Research Article
Multifunctional Silver Nanoparticles by Fruit Extract of Terminalia belarica and their Therapeutic Applications: A 3-in-1 System
Sucharitha Kumaram Venkata 1†*, Susmila Aparna Gaddam 2†*, Venkata Subbaiah Kotakadi 3†*, Divi Venkata Ramana Sai Gopal 2, 3
1 Department of Home Science, Sri Venkateswara University Tirupati-517502, Andhra Pradesh, India.
2 Department of Virology, Sri Venkateswara University Tirupati-517502, Andhra Pradesh, India.
3 DST-PURSE Centre, Sri Venkateswara University Tirupati-517502, Andhra Pradesh, India.
† Authors contributed equally to the work.
* Corresponding authors. E-mail: suchivenkata@gmail.com; susmilaaparna@gmail.com; kotakadi72@gmail.com
Received: Apr. 28, 2018; Accepted: Aug. 3, 2018; Published: Sep. 11, 2018
Citation: Sucharitha Kumaram Venkata, Susmila Aparna Gaddam, Venkata Subbaiah Kotakadi, and Divi Venkata Ramana Sai Gopal, Multifunctional Silver Nanoparticles by Fruit Extract of Terminalia belarica and their Therapeutic Applications: A 3-in-1 System. Nano Biomed. Eng., 2018, 10(3): 279-294.
DOI: 10.5101/nbe.v10i3.p279-294.
Abstract
The use of nanoparticles in treating dreadful diseases like cancer is the emerging field of research in cancer therapy. In the present investigation, the green biosynthesis of silver nanoparticles (AgNPs) with aqueous fruit extract of Terminalia belarica has been carried out, and ultraviolet-visible spectroscopy (UV-Vis) analysis was done which revealed an intense surface plasmon resonance (SPR) band at 430 nm, thus confirming the formation of Tb-AgNPs. The AgNPs were further characterized by Fourier transform-infrared spectroscopy (FTIR), showing the reduction of silver nitrate into Tb-AgNPs by the reduction of different functional groups such as hydroxyl, phenols, stretch of aldehydes, alkenes and aromatics. Transmission electron microscopy (TEM) and diffraction light scattering (DLS) study showed that the nanoparticles were round in shape with an average size of 46.5 nm. The atomic force microscopy (AFM) analysis also revealed similar results to facilitate that the AgNPs were round in shape, and the size was calculated by Z-coloration method. Furthermore, the X-ray diffraction (XRD) data confirmed that Tb-AgNPs were crystalline with face centered cubic (fcc) structure and were very stable with -29.1 mV Zeta potential. Tb-AgNPs showed efficient free radical scavenging activity against 2,2-diphenyl-1-picrylhydrazyl, hydrogen peroxide and nitric oxide. The 2,2-diphenyl-1-picrylhydrazyl (DPPH) method was the best among the three antioxidant methods with the half maximal inhibitory concentration (IC50) value of 47.25 ± 0.17. Tb-AgNPs also showed superior and efficient antibacterial activity greater than the control antibiotic, and showed effective anti-proliferative and cytotoxic effect on human breast cancer cells with IC50 value of 73.18 µg/mL. The biosynthesized Tb-AgNPs with multifunctional properties could be employed as a source for the exploration of novel therapeutic antioxidant, antibacterial and anticancer agent.
Keywords: Green synthesis; Terminalia belarica; Spectral characterization; AFM Z coloration analysis; Antioxidant activity; Antimicrobial activity; Anticancer activity
Introduction
The focus on traditional and modern nanomedicine leads to evolved green synthesis of silver nanoparticles (AgNPs) from different medicinal plant extracts. For the past two decades, understanding the properties of these nanoparticles has contributed to their applications in medicine. Synthesis of nanoparticles using physical methods such as photo chemical reduction, laser irradiation technique, aerosol technique, etc. [1-3], and chemical methods such as reduction methods using chemical reductants including NaBH4, N2H4, NH2OH, ethanol, etc. [4-8] has been carried out for years. Sometimes, these methods were expensive and hazardous. So, green chemistry methods were found to be a good alternative for biosynthesis of metallic AgNPs; they were nonhazardous, simple, inexpensive and environment friendly. Green silver nanoparticles gained their potential in antibacterial, antifungal, anticancer and biomedical applications [9-15]. The plant extracts have more potential in the synthesis of metal nanoparticles as they contain polyphenols, alkaloids, fatty acids, proteins, flavonoids which help reducing and capping agents. Silver nanoparticles have been used as an effective antimicrobial agents due to their ability to induce the production of reactive oxygen species (ROS) which lead to apoptosis of the microbial organisms [16-17]. Similarly, AgNPs have been reported to induce oxidative stress in mammalian cells and also induce toxicity in cancer cells by triggering reactive oxygen species (ROS) production which can lead to apoptosis of the cancer cells [18-19]. It was reported that biosynthesized AgNPs from a natural plant extract had exhibited anticancer activity in lung cancer cells [20]. As of the above findings, it is evidently explicit that the green AgNPs synthesized from natural sources might be used as an anticancer agent. Hence, the present study was initiated to study the effect of AgNPs on antibiotic resistant bacteria and human breast adenocarcinoma cell line (MCF-7). This work aimed at synthesizing AgNPs from aqueous fruit extract of Terminalia belarica and to characterize Tb-AgNPs by different advanced spectroscopic methods and to study their antioxidant, antibacterial and anticancer activities. Terminalia belarica belongs to the Combretaceae family, genes Terminalia species Terminalia belarica, called as vibhitaka in Sanskrit. The fruit of Terminalia belarica is used as a herb in the popular Indian traditional ayurvedic medicine, called as Triphala. In this study, aqueous extract of the fruit was used to synthesize Tb-AgNPs, Spectral characterization was carried out to study their antioxidant, antibacterial and anticancer activities in MCF-7 cell lines, which is clearly illustrated in Schematic 1
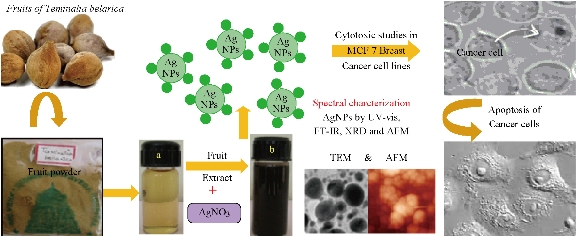
Schematic 1 Green synthesis of Tb-AgNPs and their characterization and applications.
Experimental
Preparation of Terminalia belerica fruit extract
The Terminalia belerica fruit powder was provided by a famous Ayurveda College located near Srinivasa Mangapuram, Tirupati. The aqueous fruit powder extract was ready by mixing 3 g of finely ground fruit powder in a 500 mL Erlenmeyer flask, with 300 mL of Milli-Q water. The blend was heated at 70 ℃ for 10 min and filtered with germ-free filter cloth, followed by Whatmann Grade 1 filter paper. The filtrate solution obtained (Fig. 1(a)) was a new source of aqueous extract for the biosynthesis of green AgNPs.
Biosynthesis of AgNPs
To the 10 mL diluted filtrate (2 mL extract + 8 mL distilled water), 20 mL of 0.025 mM AgNO3 was added, and the sample was kept at room temperature, till the color of reaction solution altered from slight pale yellow to dark brown (Fig. 1(b)). This color change proved the presence of AgNPs. In the current process, the biosynthesis of silver nanoparticles was done by the aqueous fruit extract of Terminalia belerica without any external toxic chemicals.
Purification of AgNPs
The solution containing AgNPs was centrifuged at 27,000 rpm for 1 h to obtain the nanoparticle pellets. The AgNPs pellets were redispersed in sterile Milli-Q water to get rid of any biological molecules. The process of centrifugation and re-dispersion in ultrapure sterilized Milli-Q water was repeated three times to obtain better separation of entities from the metal nanoparticles and to get rid of unbound fruit extract residues. The biosynthesized and purified Tb-AgNPs pellets were used for subsequent studies including Fourier-transform infrared spectroscopy (FTIR), X-ray diffraction (XRD) and energy-dispersive X-ray spectroscopy (EDX), etc.
Characterization of AgNPs
The biosynthesized and bio-reduced pure aqueous Tb-AgNPs solution with the fruit extract of Terminalia belerica was measured from time to time by sampling 1 ~ 3 µL of Tb-AgNPs solution. The optical density (OD) values of Tb-AgNPs were recorded on Nanodrop 8000, UV-visible spectrometer (Thermo Scientific) in wavelength range of 220 ~ 770 nm. The reading of the Tb-AgNPs solution was done at room temperature on Nanodrop Spectrophotometer at the resolution of 1 nm. After bioreduction and confirmation of the biosynthesis of Tb-AgNPs, they were separated by centrifugation from the colloidal solution. Then, Tb-AgNPs pellets were re-dispersed in sterile distilled water and purified by repetitive centrifugation for thrice. The final Tb-AgNPs pellets obtained were dried up in hot air oven at 70 ℃ to get fresh, pure and finer particles of Tb-AgNPs which were used for advance spectral characterization and bioactivity. The FTIR spectrum investigation was carried out to disclose the possible biomolecules of fruit extract of Terminalia belerica responsible for the biosynthesis and stabilization of Tb-AgNPs. The analysis was done by using Alpha T model, FTIR Spectrophotometer (Bruker Company, Switzerland). EDX analysis was done by using scanning electron microscope (200 kV) machine (Oxford Inca Penta FeTX3 EDS Instrument) with a line resolution of 2.32 in Å which was equipped with Carl Zeiss EVO MA 15 lens. The analysis of AgNPs was done with the help of a drop of coating on a small square bit of aluminum foil. Transmission electron microscopy (TEM) study was used to reveal the morphology, shape and size of AgNPs, by coating a drop of the purified aqueous AgNPs on a carbon-coated copper grid, and the analysis was carried out by using FEI Tecnai F12 (Philips Optics Ltd, Holland) operated at 100 kV. Furthermore, selected area electron diffraction (SAED) pattern of AgNPs was also carried out using TEM. The same purified AgNPs solution was also used to detect surface charges, i.e. Zeta potential and also particle size in colloidal form by means of dynamic light scattering (DLS) technique by using Horiba Nanopartica analyzer, SZ-100. Morphological and topological studies of AgNPs were done by using atomic force microscopy (AFM). A tiny drop of purified aqueous Tb-AgNPs solution was coated on a tiny square piece of aluminum sheet; it was assured that the sample was air dried completely without any moisture, prior to examination of the sample in AFM (Solver Next, NT-MDT, Russia). XRD study was conceded to prove the crystalline nature of the Tb-AgNPs using Ultima IV X-ray powder diffractometer (Rigaku Ltd, Tokyo, Japan) by means of Cu Kα radiation source.
Free radical scavenging activity
DPPH antioxidant activity
The in-vitro antioxidant activity of the AgNPs was measured by 2, 2’- diphenyl-1-picrylhydrazyl (DPPH) radical scavenging assay as described earlier [21]. 4 mg of DPPH was dissolved in 100 mL of methanol and stored at 20 ℃. From the stock solution, 2 mL of the solution was added to 1 mL of methanol solution containing test samples of Terminalia belarica fruit extract and Tb-AgNPs at different concentrations of 25, 50, 75 and 100 μg/mL. DPPH free radical scavenging activity (RSA) was measured at the absorbance of 517 nm. The antioxidant activity was also expressed as IC50. Ascorbic acid was used as standard in the present study. The percentage of free radical scavenging activity was calculated by using Eq. (1) as follows:
RSA (%) = [(control absorbance – sample absorbance) / control absorbance] × 100. (1)
Hydrogen peroxide (H2O2) scavenging activity
H2O2 scavenging ability of the test compound was determined according to the literature method [22]. A solution of H2O2 (40 mM) was prepared in phosphate buffer (pH 7.4). 25, 50, 75 and 100 µg/mL concentrations of Tb-AgNPs in 3.4 mL phosphate buffer were added to H2O2 solution (0.6 mL, 40 mM). The absorbance value of the reaction mixture was recorded at 230 nm. The percent of scavenging of H2O2 was calculated using Eq. (2) as below:
RSA (%) = [(control absorbance – sample absorbance) / control absorbance] × 100. (2)
Nitric oxide (NO) scavenging activity
The nitric oxide scavenging activity was measured by slightly modified methods [23]. The nitric oxide radicals (NO) were generated from sodium nitroprusside. 1 mL of sodium nitroprusside (10 mM) and 1.5 mL of phosphate buffer saline (0.2 M, pH 7.4) were added to different concentrations as of 25, 50, 75 and 100 μg/mL of Tb-AgNPs and incubated for 150 min at 25 ℃; 1 mL of the reaction mixture was treated with 1 mL of Griess reagent (1% sulfanilamide, 2% H3PO4 and 0.1% naphthylethylenediamine dihydrochloride). The absorbance of the chromatophore was measured at 546 nm. Nitric oxide scavenging activity was calculated using Eq. (3):
RSA (%) = [(control absorbance – sample absorbance) / control absorbance] × 100. (3)
Anti-bacteriological activity
The anti-bacteriological activity of Terminalia belerica fruit mediated Tb-AgNPs was studied against antibiotic resistant bacteria, two different strains of Escherichia coli (E.coli Strain-I (Donor) rifampin resistant, E.coli AB1157 (Mutant) streptomycin resistant), and also against gram positive bacterium Pseudomonas aeruginosa and gram positive bacterium Staphylococcus aureus by disc procedure. Freshly prepared bacterial culture of all the above bacterial samples were grown in sterile nutrient broth (Himedia, gm/L) for overnight at 37 ℃. 200 μL of respective microbial cultures was spread on nutrient agar plates; sterile discs were placed on nutrient agar plates; the discs were prepared with Whatmann Grade 1 filter paper. The biosynthesized green Tb-AgNPs were added onto the sterile discs with the help of micropipette as of 5 μL (5 mcg), 10 μL (10 mcg), 20 μL (20 mcg) and 30 μL (30 mcg), and Amoxyclav (30 mcg) disc was used as standard drug. The nutrient agar plates with the above samples were incubated at 37 ℃ for overnight.
Cell culture
Cytotoxic activity of AgNPs by 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT assay)
MCF-7 cells were purchased from the National Centre for Cellular Sciences (NCCS), Pune, India. MCF-7 cells were cultured in Eagle's minimum essential medium (EMEM), which consisted of 10% fetal bovine serum heat inactivated, 2 mM glutamine, 1 mM NaHCO3, 100 μg/mL streptomycin and 100 units/mL penicillin. The MCF-7 cell lines were maintained in culture at 37° C in presence of 5% CO2 atmosphere.
The cytotoxic activity of biosynthesized Tb-AgNPs was measured by using MTT assay [24]. Momentarily, 2 × 104 MCF-7 cells were seeded in 96-well plates containing 100 µL of EMEM in each well. After overnight incubation of the MCF-7 cells, exactly 100 µL of AgNPs at different concentrations (0, 10, 50, 100, 150 and 200 µg/mL) were added to the cells respectively. The plates were incubated for 24 h; the viability of cells was examined by adding 10 μL of MTT (5 mg/mL) per well and the plate was incubated at 37°C for 3 h. The 96-well plates were centrifuged at 1000 xg for 10 min at room temperature. The insoluble Formazan blue formed in the cells was dissolved by using 100 µL of DMSO. The color intensity was measured at 570 nm wavelength. The percentage of inhibition of cell viability was determined and the IC50 value at which cell viability decreased by 50% were also calculated.
Results and Discussion
More than 80% of the world population rely on herbal medicines as over the counter herbal formulations and proprietary herbal drugs. The bioactive phyto-constituents from important and rare medicinal plants are widely used directly or indirectly in new drug formulations. So in the present study the Terminalia belerica fruit extract was used for the synthesis of Tb-AgNPs. Terminalia belerica belongs to the family Combretaceace. The dried fruit has been used for centuries in Ayurveda, a holistic system of medicine originating from India [25]. The fruits are astringent, acrid, digestive, antihelmintic, expectorant, narcotic, ophthalmic, antipyretic, antiemetic and rejuvenating. The oil extract from the seed pulp is used for the treatment of leucoderma and alopecia [26]. It has important phytoconstituents; these compounds were found to be responsible for many of the pharmacological activities such as antimicrobial, antioxidant, antidiabetic, antidiarrhoeal, analgesic, immunomodulatory activities, etc. [27]. The biosynthesis of Tb-AgNPs was carried out by green process using Terminalia belarica fruit powder extract by reduction process. Under ambient conditions, the silver nitrate solution was mixed with Terminalia belarica fruit extract; the reaction mixture turned from light yellow color to dark brown color within 2-5 min (Fig. 1), which indicated the formation of Tb-AgNPs. The bio-reduction was further confirmed by ultraviolet-visible spectroscopy (UV-Vis).

Fig. 1 (a) Fruit extract of Terminalia belarica; (b) Biosynthesized Tb-AgNPs by fruit extract of Terminalia belarica.
Ultraviolet-visible spectroscopy (UV-Vis) analysis of Tb-AgNPs
UV-Vis is a significant method for analysis and detection of the formation of Tb-AgNPs in aqueous solutions. From the earlier reports, it was clearly understood that the silver nanoparticles exhibited different colors in aqueous solutions due to its characteristic surface plasmon resonance (SPR) vibrations [28-29]. The color of Tb-AgNPs in solution was due to the excitation of nanoparticles in UV-Vis, The color of Tb-AgNPs colloidal solution changed from light brown to dark with the increase in time interval. The UV-Vis spectrum was depending on particle sizes and due to SPR absorption spectrum exhibited by the metal nanoparticles in the visible range [30]. The UV-Vis absorption (SPR) spectra of the biosynthesized Tb-AgNPs by using Terminalia belarica fruit extract was observed at 430 nm (Fig. 2). The SPR peak reflected many physical factors such as size, shape and particle stability. Previous studies also revealed that the particles in SPR range of above 420 ~ 450 nm were attributed to AgNPs of varying size ranges between 2 and 100 nm [31]. If SPR of AgNPs was between 410 and 450 nm, it indicated that the biosynthesized AgNPs were spherical in shape [32-33]. Similar results have already been reported in the case of stabilization effect of biological extracts on the formation of metal nanoparticles [34, 10-11]. Thus, in these studies, the formation and stability of nanoparticles were clearly understood by UV-Vis spectrum and SPR graph. Furthermore, the FTIR analysis confirmed the participation of different phyto-constituents in the synthesis of Tb-AgNPs. EDX also revealed the percentage of elemental silver ions.
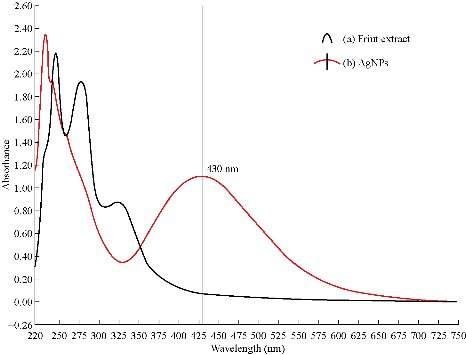
Fig. 2 (a) UV-Vis absorption spectra of Terminalia belarica fruit extract; (b) UV-Vis absorption spectra of Tb-AgNPs synthesized from Terminalia belarica fruit extract with 2×10-3 M silver nitrate.
FTIR analysis of Tb-AgNPs
Terminalia belarica fruit extract consists of 40% tannins in beleric and 60% tannins in beleric myrobalan as active ingredient. The main chemical constitutes of tannins mainly include ß- sitosterol, gallic acid, ellagic acid, ethyl gallate, galloyl glucose and chebulaginic acid apart from tannins. It also consists of different chemical constituents such as glycosides or sugars (fructose, sucrose and galactose), terpenoid (belleric acid and chebulagic acid) and saponin (bellericoside and bellericanin) [27]. So different concentration of different functional phytoconstituents of Terminalia belarica fruit may be responsible for capping and stabilizing the reduced nanoparticles. In the present study, the FTIR spectra of Terminalia belarica fruit extract and Tb-AgNPS are presented in Fig. 3. The Tb-AgNPs exhibited clear infrared radiation (IR) absorption bands at wave numbers 3408.88, 2926.02, 1634.66, 1414.68, and 1010.10. The sharp bands observed at 3408.88 and 2926.02 were attributed to stretching of –O-H- hydroxyl and C-H stretching modes in phenol vibrations. The IR absorption bands 1634.66 and 1414.68 were attributed to the COO asymmetric and symmetric stretching of aldehydes, alkenes and aromatics. From the above results, it was concluded that tannins and sugars were responsible for the reduction and stabilization of Tb-AgNPs. The other IR functional groups present in the fruit extract might also be responsible for the reduction Tb-AgNPs [35- 39, 12-14].
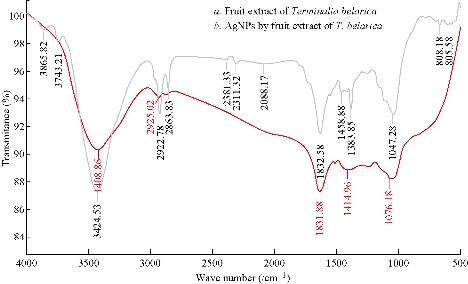
Fig. 3 (a) FTIR spectrum of Terminalia belarica fruit extract; (b) FTIR spectrum of the synthesized Tb-AgNPs.
Energy-dispersive X-ray spectroscopy (EDX) analysis of Tb-AgNPs
EDX revealed a strong signal in the silver region and confirmed the formation of AgNPs which might have originated from the biomolecules bound to the surface of the AgNPs. The EDX analysis represented the presence of the elemental silver (Fig. 4). The EDX profile showed strong silver signal along with moderate peaks of carbon and oxygen and weak peaks of silicon, chloride and potassium which might originate from the biomolecules that were bound to the surface of Tb-AgNPs, confirming the complete reduction of silver ions. Metallic silver nanocrystals generally showed typical optical absorption peak, approximately at 3 keV due to surface plasmon resonance [45]. It has been reported that nanoparticles synthesized using plant extracts are surrounded by a thin layer of some capping organic materials from the plant leaf broth, so they are stable in solution up to 4 weeks after synthesis [46]. Silver (34.64%) was the major constituent element compared to carbon (30.54%) oxygen (31.18%), silicon (1.04%), chloride (0.91%), and potassium (1.71%) (Fig. 4). The spectrum around 3 keV indicated a characteristic strong signal for nano-sized particles of silver. There were no peaks for silver compounds observed, which suggested that the silver compound was reduced completely to Tb-AgNPs as determined by the spectrum.
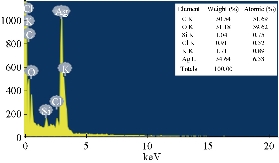
Fig. 4 EDX analysis of biofabricated Tb-AgNPs by fruit extract of Terminalia belarica.
Particle size determination of Tb-AgNPs
The DLS data revealed information about the size distribution of biosynthesized Tb-AgNPs by measuring their effects in light scattering due to Brownian motion of silver nanoparticles in liquid solution. The size of biosynthesized Tb-AgNPs was detected by dynamic light intensity and laser diffraction method. The biosynthesized Tb-AgNPs were poly-dispersed in nature. The DLS particle size data analysis revealed that the Terminalia belarica fruit extract Tb-AgNPs formed in the range of 15 ~ 90 nm. The average size (hydro dynamic radius) of the biosynthesized Tb-AgNPs was detected to be 46.5 nm (Fig. 5). Similar results were observed in TEM analysis and the average size of the Tb-AgNPs was in the range of 15 to 45 ± 5 nm.
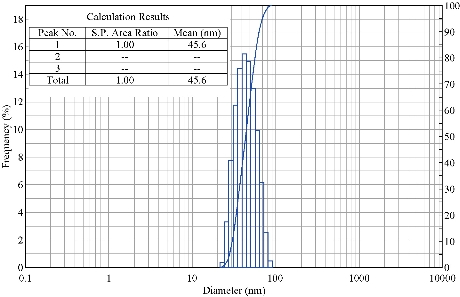
Fig. 5 Particle size distribution curve for Tb-AgNPs.
Analysis of Zeta potential of green Tb-AgNPs
Zeta potential is an important parameter which indicates the charge of the nanoparticles in a particular medium. In general, nanoparticles have charge on the surface, which generates repulsive forces between nanoparticles. The value recorded for the Terminalia belarica fruit extract biosynthesized silver nanoparticles was -29.2 mV (Fig. 6). This indicated that the AgNPs surface was negatively charged. Due to the presence of negative charge on the surface, agglomeration of Tb-AgNPs in aqueous solution was prevented, and long-term stability was also allowed. This clearly indicated that the nanoparticles were stable because the minimum charge required to stabilize the nanoparticles was +30 mV or -30 mV [40, 13-14]. The results revealed and concluded that the biosynthesized Tb-AgNPs with the fruit extract of Terminalia belarica were very stable in aqueous environment.
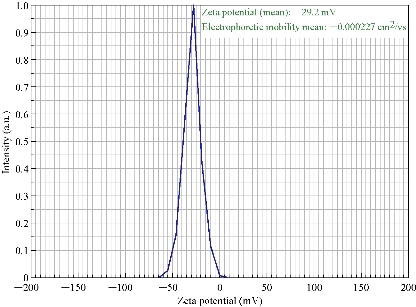
Fig. 6 Zeta potential of synthesized Tb-AgNPs.
Transmission electron microscopy (TEM) analysis and selected area electron diffraction (SAED) pattern of Tb-AgNPs
Further studies were carried out with TEM to determine the structural properties, such as the size and the shape, of biosynthesized Tb-AgNPs by the fruit extract of Terminalia belarica. From the TEM micrographs at different magnifications, it was clearly understood that Tb-AgNPs were spherical in shape, their sizes ranged from 10 to 45 ± 5 nm, and they were poly-dispersed in nature. The scale bar of TEM micrograph of biosynthesized Tb-AgNPs at 50 nm was represented in Fig. 7. The TEM images were in good agreement with the results obtained by UV-Vis spectroscopy. The biosynthesized Tb-AgNPs in aqueous solution were aggregated to some extent, but were unvarying in size and stable. The results were consistent with earlier reports of biosynthesized AgNPs from different plant-mediated sources [41, 15]. The SAED pattern of biosynthesized Tb-AgNPs by the fruit extract of Terminalia belarica shown in Fig.7(d) represented polycrystalline diffraction rings corresponding to distinct diffraction rings of the (111), (200), (220), (311) and (222) planes, indicating that the biosynthesized Tb-AgNPs were highly crystalline in nature.
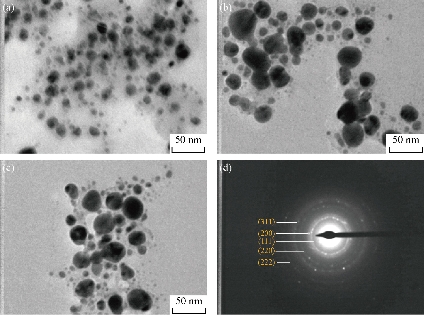
Fig. 7 TEM images of Tb-AgNPs at magnification of (a) × 200 K, (b) × 360 K and (c) × 500 K; (d) SAED pattern showed five diffraction rings.
X-ray diffraction (XRD) analysis of Tb-AgNPs
The size and crystalline nature of biosynthesized Tb-AgNPs of Terminalia belarica fruit extract were evaluated using XRD. The results of XRD analysis is shown in Fig. 8 which reveals five distinctive diffraction peaks at 38.310, 44.610, 64.500 , 77.580 and 83.350, corresponding to planes of (111), (200), (220), (311) and (222), respectively. The planes were indexed according to the facets of face centered cubic (fcc) crystal structure of silver (JCPDS card no. 87-0719). The XRD pattern was consistent with previous reports [42, 29]. At the same time, the results were in accordance with SAED pattern analysis of TEM, representing the planes of crystalline diffraction rings corresponding to (111), (200), (220), (311) and (222) (Fig. 8). That the diffraction peaks were consistent indicated the Tb-AgNPs were crystalline in nature. These results were also in accordance with studies of other researchers [43-44].
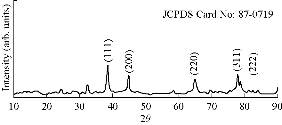
Fig. 8 XRD spectral data of biosynthesized Tb-AgNPs.
Atomic force microscopy (AFM) studies of Tb-AgNPs
AFM is an advanced spectroscopic technique used to explore and determine the morphology and topology of biosynthesized Tb-AgNPs. The results disclosed that biosynthesized Tb-AgNPs appeared to be spherical in shape by showing the nanoparticle’s topology and morphology (Fig. 9). Nova-Px 3.2.0. Rev software provided by NT-MDT was used to detect the grain size of the AFM image. An analysis of the results revealed that Tb-AgNPs were varied in size from 6 ± 5 nm to 45 ± 5 nm, whereas the average size of grains was found to be 46.5 nm. We also carried out grain analysis of the AFM 3D image using Nova-Px 3.2.0. Rev software by means of Z coloration technique which showed the range of nanoparticle size distributed within the 3D image by color differentiation (Fig. 10). No previous studies reported the Z coloration technique; we were the first to have reported this type of methodology for analysis of grain size of biosynthesized Tb-AgNPs.
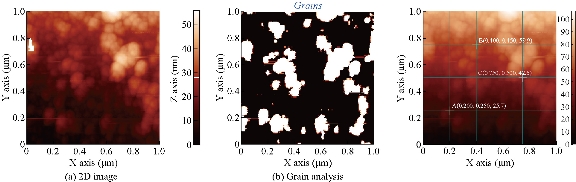
Fig. 9 2D images of (a) synthesized Tb-AgNPs and (b) grains detected.
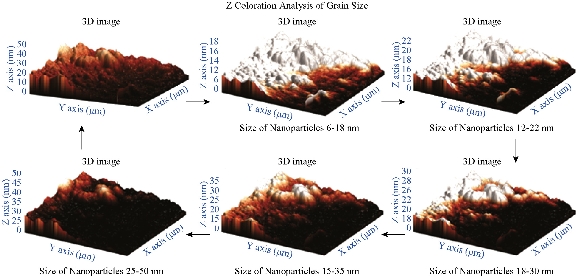
Fig. 10 3D images of synthesized AgNPs and grain analysis of Tb-AgNPs by Z coloration method.
2,2-diphenyl-1-picrylhydrazyl (DPPH) antioxidant activity of Tb-AgNPs
The antioxidant activity of biosynthesized Tb-AgNPs was detected by DPPH method. The DPPH free radical activity depends on the reduction of DPPH radical from DPPH to DPPH–H, a hydrogen-donating antioxidant. The in-vitro antioxidant activity and half maximal inhibitory concentration (IC50) values of the Terminalia belarica fruit extract biosynthesized Tb-AgNPs were reported in Table 1. The results revealed that the antioxidant free radical scavenging activity increased with the concentration of test samples. In the present experiment, it was determined that the free radical scavenging activity of biosynthesized Tb-AgNPs at the concentration of 100 µg/mL was 72.21; the value is 67.43 ± 0.67 for standard ascorbic acid and 68.10 ± 1.24 for aqueous fruit extract of Terminalia belarica. Therefore, it was concluded that the biosynthesized Tb-AgNPs proved to be the most effective scavenging agent. The oxidant activity of Tb-AgNPs was on par with standard ascorbic acid. The IC50 value of all the test samples was also calculated, which was equivalent to 50% of the free radicals in tested samples. In the present study, the IC50 values of biosynthesized Tb-AgNPs and fruit extract of Terminalia belarica were found to be 47.25 ± 0.17 µg/mL and 60.29 ± 1.38 µg/mL, respectively (Fig. 11(a)). It was previously known that the lesser the IC50 value, the more the scavenging activity. So it could be concluded that the biosynthesized AgNPs had effective free radical scavenging activity when compared with the fruit extract. The biosynthesized Tb-AgNPs by Terminalia belarica aqueous fruit extract actually consist of different secondary metabolites including flavonoids, terpenoids, polyphenols, proteins, etc. The secondary metabolites can easily donate hydrogen atoms, thus increasing the free scavenging activity of biosynthesized Tb-AgNPs, which in turn are actively involved in the stabilization of Tb-AgNPs. This was also confirmed by FTIR results in the present study. The results were similar to those that of earlier reports on biosynthesis of AgNPs [47]. It is also understood that less antioxidants lead to imbalance of ROS, which can increase oxidative stress in order to produce inflammation, atherosclerosis, neurodegenerative disorders, aging and cancer [47].
Table 1(i) Free radical scavenging activity of silver nanoparticles by DPPH method; (ii) In-vitro antioxidant activity by H2O2method; and (iii) In-vitro antioxidant activity by nitric oxide (NO) method.
|
(i) DPPH method |
Free radical scavenging activity ± SD (%) |
|
|||
|
Sample name |
25 µg/mL |
50 µg/mL |
75 µg/mL |
100 µg/mL |
IC50 |
|
Ascorbic acid |
45.07 ± 0.26 |
54.73 ± 0.07 |
63.54 ± 0.56 |
67.43 ± 0.67 |
33.93 ± 0.12 |
|
Terminalia belarica fruit extract |
15.25 ± 1.25 |
41.46 ± 1.84 |
65.48 ± 1.32 |
68.10 ± 1.24 |
60.29 ± 1.38 |
|
AgNPs |
27 ± 1.45 |
52.91 ± 0.36 |
66.24 ± 1.45 |
72.21 ± 0.19 |
47.25 ± 0.17 |
|
(ii) H202 method |
Free radical scavenging activity ± SD (%) |
|
|||
|
Sample name |
25 µg/mL |
50 µg/mL |
75 µg/mL |
100 µg/mL |
IC50 |
|
Terminalia belarica fruit extract |
9.27 ± 0.41 |
21.6 ± 1.29 |
37.45 ± 1.45 |
44.21 ± 1.33 |
21 ± 1.44 |
|
AgNPs |
18.03 ± 0.27 |
36.18 ± 0.9 |
47.54 ± 0.56 |
52.33 ± 0.7 |
24.5 ± 0.58 |
|
(iii) NO method |
Free radical scavenging activity ± SD (%) |
|
|||
|
Sample name |
25 µg/mL |
50 µg/mL |
75 µg/mL |
100 µg/mL |
IC50 |
|
Terminalia belarica fruit extract |
10.73 ± 1.7 |
27.3 ± 0.17 |
42.51 ± 0.7 |
57.33 ± 0.88 |
28.6 ± 0.76 |
|
AgNPs |
27.39 ± 1.13 |
33.22 ± 1.51 |
54.27 ± 1.3 |
61.81 ± 0.69 |
26 ± 01.44 |
Hydrogen peroxide scavenging assay of Tb-AgNPs
Accumulation of uninhibited H2O2 in living cells leads to the increase of reactive oxygen species or oxygen free radicals such as peroxides and hydroxyl, which causes vast injure to cell membranes. In the present study, hydrogen peroxide scavenging activity of Tb-AgNPs was carried out and quantified, as shown in Table 1 and Fig. 11(b). The inhibition concentrations at 100 µg/mL were found to be 52.33 ± 0.7% and 44.21 ± 1.33% for the biosynthesized Tb-AgNPs and fruit extract of Terminalia belarica, respectively. The H2O2 free radical was consistently lower than those obtained for DPPH scavenging activity. The biofabricated Tb-AgNPs dispersed in the presence of hydrogen peroxide could stimulate reactive oxygen species such as hydroxyl radicals and produce much stronger oxidative stress. The present results were in accordance with earlier reports of H2O2 scavenging effect of different plant extracts [48-50]
Nitric oxide (NO) radical scavenging assay of Tb-AgNPs
The NO scavenging activity of biosynthesized Tb-AgNPs and fruit extract of Terminalia belarica, showed the highest activity at the concentration of 100 µg/mL, i.e. 61.81 ± 0.69 and 54.27 ± 1.3, respectively (Table 1, Fig. 11(c)). The above observed NO activity was higher than that of the H2O2 scavenging activity, whereas was lower than that of DPPH activity. In the biology system, nitric oxide is a very important bio-regulatory molecule in various systems like immune system, nervous system and cardiovascular system [51]. The nitric oxide free radicals are very unstable in nature, since the production of free radicals may be due the interaction of AgNPs and nitric oxide under anhydrous condition at room temperature [52]. So we could conclude that the antioxidant potential of Tb-AgNPs could be attributed to functional groups adhered to them which were originated from the fruit extract of Terminalia belarica. Likewise, several other scientists studied the antioxidant activity of AgNPs biosynthesized from different parts of different plant materials [53]. Nitric oxide radical scavenging activity results revealed that the biosynthesized Tb-AgNPs from fruit extract of Terminalia belarica could be used in future antioxidant formulations.
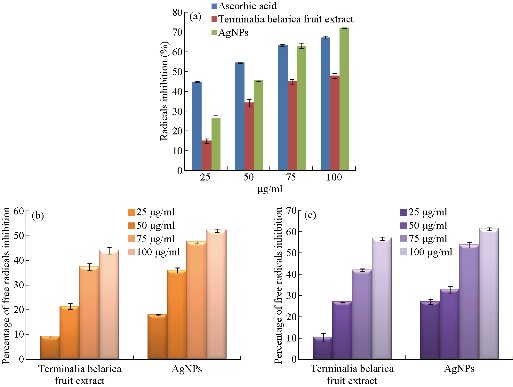
Fig. 11 (a) Free radical scavenging activity of biofabricated Tb-AgNPs by DPPH method; (b) Free radical scavenging activity of biofabricated Tb-AgNPs by H2O2 method; (c) Free radical scavenging activity of biofabricated Tb-AgNPs by no method.
Antimicrobial activity
The bio-fabricated Tb-AgNPs were found very effective against four different bacterial species at different concentrations of 5, 10, 20 and 30 μL of AgNPss on different gram +ve and gram –ve bacterial strains. The gram –ve bacteria Escherichia coli is antibiotic resistant bacteria; Pseudomonas aeruginosa and gram +ve Staphylococcus aureus bacteria are non-antibiotic resistant bacteria. The results showed excellent antimicrobial activity against Escherichia coli (E. coli) donor strain, Escherichia coli mutant strain and Pseudomonas aeruginosa; moderate activity was observed against Staphylococcus aureus gram +ve strain. The inhibitory behavior of Tb-AgNPs is shown in Table 2 and Fig. 12, by comparing with standard drug Amoxyclav. Hence, in the present study, Tb-AgNPs synthesized using silver nitrate and fruit extract of Terminelia belarica showed excellent bactericidal activity. Moreover, recent studies have revealed that the biosynthesized Tb-AgNPs of round or spherical shape, size of and above 20 nm showed competent antibacterial activity against Escherichia coli and Staphylococcus aureus [54]. AgNPs have unique chemical and physical properties and high surface area to volume ratio due to the above properties; AgNPs have emerged as new antimicrobial agents [55]. In the current study, biosynthesized Tb-AgNPs with the fruit extract of Terminelia belarica were found to have excellent bactericidal activity. When added to different bacterial strains, the Tb-AgNPs enter the bacteria by endocytosis and diffusion through the cell wall into the cytoplasm. The Tb-AgNPs induce toxicity in the bacterial cell. In sequence, the ROS along with Tb-AgNPs acts on nucleus of the bacteria, which stimulates the oxidation of nucleus and also induces chromosomal aberrations, eventually causing the death of the bacteria. Our present study led to the progress of new antibiotics against multidrug resistant bacteria with simple economical, eco-friendly green synthesis.
Table 2 Antibacterial activity of biosynthesized Silver nanoparticles
|
|
Different concentrations of AgNPs by Terminalia balerica fruit extract |
Control: Amoxyclav antibiotic |
|||
|
Concentration of AgNPs (mcg) |
5 |
10 |
20 |
30 |
30 |
|
Bacterial species |
Zone of inhibition (mm) |
||||
|
Escherichia coli strain I (donor) rifampin resistant |
12 |
13 |
15 |
20 |
15 |
|
Escherichia coli AB 1157 (mutant) streptomycin resistant |
10 |
10 |
14 |
21 |
15 |
|
Pseudomonas aeruginosa |
9 |
12 |
15 |
21 |
14 |
|
Staphylococcus aureus |
9 |
10 |
12 |
14 |
12 |
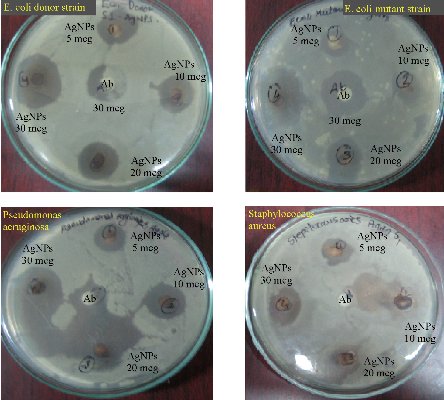
Fig. 12 Antimicrobial activity of Tb-AgNPs on gram +ve and gram -ve bacteria
Cytotoxic activity of Tb-AgNPs on MCF-7 cancer cells
Biocompatibility is crucial for the successful application of AgNPs in biomedical field. Hence, the Tb-AgNPs were evaluated for their cytotoxic effects on cancer cells breast cancer cell lines MCF-7. The percentage of cell viability of MCF-7 cell lines used in this study is depicted in (Fig. 13). Tb-AgNPs did show dose dependant increase in cytotoxicity against MCF-7 cancer cells. It was observed that the percentage of cell viability decreased as the dosage of Tb-AgNPs increased against cancer cells. Increase in the concentration of Tb-AgNPs reduced the cell viability and increased the cytotoxicity against MCF-7 cell lines, Tb-AgNPs effectively exhibited dose dependant cytotoxicity against cancer cells. Decrease in the cell viability and increase in the cytotoxicity were clearly observed as the concentration of Tb-AgNPs increased against cancer cells. Tb-AgNPs showed 73.3% inhibition of MCF-7 cells at 100 µg/mL concentration of Tb-AgNPs in the present study. IC50 of Tb-AgNPs against MCF-7 cancer cells was found to be 73.18 µg/mL. From the results, it was clear that MCF-7 cells were more susceptible to Tb-AgNPs as tested in this study. The cytotoxic activity of Tb-AgNPs at different concentrations against MCF-7 cells is depicted in Fig. 13. It clearly showed the increase in cytotoxicity with the increase in Tb-AgNPs concentration. The conceivable mechanism of the anticancer activity of Tb-AgNPs is explained as follows and illustrated in Schematic 2. The AgNPs entered into mammalian cells through various mechanisms such as endocytosis, pinocytosis and phagocytosis. Inside the cells, AgNPs interrupted various metabolic pathways by interacting with essential proteins; they could interact with the cellular DNA and cause DNA damage and finally lead to cell death [56]. It was also reported that the inhibitory effects of AgNPs on different cell lines mainly depended on the size, the morphology and the plant compound coated around AgNPs (proteins, polyphenols, tanins and flavonoids, etc). Our results are in line with other Tb-AgNPs synthesized from different plant extracts. AgNPs synthesized from leaf extract of Annona squamosa showed cytotoxicity against MCF-7 [57]. Based on the above results, it is apparent that the biosynthesized Tb-AgNPs are biocompatible and toxic to cancer cells, and therefore AgNPs can be useful as potential anticancer agents.
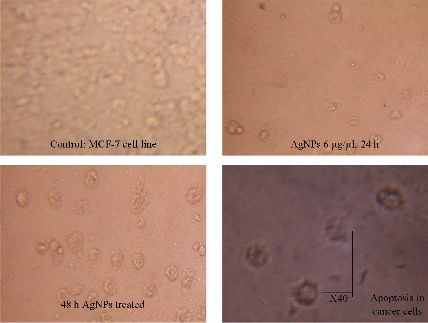
Fig. 13 Cytotoxic effect of Tb-AgNPs on MCF-7 human breast cancer cell.
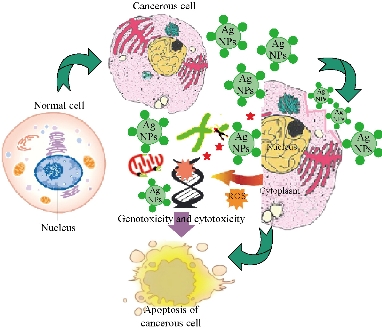
Schematic 2 Mechanism of the anticancer activity of Tb-AgNPs.
Conclusions
The current study revealed that the biosynthesized Tb-AgNPs with the fruit extract of Terminelia belarica showed excellent multifunctional therapeutic properties such as antioxidant, antibacterial and anticancer activities. It was clearly evident that the metal nanoparticles smaller than 100 nm had very good biomedical applications.
Acknowledgement
The author Venkata Subbaiah Kotakadi is grateful to Department of Science and Technology-Promotion of University Research and Scientific Excellence (DST-PURSE) Programme, sponsored by Department of Science and Technology, New Delhi, for providing fellowship.
Authors’ contributions
Sucharitha Kumaram Venkata, Susmila Aparna Gaddam, and Venkata Subbaiah Kotakadi contributed equally to the work.
Conflict of Interests
The authors declare that no competing interest exists.
References
Copyright© Sucharitha Kumaram Venkata, Susmila Aparna Gaddam, Venkata Subbaiah Kotakadi, and Divi Venkata Ramana Sai Gopal. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.