEM images of the raw bovine femur bone (B0) and calcined bovine femur bone at 900 °C (B900), 950 °C (B950), 1000 °C (B1000), 1050 °C (B1050) and 1100 °C (B1100). The uncalcined sample (B0) exhibited the presence of fat, protein and the interconnected porousness that constituted the organic phase. The morphology changed at the surface as calcination temperature increased [22]. For samples calcined at 900 °C, all the fat and protein was removed. When the calcination temperature increased from 900 °C to 1100 °C, the grain size increased. This grain growth may be associated with the absorption of heat energy by the particles [19]. The image of SEM showed the formation of micro and sub-micro grains of HAp for calcined sample [23]. The result of EDS (Fig. 5) referred to the presence of elements such as O, Mg, Na, and C, and these results could be used to determine the value of Ca/P as shown in Table 2.
Table 2 Results of EDS for raw bovine femur bone and at different calcination temperatures
|
Sample Condition |
Elements (mol) |
Molar ratio |
|
|
Ca |
P |
Ca/P |
|
|
Raw |
0.2924 |
0.1785 |
1.6380 |
|
900 °C |
1.0843 |
0.5039 |
2.1518 |
|
950 °C |
0.8408 |
0.4929 |
1.7058 |
|
1000 °C |
1.1891 |
0.3748 |
3.1726 |
|
1050 °C |
1.1407 |
0.4878 |
2.3384 |
|
1100 °C |
0.8146 |
0.4652 |
1.7510 |
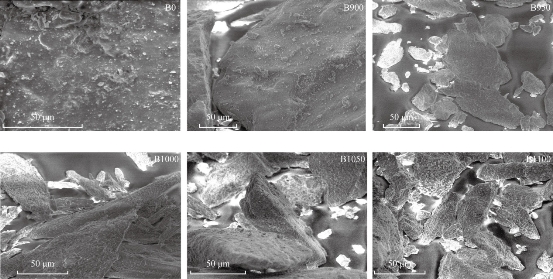
Fig. 4 SEM of raw bovine femur bone (B0) and calcination at different temperatures: 900 °C (B900), 950 °C (B950), 1000 °C (B1000), 1050 °C (B1050) and 1100 °C (B1100).
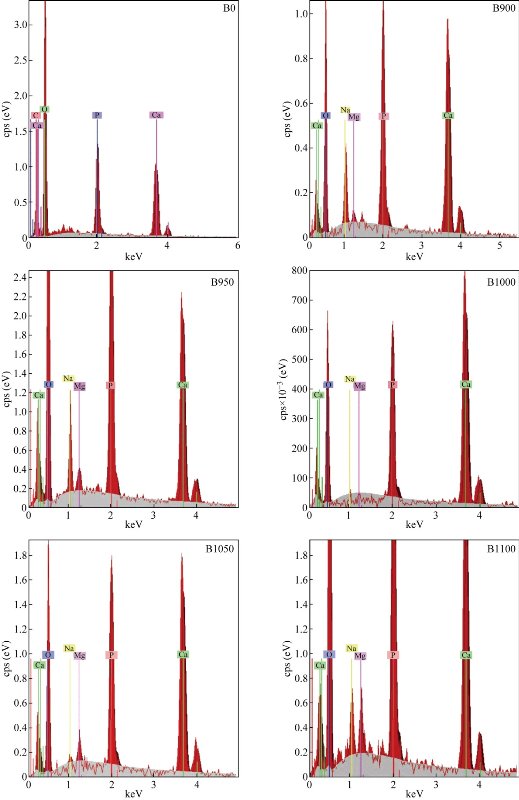
Fig. 5 EDS for raw bovine femur bone (B0) and calcination at different temperatures: 900 °C (B900), 950 °C (B950), 1000 °C (B1000), 1050 °C (B1050) and 1100 °C (B1100).
Atomic force microscopy (AFM)
AFM images of the surface topography of the raw bovine femur bone (B0) and calcined bovine femur bone at 900 °C (B900), 950 °C (B950), 1000 °C (B1000), 1050 °C (B1050) and 1100 °C(B1100) are shown in Fig. 6. The results revealed an increased particle size with increasing temperatures: 900, 950, 1000, 1050 and 1100 °C. And the size of particles changed into micro- and nanoparticle; the shape of particles was irregular before calcination and tended to be regular after calcination.
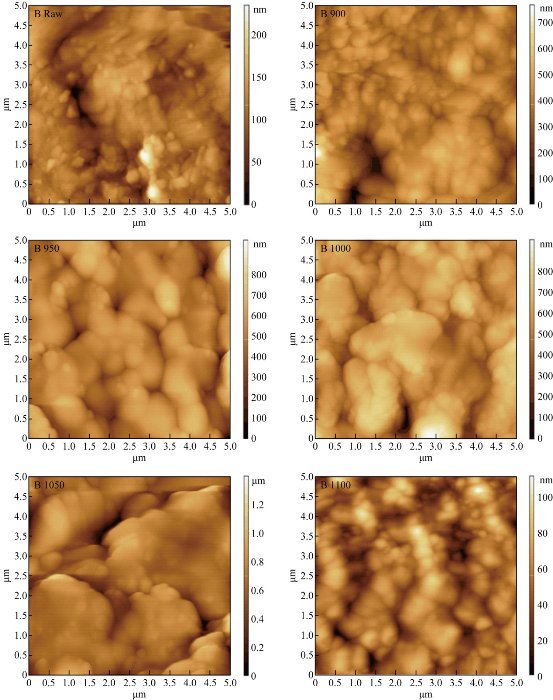
Fig. 6 AFM image for raw bovine femur bone (B0) and calcination at different temperatures: 900 °C (B900), 950 °C (B950), 1000 °C (B1000), 1050 °C (B1050) and 1100 °C (B1100).
Conclusions
In this study, through the calcination process of different temperatures at 900, 950, 1000, 1050 and 1100 °C, natural HAp was obtained from the bovine femur bone. FTIR, XRD and Raman spectra results showed that calcination of bovine femur bone at 900 °C and above could produce organic free and crystalline natural HAp. SEM results revealed that the grain size increased and the best results were obtained at 950 °C, because the Ca/P ratio was 1.7058, near to the stoichiometric HAp ratio (Ca/P: 1.67). The values of c and a were near to the standard values of c = 0.6884 nm and a = 0.9418 nm. AFM result showed that the shape of particles tended to be regular after calcination.
Acknowledgments
The authors gratefully acknowledge the staff of Materials Research Center, Ministry of Science and Technology- Iraq for the characterization facility provided there.
Conflict of Interests
The authors declare that no competing interest exists.
References
Copyright© 2017 Ali Sabea Hammood, Sora Salem Hassan, and Mohammed Talib Alkhafagy. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.