Research Article
Formulation and Evaluation of Etoricoxib Niosomes by Thin Film Hydration Technique and Ether Injection Method
Veldurthi Ravalika, Abbaraju Krishna Sailaja *
Department of pharmaceutics, RBVRR Women’s College of Pharmacy, Barkatpura, Hyderabad, India.
* Corresponding author. E-mail: shailaja1234@rediffmail.com
Received: Aug. 3, 2017; Accepted: Sep. 28, 2017; Published: Sep. 30, 2017
Citation: Veldurthi Ravalika, Abbaraju Krishna Sailaja, Formulation and Evaluation of Etoricoxib Niosomes by Thin Film Hydration Technique and Ether Injection Method. Nano Biomed. Eng., 2017, 9(3): 242-248.
DOI: 10.5101/nbe.v9i3.p242-248.
Abstract
The main objective of this study is to formulate etoricoxib niosomes as vesicular carriers for site specific drug delivery. Niosomes are novel vesicular carriers, in which the drug is incorporated in a vesicle. Niosomal vesicles are formed by hydrating mixture of cholesterol and nonionic surfactants. Niosomes can increase the permeability of the skin (stratum corneum and epidermis), by avoiding the first pass metabolism and also reduce the side effects. Etoricoxib is a potent new COX-2 inhibitor used in the treatment of osteoarthritis, rheumatoid arthritis, ankylosing spondylitis, acute gout arthritis etc. Two formulations were prepared by thin film hydration technique using the drug, cholesterol and surfactants Tween 80 (F1) and Span 60 (F2). Another two formulations were prepared by ether injection method using cholesterol and surfactants Tween 80 (F3) and Span 60 (F4). Each formulation was evaluated for drug content, entrapment efficiency, mean vesicular diameter, zeta potential and In-vitro drug release studies. Among the four formulations, F2 formulation containing the drug and Span 60 showed maximal drug content of 95.57%, entrapment efficiency of 96.40%, mean vesicular diameter of 463.7 nm, zeta potential of -80.5 mV, in-vitro drug release of 95.14% in 12 h, and the drug release followed the first order with non-fickian diffusion mechanism by thin film hydration technique. Hence, the thin film hydration technique is an optimized technique for the preparation of etoricoxib niosomes.
Keywords: Niosomes; Cholesterol; Span 60; Tween 80; Etoricoxib; Entrapment efficiency
Introduction
Niosomes are one of the promising drug carriers that have a bilayer structure and are formed by self-association of nonionic surfactants and cholesterol in an aqueous phase (Fig. 1) [7, 8]. Niosomes are alternative carriers to liposomes, usually made up of synthetic, non ionic surfactant of alkyl or di-alkyl poly glycerol ether class. The uni- or multilamellar niosomes can entrap solutes and have more stable in-vitro release and can improve the stability and duration of action of entrapped drug as compared to the stability of conventional dosage forms.
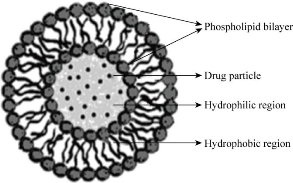
Fig. 1 Structure of niosome.
Advantages of niosomes
Niosomes are osmotically active and stable, and also they increase the stability of entrapped drug. Nisomes improve oral bioavailability of poorly absorbed drugs and enhance skin penetration of drugs; they can be made to reach the site of action via oral, parenteral as well as topical routes. Niosomes containing the surfactants are biodegradable, biocompatible and non-immunogenic. Niosomes improve the therapeutic performance of the drug molecules by delayed clearance from the circulation, protecting the drug from biological environment and restricting effects to target cells [1, 2].
Applications of niosomes
Niosomal drug delivery has a number of potential advantages over conventional dosage forms. Niosomes are potentially applicable to many pharmacological agents for their action against various diseases. An increase in the penetration rate has been achieved by transdermal delivery of drugs incorporated in niosomes. The incorporation of drugs with low therapeutic index and low water solubility will sustain the drug release action of niosomes; the localized drug action of niosomes results in enhancement of efficacy of potency of the drug and reduces its systemic toxic effects. Niosomes can be used as a carrier for hemoglobin and also used for studying the nature of the immune response provoked by antigen [3, 5]. Etoricoxib (5-chloro-2-(6-methylpyridin-3-yl)-3-(4-methylsulfonylphenyl) pyridine) is a novel orally active agent that selectively inhibits COX-2 (cyclooxygenase-2). Etoricoxib is used in the treatment of rheumatoid arthritis, osteoarthritis, acute gout, chronic musculoskeletal pain (including chronic low back pain), postoperative dental pain and primary dysmenorrhoea. The recommended dosage of the drug is between 60 and 120 mg/day as oral tablets. It is a poorly soluble, lipophilic dug with estimated logP of 3.14 and pKa of 4.6. Its aqueous solubility is low and highly pH-dependent. Etoricoxib is completely and rapidly absorbed, with an oral bioavailability of up to 100% [16]. The purpose of this work is to formulate etoricoxib niosomes as carriers to reduce the risk of side effects and to achieve the localized action. Etoricoxib is available as tablets, capsules and emulgels. The long term use of this drug, when taken as oral formulations leads to severe adverse drug reactions such as constipation, diarrhoea, nausea, vomiting, oesophagitis, flatulence, gastritis, heartburn/acid reflux, dyspepsia/epigastric discomfort, oral ulcer, alveolar osteitis, oedema/fluid retention, dizziness, headache, palpitations, arrhythmia, HTN, asthenia/fatigue, flu-like disease, bronchospasm, Stevens-Johnson syndrome, exfoliative dermatitis and toxic epidermal necrolysis, upper gastrointestinal (GI) ulceration, perforation and bleeding. Hence there is a need to develop site specific drug delivery system to avoid side effects of etoricoxib [16].
Experimental
Materials
Etoricoxib was purchased from Hetero Chemicals Pvt. Limited, Hyderabad. Cholesterol, Span 60, Tween 80, chloroform, ethanol, pH 7.4 buffer, diethyl ether and methanol were from SD Fine-Chem. Limited, Mumbai.
Methods of preparation of niosomes
Thin film hydration technique
The mixture of vesicles forming ingredients like surfactant and cholesterol were dissolved in a volatile organic solvents chloroform and ethanol (1:2) in a round bottom flask. The organic solvent was then removed above the lipid transition temperature by using rotary evaporator, leaving a thin layer of solid mixture deposited on the wall of the flask. The dried surfactant film could be rehydrated with 10 mL of aqueous phase (pH 7.4 buffer) at 0 - 60 ºC with gentle agitation. This process formed typical multilamellar niosomes (Table 1) [4, 5].
Table 1 Formulations for thin film hydration technique
|
Formulation code |
Surfactant |
Method of preparation |
Drug : surfactant : cholesterol |
|
F1 |
Tween 80 |
Thin film hydration |
1 : 1 : 1 |
|
F2 |
Span 60 |
Thin film hydration |
1 : 1 : 1 |
Ether injection method
This method provided a means of making niosomes by slowly introducing nonionic surfactant and cholesterol dissolved in diethyl ether mixed with 2 mL methanol previously containing weighed quantity of drug. The resulting solution was slowly injected using microsyringe into 10 mL hydrating phosphate buffer at a rate of 1 mL/min on magnetic stirrer, and the temperature maintained at 60 - 65 ºC. Then the lipid solution was injected slowly into aqueous phase. Differences in temperature between the phases caused rapid vapourization of ether and resulted in the formation of niosomal vesicles (Table 2) [11, 12].
Table 2 Formulations for ether injection method
|
Formulation code |
Surfactant |
Method of preparation |
Drug: surfactant: cholesterol |
|
F3 |
Tween 80 |
Ether injection |
1 : 1 : 1 |
|
F4 |
Span 60 |
Ether injection |
1 : 1 : 1 |
Charecterization of niosomes
Microscopic evaluation of niosomes
One drop of niosomal dispersion was taken on the glass slide and observed under projection microscope with 10 × magnification (Fig. 2).
Fig. 2 Optical images of niosomal formultion.
Mean vesicular diameter
A small amount of optimized niosomal formulation was mixed with 5 mL double distilled water and kept for 1 h sonication. Then the sample was analyzed by using nanoparticle analyzer (HORIBA Nanopartica SZ-100) for particle size determination [10, 13].
Zeta potential
The optimized formulation was analyzed for zeta potential value to determine the stability of the formulation. The analysis was carried out by using double distilled water as dispersion medium at the temperature of 25 °C [11, 14].
Drug content
Niosomal suspension equivalent to 5 mg was taken in a 10 mL volumetric flask, and the volume was made with 7.4 pH buffer to disrupt the vesicle by sonication for 40 min. 1mL of the solution was pipetted out in 50 mL volumetric flask; the volume was made with buffer and kept for sonication. Then the concentration of drug was analyzed by UV spectrophotometer at 235 nm [15, 17].
Entrapment efficiency
After preparing niosomal dispersion, unentrapped drug was separated by centrifugation using pH 7.4 phosphate buffer for 45 min at 17,000 rpm. The resulting solution was analyzed by UV spectrophotometer at 235 nm for the total amount of entrapped drug [9, 13].
![]()
In-vitro drug release studies
In-vitro drug release studies of transferosomes were carried out by Franz diffusion cell. 1 mL of niosomal formulation was placed on cellophane membrane between the donor compartment and the receptor compartment containing 50 mL of pH 7.4 buffer. The samples were withdrawn using microsyringe at particular interval of time with replacing fresh buffer to maintain the sink condition. The absorbance of each sample was analyzed by UV spectrophotometer at 235 nm, and the in-vitro drug release was calculated [18, 19].
Results and Discussion
Comparison of drug content of niosomal formulation prepared by both the techniques
The prepared niosomal formulations F1, F2, F3 and F4 were evaluated for drug content. As shown in Table 3, the percentage of drug content was found to be 94.96% , 95.57%, 89.02% and 93.12% respectively. Among the four formulations, F2 showed maximal drug content as of 95.57% (Fig. 3).
Table 3 Percentage of drug content of niosomal formulations
|
Formulation code |
Percentage of drug content |
|
F1 |
94.96% |
|
F2 |
95.57% |
|
F3 |
89.02% |
|
F4 |
93.12% |
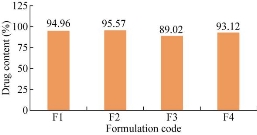
Fig. 3 Comparison of drug content of niosomal formulations.
Comparision of entrapment efficiency of niosomes prepared by ether injection method and thin film hydration technique
The prepared niosomal formulations F1, F2, F3 and F4 were evaluated for entrapment efficiency. As shown in Table 4, the percentage of entrapment efficiency was found to be 92.31%, 96.4%, 84.51% and 93.23% respectively. Among the four formulations, F2 showed maximal entrapment efficiency as of 96.4% (Fig. 4).
Table 4 Percentage of entrapment efficiency of niosomal formulations
|
Formulation code |
Entrapment efficiency |
|
F1 |
92.31% |
|
F2 |
96.40% |
|
F3 |
84.51% |
|
F4 |
93.23% |
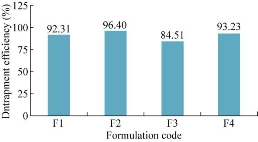
Fig. 4 Comparison of entrapment efficiency of niosomal formulations.
Mean vesicular diameter
The mean vesicular diameter of all formulations was evaluated by using HORIBA Nanopartica. Among the four formulations, F2 (with Span 60) showed a mean vesicular diameter of 463.7 nm (Fig. 5).
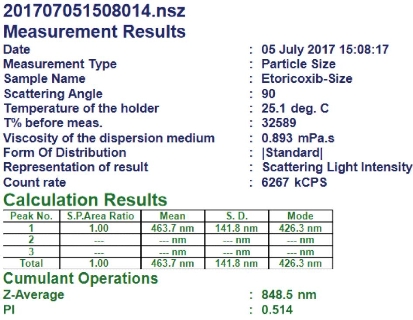
Fig. 5 Mean vesicular diameter of formulation F2 prepared by thin film hydration technique using surfactant Span 60.
Zeta potential determination
The zeta potential of the all formulations was carried out by using zeta sizer. Among all formulations, F2 formulation was found to be -80 mV which indicated the formulation was highly stable (Fig. 6).
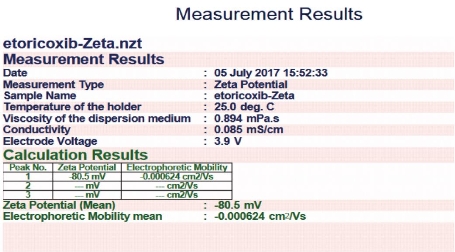
Fig. 6 Zeta potential value for formulation F2 prepared by thin film hydration technique using surfactant Span 60.
In-vitro diffusion study
The in-vitro drug release studies for all formulations were performed. The optimized formulation showed the drug release of 95.14% within 12 h (Fig. 7). The prepared niosomal formulations F1, F2, F3 and F4 were subjected to in-vitro diffusion studies. The percentage of drug release was found to be 90.97%, 95.14%, 43.62% and 29.88% respectively. Among all four formulations, F2 showed maximal drug release as of 95.14% in 12 h.
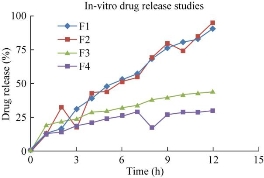
Fig. 7 In-vitro drug release for four formulations F1, F2, F3 and F4 as prepared by thin film hydration technique and ether injection method.
Kinetics of drug release
Zero order, first order, Higuchi and Korsmeyer-Peppas plots were drawn for the optimized formulation F2, in order to demonstrate the release kinetics of the drug (Fig. 8) [4,11]. According to the kinetic plots, the formulation was following first order release with non-fickian diffusion. The four formulations of etoricoxib niosomes were prepared by thin film hydration technique and ether injection method using surfactants including Span 60 and Tween 80 and by adding equal amount of cholesterol and drug. Among all formulations, formulation F2 showed the maximal drug content, entrapment efficiency and mean vesicular diameter. Previously, works have been done for the preparation of niosomes containing etodolac for topical drug delivery using cholesterol and Span 60 by thin film hydration technique, which showed good entrapment efficiency as of 96.72% and drug release as of 94.91% after 24 h [19]. Rahman et al. developed curcumin loaded niosomal transdermal gel using Span 60 and cholesterol in the ratio of 7 : 3 mmol. After evaluation, it was found that the formulation containing Span 60 showd better entrapment efficiency as of 61.22% and particle size as of 1-5 µm [20]. Diclofenac sodium niosomes were developed by using Span 20, 40, 60, 80, 85 and Tween 20, 40, 60, 80, 85 and by hand-shaking method. Among all formulations, the formulation containing Span 60 showed better entrapment efficiency as of 30.52% [21, 22]. These works proved that Span 60 was used as a good surfactant for the formation of niosomes, and the thin film hydration technique was a better technique to formulate good nisomal vesicles. Hence, the thin film hydration technique was selected for the preparation of etoricoxib niosomes [20, 21]. Usually, surfactant with a hydrophilic-lipophilic balance (HLB) value between 4 to 8 is mostly preferred for the vesicle formation. Increasing the HLB value will moderately decrease the entrapment efficiency, vesicle formation and also influence the stability of the drug. The HLB value of sorbitan monostearate Span 60 is 4.7, and it is solid at room temperature, having the highest phase transition temperature of 50 °C [12]. The surfactant has the highest transition temperature and produces the highest entrapment efficiency. Hence, the formulation F2 containing Span 60, cholesterol and drug showed entrapment efficiency as of 96.40%, drug content as of 95.57%, mean vesicular diameter as of 463.7 nm and zeta potential as of -80.5mv. The drug release was found to be 95.14% in 12 h. The optimized formulation followed the first order kinetics with non- fickian diffusion mechanism.
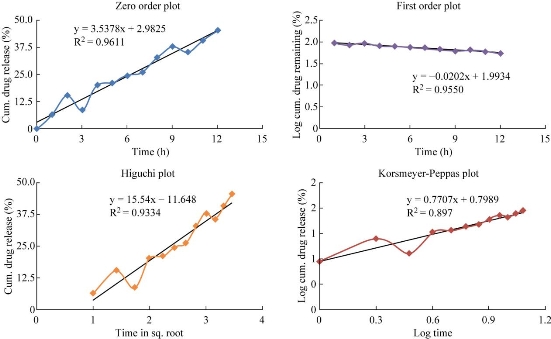
Fig. 8 Kinetic release data for optimized formulation F2.
Table 5 Kinetic release data for optimized formulation F2
|
Formulation |
Zero order (R²) |
First order ( R²) |
Higuchi plot (R²) |
Peppas plot (n) |
|
F2 |
0.947 |
0.955 |
0.933 |
0.770 |
Conclusions
Etoricoxib niosomes were successfully developed as a carrier for site-specific drug delivery by thin film hydration technique using the drug, cholesterol and surfactants. The formulation containing surfactant Span 60 showed maximal drug content as of 95.57%, better entrapment efficiency as of 96.40%, minimal mean vesicular diameter as of 463.7nm, and zeta potential as of -80.5 mV. Hence, Span 60 was used as a good surfactant to form niosomes and thin film hydration technique was an optimized technique for the preparation of etoricoxib niosomes.
Conflict of Interests
The authors declare that no competing interest exists.
References
Copyright© Veldurthi Ravalika, Abbaraju Krishna Sailaja. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.