Research Article
Interaction of Colloidal Gold Nanoparticles with Protein
Sami Waheed Radhi *
Department of Chemistry, Faculty of Science, University of Kufa, Najf, Iraq.
* Corresponding author. E-mail: samiche100@gmail.com
Received: Nov. 2, 2017; Accepted: Dec. 5, 2017; Published: Dec. 25, 2017
Citation: Sami Waheed Radhi, Interaction of Colloidal Gold Nanoparticles with Protein. Nano Biomed. Eng., 2017, 9(4): 298-305.
DOI: 10.5101/nbe.v9i4.p298-305.
Abstract
The interaction between nanoparticles (NPs) with biomaterials has many applications. In the present research, the interaction between colloidal gold nanoparticles and human protein, human chorionic gonadotropin (hCG) was studied to find out the changes in the structure of protein at the presence of colloidal gold nanoparticles and the quantity of protein that colloidal gold nanoparticles absorbed on the hCG surface. Colloidal gold nanoparticles were synthesized through the reduction of HAuCl4 using ammonium hydroxide. The gold nanoparticles were estimated at 20 nm in diameter. The results showed that there was a significant adsorption of colloidal gold nanoparticles absorbed on the hCG surface. Sips equation was used to express the absorption of isotherms and to calculate the absorption parameters and thermodynamic constant. Fluorescence study indicated a mild change in the tertiary structure near the microenvironment of the aromatic amino acids tyrosine and phenyl alanine, which was due to the interaction forces between the colloidal gold nanoparticles and hCG protein that affected the environment. It can be concluded that the colloidal gold nanoparticles had effects on the tertiary structure of proteins and affected the availability of hCG concentration.
Keywords: Nanoparticles; Gold; Absorption; Protein; hCG
Introduction
Nanotechnology is defined as “the design, characterization, production and application of structures, devices and systems by controlling shape and size at the nanoscale (<100 nm)” [1]; it investigated the interactions between nanomaterials with biological systems, known as nano-bio interactions [2-6]. Nanotechnology has the potential to create new materials and devices with many applications in medicinal chemistry, agriculture [7-10] and energy or electronic devices, [11, 12]. The size of nanoparticles (NPs) is dependent on optical, electrical and magnetic properties, making nanotechnology science a promising candidate for biomaterials and applications such as in-vivo imaging, sensing, therapeutics and catalysis [13, 14]. Based on different scientific fields, biologists, physicists, chemists as well as bioengineers share a common advantage to treat severe diseases through nanotechnology [15]. Theoretically, NPs can be tailored to reach the right target at the right time [16, 17]. Human chorionic gonadotropin (hCG) is synthesized primarily in the placenta (placental hCG) as heterodimeric hormone, pituitary, produced by differentiated syncytiotrophoblast cells or more specifically villous syncytiotrophoblast cells during the pregnancy progresses [18, 19]. hCG is a glycoprotein that contained 4-N-linked and 4-O-linked oligosaccharides [20, 21]. The molecular weight of hCG is approximately 37,500 Da. It is an unusual molecule in that 25-41% of the molecular weight is derived from the sugar side-chains (25-30% in regular hCG and 35-41% in hyperglycosylated hCG). All the molecules share a common hCG β-subunit amino acid sequence) [22]. The interaction of specific proteins with nanoparticles led to a vast improvement of in vivo gene delivery, clinical diagnosis, medical/cancer imaging, receptor-targeted delivery [23] The interaction between NPs and proteins surface leads to the formation of proteins “corona” around NPs that largely defines their biological identity as well their potential toxicity [24, 25]. According to the type of the interaction between protein and NPs, there are two main types of interactions between proteins and NPs: covalent protein-NP conjugation and non-covalent protein-NP conjugation. The supramolecular interactions between proteins and NPs are central to the applications of NPs in vivo.
Experimental
Instruments
All chemicals were provided by Sigma Aldrich. Transmission electron microscope (TEM), Jem. 2010F was from Jeol, USA; Fluorescence spectrophotometer, A665375 from Cary Eclipse, USA; UV-visible spectrophotometer, 50 Bio UV-VIS from Varian Cary, USA; ultra centrifuge, A666037 from Sorvall, USA; Thermo Scientific Nicolet from USA; and vacuum oven, A 280 from Isotemp, USA.
Synthesis of (20 nm) colloidal gold NPs
Gold Nps with particle size around (20nm) was synthesized according to the following procedure: In the conical flask, insert a magnetic stirrer bar into 500 mL of boiling distilled water; add 2 mL of 1% gold chloride, and then add in 1 portion of ammonium hydroxide and speedily 10 mL of ammonium hydroxide (0.5 mol). After a few minutes, the color of colloid (red) was changed. Leave colloid to 20 mint and obtained 500 ml (40 ppm) of colloidal gold Nps.
Interaction of colloidal gold Nps with hCG hormone
1.5 ml of colloidal gold Nps (20 nm) was dispersed in 40 mL of phosphate buffer solution, The mixture was then mixed and ultrasonicated for 10 min twice. The hCG concentrations of hCG powder in phosphate buffer were prepared, (40, 35, 30, 25, 20, 15 and 10 µg/mL).Then 500 mL of the colloidal gold Nps suspension was mixed with 0.5 mL of different concentrations of hCG solution. The mixtures were stirred for 30 min at different temperatures (15, 25, 35 and 45 ˚C). The mixtures were then ultracentrifuged at 20000 rpm. The isotherms were constructed between the hCG concentration in solution at equilibrium (Ce) and the amount of hCG that Np (Qe) absorbed on it:
Qe = V(Co-Ce)/m,
where Qe refers to the amount of adsorption (µg/g), Co refers to the initial concentration (µg/mL), m refers to the weight of absorbent (mg), V refers to the volume of solution (mL), and Ce refers to the concentration at equilibrium (µg/mL). The isotherms were studied to find out the best equation applied for the practical results. The thermodynamic parameters ∆H, ∆S and ∆G were calculated firstly using Vant-Hoff’s equation [26]:
LnKe = -∆H/RT+ constant,
where ∆H refers to the enthalpy in reaction, Ke refers to the equilibrium constant, R refers to the gas constant which was 8.314 J/K/mol. By plotting Ln Ke against 1/T, a straight line should be formed with a slope of -∆H/R. In order to calculate the free energy change (∆G) of the adsorption process at a certain temperature, equilibrium constant of the adsorption-desorption process of Au NPs on hCG should be calculated according to:
Ke = Qe×M/Ce×V(mL),
where Ke refers to the equilibrium constant, Qe refers to the adsorption amount at equilibrium (µg/mg), M means the mass of NPs (mg), Ce means the concentration of hCG (µg/mL) at equilibrium, and V(mL) is the volume of solution (mL). Then, free energy change can be calculated from the following equation:
∆G = -RT lnKe
Entropy (∆S) was then calculated from the following formula [26,27]:
∆S= (∆G – ∆H)/T
Preparation of NPs for transmission electron microscope (TEM)
TEM micrographs of Au NP samples were obtained by diluting the nanoparticle and, after brief sonication, depositing approximately 0.1 mL of samples on a carbon-coated copper grid. The samples of Au NPs were dried under vacuum and left overnight before analysis. A variety of drying conditions were tested, but the preparation of samples caused the particles to clump. At least three separate Au NPs solutions of each morphology, prepared in the method outlined above, were imaged using TEM and used to determine the nanoparticle size distributions.
Absorption procedure
In test tubes were prepared solution containing 0.5 mL (0.5 mg/mL) Au NPs and 0.5 mL (0.5 µg/mL) hCG solution in order to form an Au NP-hCG conjugate. The mixture was stirred for 35 min at room temperature. After centrifuge, the concentration of hCG in the supernatant was measured by ELISA method. The precipitates in each tube were mixed with 2 mL of phosphate buffer and the mixture was stirred for 35 min at different temperatures (15, 25, 35 and 45 ˚C).
Calculation of Au NP surface area and hCG hormone volume
Au NP surface area and hCG volume
Au NP surface area and hCG volume were calculated in order to estimate theoretically the number of Au NPs enough to cover hCG in one layer manner.
Surface area of Au NPs = A = 4πr2 = 125.664×10-6 cm2
Volume of each NP = V = 4/3πr3 = 4/3π103 = 4188.79 nm3
hCG volume and surface area.
The calculation of hCG volume was based on the assumption that the protein had the simplest shape as a sphere, the radius of which was calculated according to:
Volume of hCG molecules V (nm3) = 1.212×10-3(nm3/Da) ×M.wt (Da) [28] = 1.212×10-3×37×103 = 44.844 nm3
(Da=Dalton; M.wt=Molecular weight)
Radius rnm = (3V/4π)1/3 = (3×30.3 nm3/4π)1/3 = 1.9339 nm
Surface area = πr2 = π2.2032 nm2 = 1.5239×10-6 cm2
Mass of one hCG = M.wt/6.02×10+23 = 4.528×10-20 g
Calculation of absorption for monolayer
Number of Au NPs that could cover one hCG in a monolayer manner
= Surface area of one hCG (cm2)/Surface area of one Au NP (cm2) = 82.51
Mass of Au NPs on one hCG = Number of Au NPs per hCG×Mass of one hCG molecule = 37×103×6.02×10+23 = 6.146×10-20 g
Results and Discussion
Preparation and identification of Au NPs
The method used for the preparation of Au NPs was carried out in aqueous environment. The particle morphology and microstructure of the synthesized Au NPs were examined by TEM. The images of Au NPs were taken using an accelerating voltage 200 keV field emission analytical TEM equipped with an Oxford energy dispersive X-ray spectrometer (EDS) and a charge coupled camera (type Gatan 794CCD). The dimensions and the shape of NPs were estimated using a program existing in the TEM device; the shape of the particle was spherical with a radius of 20 nm, as shown in Fig. 1(a)-(c). The data from EDS revealed the percentage of gold equalled 98.88±2.17%. This result confirmed the conformation of Au NPs. The interaction of an electron beamwith a sample target (NP) produced a variety of emissions, including X-rays. The EDS results of Au NPs are shown in Fig. 2. The use of Au NPs to enhancing permeably of cell membrane in drug delivery of compounds into cells is possible if they are suitably stabilized in colloidal form by charged molecules (including oligonucleotides) or polymers (including DNA and polyelectrolytes) [29].
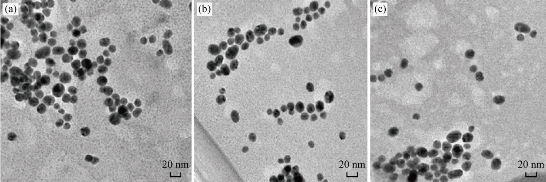
Fig. 1 TEM images of the prepared Au NPs in different areas of (a), (b) and (c).
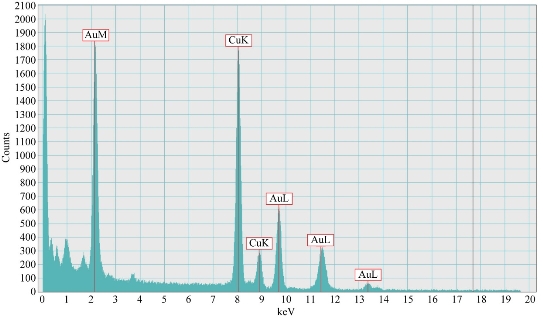
Fig. 2 EDS of Au NPs.
TEM study for Au NP-hCG complex
The morphologies of Au NPs absorbed on the surface of hCG in different areas are presented in Fig. 3(a)-(c). The figure shows several interesting conclusions, particularly in the formation of the Au NP layers on the surface of hCG. Furthermore, hCG aggregated with each other after the adsorption of Au NPs, which indicates the cement-like action of the absorbed layer of Au NPs and the cooperative interaction among the absorbed Au NPs molecules. This finding indicates the high possibility of forming more the one absorbed layer of Au NPs on the surface of hCG. The interaction of the absorbed Au NP molecules with each other indicates that more absorbed solutes resulted in easier accumulation of additional amounts. This finding suggests a lateral association among the absorbed molecules that holds them on the surface. This process is called "cooperative adsorption". The adsorption of Au NPs onto proteins (hCG) is interesting because the interaction between proteins and Au NPs is fundamental to understand bio mineralization, a higher concentration of Au NPs on the surface of protein. The effective force among the absorbed protein molecules were stronger and more long-ranged than that in the solution; thus, the Au NPs aggregate on the surface protein molecules at similar conditions [30]. These explanations may be applied for the adsorption process of Au NPs on the surfaces of hCG. The incubation of hCG with Au NPs reduced the hCG concentration in the solution, which indicates that some hormones were bound to the NP surface with different surface phenomena, namely, adsorption processes. Adsorption refers to the accumulation of a dissolved solute onto the surface of a solid-absorbing material. Adsorption is achieved by establishing a contact between the solution and the adsorbing material (absorbent). The absorbed substance is called absorbate, whereas the surface that absorbs this substance is called absorbent. Adsorption is classified into chemical (chemisorption) and physical (Van der Waals adsorption) [31]. The energy distribution of the adsorption of Au NPs on the protein (hCG) showed a heterogeneous behavior. This behavior resulted from the surface heterogeneity and the lateral effect between the absorbed molecules. The Sips isotherm model is a combination of the Freundlich and Langmuir isotherm-type models and describes the heterogeneous surface more accurately [32]:
Qe = Qm × Ks × Cte/(1 + Ks × Cte).
The Sips isotherm approaches the Freundlich isotherm at low absorbate concentrations, whereas approaches the Langmuir isotherm at high concentrations. Ks is a Sips constant related to the energy of adsorption, and parameter t is the parameter characterizing the system heterogeneity. The adsorption isotherms of different hCG concentrations from aqueous solutions on a constant weight of Au NPs at 25 °C and pH 7.4. The fitting of the Sips equation for the adsorption isotherm of the Au NPs adsorption on hCG hormone is consistent with the general finding in the ability of Sips equation to explain the adsorption of NPs on different proteins [33]. Other studies confirmed the ability of the Sips isotherm to predict the adsorption of large molecules and NPs on the surface of proteins [34]. The adsorption of NPs on protein is the first step of a complex series of biophysical and biochemical processes that determine the biocompatibility of the material. Adsorption is affected by many factors, including shape, energy and functional group of the NPs surface and protein properties such as charge distribution, net charge and hydrogen bonding) [32]. The present findings demonstrated the heterogeneous adsorption behavior of Au NPs on hCG. Heterogeneity is a general feature of the surface properties caused by different unsaturation of a sorption on the sites leading to their different energetic characteristics. Surface imperfection and the presence of impurities are thus important; different types of adsorption forces on different active sites result in the creation of clusters or assemblies of adsorption molecules on the surface. The Sips model for heterogeneous adsorption produces three important constants: the maximum adsorption amount (Qm), Sips constant (Ks) and the heterogeneity system (t). These constants can be estimated as part of the fitting exercise and can be used to determine the parameters affected by the general shape of the molecule [35]. The three constants extracted from the adsorption isotherm of Au NPs on hCG were: Qm = 71.3 µg/mg, t = 3.1, and Ks = 0.24. The Sips equation is also used to model the cooperative adsorption among the absorbed macromolecules. The t value for Au NPs was higher than one which represents a favorable adsorption condition; thus, the absorbed hCG affects the adsorption of other hCG molecules. The magnitude of t has been linked to the factors affecting heterogeneous adsorption. At 0 < t < 1, heterogeneity is linked to the variations in the solid surface. When the absorbed molecule has a strong affinity to other absorbent molecules (a positive cooperative effect), t is higher than one [36]. This mechanism is consistent with the low hCG adsorption at low solution concentrations followed by a rapid increase. This lateral effect (positive cooperative effect) is also consistent with the energy site distribution (ESD) analysis in many studies. In this intermediate state, the α-helix of the protein is disrupted, while the β-sheet remains fully structured [37].
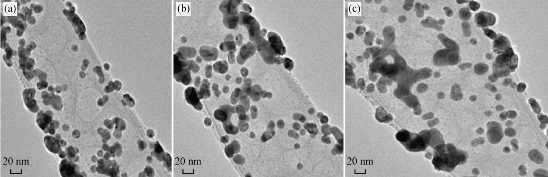
Fig. 3 TEM morphology of the Au NP- hCG conjugates in addition to the aggregation of Au NPs-hCG flakes caused by the hCG outer layer in different areas of (a), (b) and (c).
Thermodynamics of the interaction between Au NPs and hCG
The thermodynamic data (changes in enthalpy (ΔH), entropy (ΔS) and free energy (ΔG)) obtained from all protein-NP interactions can be used to detect the nature of the interaction due the fact that thermodynamics regulates most aspects of biomolecular interactions. The enthalpy of adsorption (ΔH°) provides a direct measure of the strength of the binding force between the absorbate molecules and the surface of the absorbent. To analyze the thermodynamics of the adsorption of Au NPs on hCG, adsorption processes were performed at different temperatures (288, 298, 308 and 318 °K) by using 0.5 μg/mL hCG hormone and 0.5 mg/mL Au NPs. The results showed that the amount of adsorption slightly increased with the increase in temperature. The plotting of Vant–Hoff’s equation for the adsorption process is presented in Fig. 4. The positive value of ∆H° (21.162 kJ/mol) indicates that the adsorption process was endothermic. The adsorption of Au NPs to a surface may induce conformational changes in the protein. The degree of conformational changes was determined with the combination of the native stability of a protein, the hydrophobicity and charges of the protein, and the sorbent surface. The adsorption of Au NPs can be driven by conformational entropy changes, particularly if the adsorption is endothermic. Other research found that the adsorption capacity of Au NPs increased with the increasing temperature, which indicates an endothermic process. The values of changes in free energy and entropy are presented in Table 2. Thermodynamic quantities reveal that NP-hCG complexes involved electrostatic interaction and other non-covalent forces, including hydrophobic, hydrogen bonding and π–π interaction obtained from the surface of functional groups of the NPs [38]. The adsorption energy was consistent with the adsorption energy derived as the sum of the electrostatic and Van der Waals forces as seen previously [39]. The stability of the adsorption system (NP – hCG) depended on the equilibrium between the entropy and enthalpy change on adsorption.
Table 1 Amount of the Au NPs adsorbed on hCG hormone at different temperatures
|
T (K) |
Co (µg/mL) |
Ce (µg/mL) |
Qe (µg/mg) |
|
288 |
0.5 |
19.2 |
51.13 |
|
298 |
0.5 |
17.3 |
53.34 |
|
308 |
0.5 |
15.6 |
56.64 |
|
318 |
0.5 |
12.8 |
58.87 |
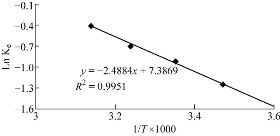
Fig. 4 Vant–Hoff's lines of the adsorption of Au NPs on hCG at different temperatures (288, 298, 308 and 318 °K).
Table 2 Thermodynamic parameters of Au NPs adsorption on hCG at four different temperatures (288, 298, 308 and 318 °K).
|
∆H (kJ/mol) |
lnKe |
∆G (kJ/mol) |
∆S (J/mol/K) |
|
21.162 |
-1.25 |
2.993 |
-63.086 |
|
|
-0.93 |
2.304 |
-63.281 |
|
|
-0.71 |
1.818 |
-62.805 |
|
|
-0.41 |
1.083 |
-63.141 |
Fluorescence spectroscopy
As shown in Fig. 5, the intrinsic fluorescence spectrum was different between the free hCG and Au NP -hCG. Fluorescence spectra were scanned between 250 and 450 nm after excitation at 260 nm at room temperature. A valuable feature of the intrinsic protein fluorescence is the high sensitivity of amino acids containing aromatic ring tryptophan to its local environment. Changes in the emission spectra of tryptophan often occur in response to conformational transitions in the three dimensional of protein, subunit association, substrate binding, or denaturation due to changes in the tertiary structure of hormone hCG by affected Au NPs. These interactions between Au NPs and hCG can affect the local environment surrounding the indole ring of tryptophan. Tyrosine and tryptophan display high anisotropies that are often sensitive to protein conformation and the extent of motion during the excited-state lifetime. Also, tryptophan appears to be uniquely sensitive to collisional quenching, apparently due to a tendency of excited-state indole to donate electrons [40]. Tryptophan can be quenched by externally added quenchers or by nearby groups within the proteins. There are many reports on the use of emission spectra, anisotropy and quenching of tryptophan residues in proteins to study protein structure and function. Valuable information regarding the local environment and the tertiary conformational variations can be achieved via fluorescence spectroscopy. The fluorescence emission spectra of the protein is produced from the contribution of the aromatic amino acids, including tryptophan, tyrosine and phenylalanine; the intrinsic fluorescence of the proteins is highly sensitive to their surrounding environment [41]. This difference can be explained through the significant changes in the microenvironment of aromatic residues after the adsorption of Au NPs on the surface of protein through different forces. The hCG used in the present experiment was a tryptophan-free protein, hence the increase in the fluorescence signal in the fluorescent spectrum was mainly due to tyrosine and phenyl alanine. Detecting tyrosine and phenylalanine fluorescence is complicated because of the interference of tryptophan with resonance energy transfer and the weak signal of phenylalanine. Therefore, the application of tyrosine and phenylalanine fluorescence is limited to tryptophan-free proteins, such as the hCG in the present work.
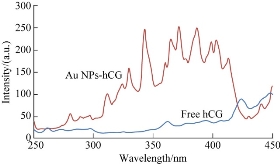
Fig. 5 Fluorescence spectra of the free hCG and Au NP- hCG.
Conflict of Interests
The authors declare that no competing interest exists.
References
Copyright© Sami Waheed Radhi. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.