Research Article
Performance Improvement of Working Electrode Using Grafted Polymer Modified with SiO2 Nanoparticles
Muhammed Mizher Radhi 1*, Ahmed Ali Moosa 2, Ishraq Abd-Alkareem Khalaf 2
1 Radiological Techniques Department, Health and Medical Technology College-Baghdad, Middle Technology University, Baghdad, (MTU) Iraq.
2 Technical Engineering College-Baghdad, Middle Technology University, Baghdad, (MTU) Iraq.
* Corresponding author. E-mail: mmradhi@yahoo.com
Received: Mar. 30, 2018; Accepted: May 17, 2018; Published: May 28, 2018
Citation: Muhammed Mizher Radhi, Ahmed Ali Moosa, and Ishraq Abd-Alkareem Khalaf, Performance Improvement of Working Electrode Using Grafted Polymer Modified with SiO2 Nanoparticles. Nano Biomed. Eng., 2018, 10(2): 156-164.
DOI: 10.5101/nbe.v10i2.p156-164.
A new modified glassy carbon electrode (GCE) with grafted polymer (GP)/SiO2 nanoparticles (SiO2 NPs) were prepared using mechanical attachment method to produce a new sensor in cyclic voltammetric technique. The new working electrode GP/SiO2 NPs/GCE was characterized by a standard solution of 1 mM K4[Fe(CN)6] with 1 M K2HPO4 as an electrolyte to study the redox current peaks of FeII/FeIII ions at different concentrations such as scan rate, pH, determination of diffusion coefficient (Df), reliability and stability of the modified GCE. It was found that the new modified electrode enhanced the redox current peaks of FeII/FeIII from 12 µA to 20 µA and -5 µA to -15 µA for oxidation and reduction peaks in GCE, repectevely. So, the current ratio (Ipa/Ipc) for the new modified electrode was 1, and the potential peak separation (ΔEpa-c) was 100 mV, which indicated good electrochemical properties as an irreversible electrode and heterogeneous reaction. Good reliability and stability of modified GCE was obseved with low detection limit. Scanning electron microscopy (SEM) and atomic force microscopy (AFM) analysis of the nano-deposit was also studied.
Keyword: Grafted polymer; Silica nanoparticles; Cyclic voltammetry; FeII/FeIII; GCE
Introduction
The new study of nanoparticles with different types of polymers is an important subject in the field of electrochemistry, especially in the conversion of insulators into conductive or semi-conductive materials [1-5]. Cyclic voltammetry chronoamperometry, electrochemical impedance spectroscopy and differential pulse voltammetry were used to identify the electrochemical behavior of mitoxantrone at the sulfonic acid-functionalized SiO2 nanoparticles (SiO2 NPs) using glassy carbon electrode (GCE). The determination of mitoxantrone were optimized by the oxidation current peak which was proportional to mitoxantrone concentration in the range of 0.5-173 μM, while the detection limit was 36.8 μM (S/N = 3) [6]. A modification of grafted polymer (GP) with carbon nanotubes was fabricated as a new working electrode. The working electrode was characterized by K3[Fe(CN)6] solution in KCl as supporting electrolyte at different concentrations, scan rates and temperatures using cyclic voltammetric technique [7]. Electrochemical sensors were used for the detection of heavy metals such as lead, cadmium, mercury, arsenic, etc. Stripping voltammetry techniques were applied on electrodes (mercury, bismuth) or electrodes modified at their surface by nanoparticles or nanostructures, carbon nanotube (CNT, graphene). Special attention would be paid to strategies using biomolecules (DNA, peptide or proteins), enzymes or whole cells [8]. A new grafting technique for the functionalized silica particles with anionically produced new polymers was reported. First, the silica nanoparticles were modified with multifunctional chlorosilanes where the original Si−OH surface groups wwere replaced by Si−Cl groups. Then, the anionically synthesized polymers were linked to the Si−Cl functionalized nanoparticle surface. The polymer linking event was accompanied by termination reactions, most likely due to residual Si−OH groups [9]. Cyclic voltammetry, differential pulse voltammetry and linear sweep voltammetry were used to evaluate some electrochemical aspects of the nanohybrid materials of poly-proline-amino functionalized magnetic mesoporous silica-beta cyclodextrin nanohybrid on GCE [10]. A new nanocomposite based on O-aminophenol (OAP) was prepared by the electropolymerization of OAP at the surface of GCE in the presence of SiO2 nanoparticles. The cyclic voltammetry and electrochemical impedance spectroscopy (EIS) studies confirmed that the poly O-aminophenol (POAP) nanocomposite films had a higher capacitance than the pure POAP films. The presence of SiO2 led to an obvious improvement in the overall electrochemical performance of the GCE surface covered by POAP films [11]. CNTs have excellent properties such as small size with larger surface area, high electrical and thermal conductivity, high chemical stability, high mechanical strength, and high specific surface area. They are now used in the fabrication of nanostructured electrochemical sensors , immunosensors and DNA biosensors [12]. In this work, GP was modified with SiO2 NPs and doping the GCE with these polymer to study in cyclic voltammetric technique.
Experimental
Equipments and electro-analytical analysis methods
The potentiostat used in this work was EZstat series (Potentiostat/Galvanostat, NuVant Systems Inc., USA). The cyclic voltammetry experiment was performed by connecting the potentiostat to the electrochemical cell and a computer with special software. The refrence elctode was silver/silver chloride (Ag/AgCl in 3 M NaCl) with 1 mm diameter platinum wire as counter electrode. Two modified GCEs were prepared and used as working electrodes. Before using any solutions in the cyclic voltammetryic cell, the electrolyte solution was treated with nitrogen gas for 10-15 min to remove oxygen. The cyclic voltammetry cell experimental set up is shown in Fig. 1. In this work, the surface morphologies and dimeter of sample nanoparticles were investigated by scanning electron microscope (SEM) SEM–JEOL operated at 20-30 kV and atomic force spectroscopy (AFM), respectively.

Fig. 1 Cyclic voltammetry experimental set-up.
Procedure
Cyclic voltammetric cell was used in this technique by adding 10 mL of electrolyte in the quartz cell and immersing three electrodes in the electrolyte medium, GP-SiO2NPs/GCE as working electrode, Ag/AgCl as reference electrode and platinum wire as counter electrode. Then these three electrodes were connected with potentiostat to find the results by the cyclic voltammogram using personal computer.
Reagents
Silicon oxide nanoparticles (20-30 nm) was purchased from Hongwu International Group Ltd, China. Potassium ferreous cyanide, dipotassium phosphate and potassium chloride were from Sinopharm Chemical Reagent Co, Ltd. (SCRC), China, Potassium perchlorate and potassium nitrate were from British Drug Houses (BDH), Engiland. The deionize water was used to dilute all the solutions. All materials were with purity of 98-99.9% and were thus used without any further purification process.
Synthesis of grafted polymer (GP)
By gamma-irradiation technique, polystyrene was grafted with acrylonitrile using chloroform as a solvent and ferrous ammonium sulphate as a catalyst, respectively. Different percentages of the GP were collected and studied [13].
Preparation of GP/SiO2 NPs
The GP was first dissolved in chloroform and mixed with nano SiO2 powder (20-30 nm) by weight ratio of 1000:1 (GP: nano SiO2) for 72 h with constant stirring at 50 oC. After the evaporation of chloroform, the precipitate was ground with mortar and pestle into fine particles yield as GP/nanosilica.
Preparation of modified GCE (GP/SiO2 NPs/GCE)
The GCE was polished with alumina slurry (0.5 micron) and then ultrasonically cleaned for 10 min, followed by rinsing with distilled water and drying at room temperature (drying by air blower). The modification of the cleaned GCE with silica nanoparticles was done by mechanical attachment method [14]. The GCE surface was tapped (doping) about thirty times onto GP/SiO2NPs powder placed on a filter paper as shown in Fig. 2. In this work, the modified GCE with silica nanoparticles would be used as working electrode and termed as GP/SiO2 NPs/GCE.
Fig. 2 Mechanical attachment method.
Results and Discussion
Characterization of different modified electrode
Fig. 3 illustrates the cyclic voltammogram of different working electrodes (GCE and GP/SiO2 NPs/GCE) to characterize the potential area of the electrode in 1 M K2HPO4 solution as a supporting electrolyte. A wide potential area of the new modified electrode (GP/SiO2 NPs/GCE) at -2 to +2 V without any current peaks was found, while the GCE had a range of potential at -1.5 to +1.8 V with current peak at 1-1.8 V. So, the modified electrode achieved good electrochemical properties for voltammetric analysis [15]. Normally, in voltammetric analysis, the K4[Fe(CN)6] compound was chosen for standardization and calibration [16] of the new modified electrode GP/SiO2 NPs/GCE and for comparing it with SiO2 NPs/GCE as shown in Fig. 4. It was found from the oxidation-reduction current peaks of FeII/FeIII that the modified GCE with SiO2 NPs had current at 12 µA and -5 µA respectively, while the current of modified GCE with GP/SiO2 NPs was enhanced to 20 µA and -15 µA respectively. This means that GP polymer with nanoparticles acted as an electro-catalyst and increased the conductivity of the modified working GCE. Also, the current ratio of oxidation-reduction current peaks of FeII/FeIII was Ipa/Ipc ≈ 1. This demonstrates that the new modified electrode GP/SiO2 NPs/GCE functioned as a reversible electrode [17], and the potential peak separation of ΔEpa-c ≈ 100 mV suggests that the reaction at the modified electrode was a homogenous process [18].
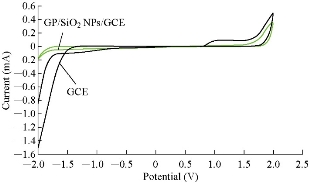
Fig. 3 Cyclic voltammogram of 1 M K2HPO4 using GCE and GP/SiO2 NPs/GCE as working electrodes versus Ag/AgCl as reference electrode and SR = 100 mV/sec.
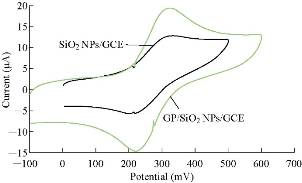
Fig. 4 Cyclic voltammogram of 1 mM K4[Fe(CN)6] in 1 M K2HPO4 using different modified GCEs as working electrodes versus Ag/AgCl as reference electrode and SR = 100 mV/sec.
Effect of different electrolytes
The effect of different supporting electrolytes was studied on the oxidation-reduction current peaks of FeII/FeIII ions to find the enhancement of the current on the modified electrode. It was found that the value of enhancement of the oxidation-reduction current in K2HPO4 electrolyte was 1.313 and 1.22 respectively, as shown in Table 1. The modified working electrode (GP/SiO2 NPs/GCE) was more sensitive to the K2HPO4 electrolyte in the electro-analysis by cyclic voltammetric technique. In general, the degree of oxidation current enhancement in varying electrolyte varied in the following order:
K2HPO4 >> KClO4 > NaCl > KCl > KNO3.
Furthermore, the reduction current enhancement was in the following order:
K2HPO4 > KClO4 > NaCl > KCl > KNO3.
Since K2HPO4 produces the highest current output, it was used in the following studies.
Table 1 Cyclic voltammetry of 1 mM potassium ferrous cyanide in different electrolytes, 1 M of KCl, KNO3, K2HPO4, NaCl and KClO4 at scan rate 100 mV/s for the SiO2 NPs/GCE and GP/SiO2 NPs/GCE
|
Electrolytes |
Ipa (µA) |
Epa (mV) |
Ipa (µA) |
Epa (mV) |
Enhancement |
|
Anodic |
|||||
|
KCl |
7.36 |
322 |
7.71 |
420 |
1.047 |
|
KNO3 |
10.6 |
332 |
10.7 |
375 |
1.009 |
|
K2HPO4 |
19.8 |
325 |
26 |
364 |
1.313 |
|
KClO4 |
12.7 |
298 |
14.8 |
320 |
1.165 |
|
NaCl |
9.16 |
363 |
10.2 |
384 |
1.113 |
|
Cathodic |
|||||
|
KCl |
4.68 |
200 |
3.75 |
126 |
0.801 |
|
KNO3 |
6.53 |
179 |
5.87 |
150 |
0.898 |
|
K2HPO4 |
12.7 |
201 |
15.5 |
179 |
1.220 |
|
KClO4 |
8.15 |
136 |
9.89 |
126 |
1.213 |
|
NaCl |
5.89 |
160 |
6.04 |
155 |
1.025 |
Effect of different concentrations
The new modified electrode (GP/SiO2 NPs/GCE) can be used to find and detect the low concentrations of ions in the aqueous solutions. This electrode is a good sensor for ions by studying the oxidation-reduction current peaks of the K4[Fe(CN)6] compound at low concentrations which enhanced the current against the increasing concentration, as shown in Fig. 5. A straight line of the relationship between the oxidation and reduction current was obtained with different low concentrations of K4[Fe(CN)6] with high sensitivity as in Fig. 6 and 7, respectively.
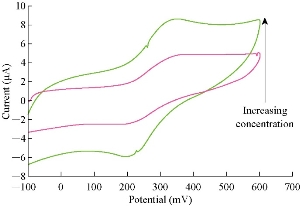
Fig. 5 Cyclic voltammogram of different concentrations of K4[Fe(CN)6] in 1 M K2HPO4 solution on the modified electrode versus Ag/AgCl as reference electrode and scan rate 100 mV/sec.
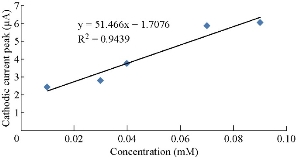
Fig. 6 Relationship between cathodic current peak against different concentrations of K4[Fe(CN)6].
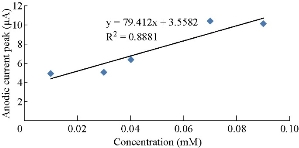
Fig. 7 Relationship between anodic current peak against different concentrations of K4[Fe(CN)6].
Effect of different pH
One of the important studies for the characterization of the modified electrode GP/SiO2 NPs/GCE is electrochemical property at different pH. Fig. 8 shows the oxidation-reduction current peaks of FeII/FeIII on the new electrode, indicating high current at acidic (pH: 2 – 6) and low value at alkaline medium (pH: 8 - 12). Thus, the modified working electrode acted in acidic medium as electro-catalyst; also, the modified electrode could used in acidic and basic media [19].
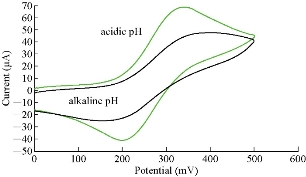
Fig. 8 Cyclic voltammogram of K4[Fe(CN)6] in K2HPO4 solution at acidic and alkaline pH on GP/SiO2 NPs/GCE versus Ag/AgCl as reference electrode.
Reliability and stability of the electrode
The stability of modified materials on the GCE was studied for ten times of oxidation-reduction current peaks of K4[Fe(CN)6] in K2HPO4 solution as shown in Fig. 9. The relative standard deviation (RSD) of anodic and cathodic peaks was determined with good value as of ± 0.4349 and ± 0.1836, respectively (Table 2).
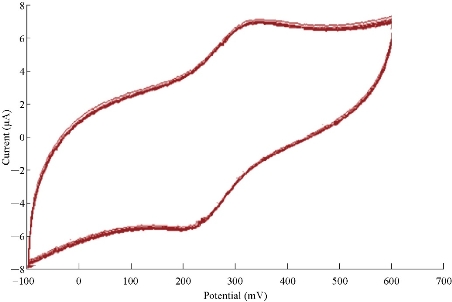
Fig. 9 Cyclic voltammogram of FeII/FeIII current peaks on the modified GCE at ten times of scan rate versus Ag/AgCl as reference electrode.
Table 2 RSD of the stability for modified working electrode (GP/SiO2 NPs/GCE) of oxidation-reduction current peaks for K4[Fe(CN)6] in 1 M K2HPO4
|
Stability and relability GP/SiO2 NPs/GCE |
||||
|
Anode |
||||
|
Ipa (X) |
X´ |
Standard |
Relative standard |
|
|
6.681 |
6.6441 |
0.00136161 |
0.0289 |
0.4349 |
|
6.68 |
0.00128881 |
|||
|
6.67 |
0.00067081 |
|||
|
6.66 |
0.00025281 |
|||
|
6.65 |
3.481E-05 |
|||
|
6.64 |
1.681E-05 |
|||
|
6.63 |
0.00019881 |
|||
|
6.62 |
0.00058081 |
|||
|
6.61 |
0.00116281 |
|||
|
6.6 |
0.00194481 |
|||
|
Cathode |
||||
|
Ipc (X) |
X´ |
(X-Xˊ)2 |
Standard |
Relative standard |
|
5.85 |
5.632 |
0.047524 |
0.0103 |
0.1836 |
|
5.8 |
0.028224 |
|||
|
5.75 |
0.013924 |
|||
|
5.7 |
0.004624 |
|||
|
5.65 |
0.000324 |
|||
|
5.6 |
0.001024 |
|||
|
5.55 |
0.006724 |
|||
|
5.52 |
0.012544 |
|||
|
5.5 |
0.017424 |
|||
|
5.4 |
0.053824 |
|||
Effect of different scan rates
Different scan rates from 0.01 to 0.1 V/sec was studied for the oxidation-reduction current peaks of FeII/FeIII in 1 M K2HPO4 solution as an electrolyte on the modified electrode (GP/SiO2 NPs/GCE) as shown in Fig. 10. It was found that the redox peaks of FeII/FeIII was enhanced with increasing the scan rate. Hence, the new modified electrode acted as electro-catalyst with the presence of silica nanoparticles in the structure of GP. Diffusion coefficient value was determined from the Randles-Seveik equation which describes it as reversible redox couple peaks [20, 21]:
Ip = (2.69 × 105) n3/2 AC Df1/2 V1/2 (1),
where Ip is the current peak (μA), n is the number of moles of electrons transferred in the reaction, A is the area of the electrode (cm2), Df is the diffusion coefficient (cm2/sec), and V is the scan rate of the applied potential (V/sec). The diffusion coefficient values of oxidation-reduction reaction for FeII/FeIII ions in K2HPO4 solution on GP/SiO2 NPs/GCE was determined as Dfa = 6.03 ×10-6 cm2/ec and Dfc = 3.122 × 10-6 cm2/sec, respectively.
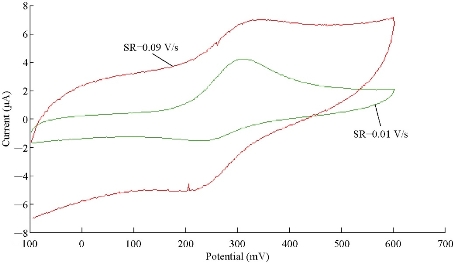
Fig. 10 Cyclic voltammogram of oxidation-reduction for FeII/FeIII in K2HPO4 solution on GP/SiO2 NPs/GCE at different scan rates of 0.01 - 0.1 V/sec.
Scanning electron microscopy (SEM) study
Fig. 11 illustrates the SEM of the GP structure with silica nanoparticles. The silica nanoparticles were incorporated inside the GP structure [22-26].

Fig. 11 SEM of GP/SiO2 NPs.
Atomic force microscpy (AFM) study
The AFM image of the GP material modified with silica nanoparticles GP/SiO2 NPs is shown in Fig. 12. The average SiO2 NPs diameter was 50 nm as shown in Fig. 13.
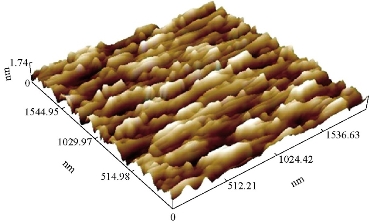
Fig. 12 AFM of GP/SiO2 NPs.
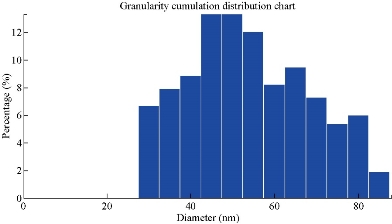
Fig. 13 Scanning prob microscop (SPM) of GP/SiO2 NPs.
Conclusions
In this study, high sensitivity to low detection limit of concentration in aqueous solution, good reliability with stability on the electrode and resistance to different pH were obtained using new working electrode GP/SiO2 NPs/GCE. The diffusion coefficient of the redox current peaks from different scan rates of FeII/FeIII was determined by using Randles-Seveik equation. And SEM and AFM images confirmed the nanoparticle structure of the GP.
Acknowledgements
The authors would like to thank the Dean of the Health and Medical Technology College-Baghdad, Dr. S. Dawood, for his support in completing the research.
Conflict of Interests
The authors declare that no competing interest exists.
References
Copyright© Muhammed Mizher Radhi, Ahmed Ali Moosa, and Ishraq Abd-Alkareem Khalaf. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.