Research Article
Associations of Specific HLA-C Loci and Sociodemographic Factors with the Prevalence of Type I Psoriasis in Iraqi Patients
Manal Mohammed Khadhim *, Alaa Irhayyim Ali
Department of Medical Microbiology, College of Medicine, University of Al-Qadisiya, Diwaniyah, Iraq.
* Corresponding author. E-mail: manal.kadhim@qu.edu.iq Tel.: +9647801085917
Received: Apr. 20, 2018; Accepted: Jul. 19, 2018; Published: Oct. 26, 2018
Citation: Manal Mohammed Khadhim, Alaa Irhayyim Ali, Associations of Specific HLA-C Loci and Sociodemographic Factors with the Prevalence of Type I Psoriasis in Iraqi Patients. Nano Biomed. Eng., 2018, 10(4): 328-333.
DOI: 10.5101/nbe.v10i4.p328-333.
Abstract
Psoriasis is an autoimmune inflammatory disease of human skin with the etiology being unknown and for which there is no cure. It is believed to be genetically and immunologically conditioned and has major negative impact on quality of life. This study aimed to determine the impact of inheritance of specific human leukocyte antigen-C loci and some sociodemographic factors on the susceptibility to early onset psoriasis (type I). The current study included psoriatic group involving 76 patients (type I) and a match of apparently healthy group comprising 87 persons as a control. A polymerase chain reaction based method (low resolution sequence specific primer) was used to detect C*06, C*07 and C*17 allele after informed consent. The study showed that the C*06 and C*07 allele were significantly associated with early onset psoriasis (p-value < 0.05), while C*17 showed no significant association. There was also a higher percentage of patients in urban districts (84.2%) than rural residents (15.8%). There was no significant association between smoking and type I psoriasis (p-value > 0.05). Both of C*06 and C*07 genotypes increased the risk of early onset psoriasis, while rural residency decreased the chance of getting type I psoriasis. Furthermore, the lack of association with smoking could not mitigate the effect of passive smoking.
Keywords: C*06; C*07; C*17; Psoriasis; Residency; Smoking; Type I
Introduction
Psoriasis is defined as a common, chronic disease, with a genetic basis, characterized by an inflammation and proliferation activity [1]. Typically, psoriasis lesions are characterized by erythematous papules which develop to form plaques that are characterized by sharp borders and increased scaling [2]. It has a complex, multifactorial nature that is influenced by genetic, environmental factors and immune components, with a worldwide prevalence of approximately 1 to 3% [3]. According to the age of onset, psoriasis is characterized by bimodal distribution as type I early onset psoriasis and type II late onset psoriasis [4]. Some other authors identified type I psoriasis as inherited and associated with Cw6, while type II psoriasis occurs sporadically [5]. Several susceptibility loci have been identified; among them PSORS1 (6p21.3) is well-confirmed, in which HLA-Cw6 is the main marker allele, and has been notified in a number of studies [6]. Susceptibility to psoriasis in different ethnic groups is associated with different HLA alleles [5]. The current study aimed at determining the impact of inheritance of specific HLA-C loci including C*06, C*07 and C*17, and some sociodemographic factors on the susceptibility to early onset psoriasis (type I).
Experimental
This was a prospective case control study comprising seventy-six patients diagnosed by dermatologist, selected from those visiting the dermatological clinic in Al-Zahra Teaching Hospital in Al- Kut city from February to August, 2017, based on the age of first infection (less than 40 years) of chronic plaque psoriasis. And a match of eighty-seven apparently healthy persons as a control. Informed consent was obtained before venous blood sample (2 mL) taken from each patient and control in K3-EDTA anticoagulated tube and subjected to a DNA extraction procedure. After that, the extracted DNA that fulfilled the required purity and concentration aliquot was stored at -20 ℃ for polymerase chain reaction amplification and detection. A polymerase chain reaction-sequence specific primer (PCR-SSP) based technique was used in the current study with the primer sequence shown in Table 1. In the current study, a primer with specific sequence for glyceraldehyde 3 phosphate dehydrogenase (GAPDH) was used as a control primer. The obtained result is shown in Fig. 1.
Table 1 Primers sequence employed in this study
|
No. |
Target |
Sequence 5ʼ-3ʼ |
Product size (bp) |
References |
|
1 |
HLA-cw6 F |
GGATCAGGACGAAGTCCCAG |
170 |
NCBI/ primer-BLAST |
|
2 |
HLA-cw6 R |
GGGGACGCGTCATGAGTATT |
||
|
3 |
HLA-cw7 F |
TTA CAT CGC CCT GAA CGA GG |
237 |
NCBI/ primer-BLAST |
|
4 |
HLA-cw7 R |
GGC CAT CCC GGG AGA TCT AT |
||
|
5 |
HLA-cw17 F |
GGA TGA GGG GTC ATG TGT CT |
400 |
NCBI/ primer-BLAST |
|
6 |
HLA-cw17 R |
AGT AAG TGC TGG CAC ACA GG |
||
|
7 |
GAPDH F |
AGA CCA CAG TCC ATG CCA TC |
498 |
NCBI/ primer-BLAST |
|
8 |
GAPDH R |
CAG GGC CCT TTT TCT GAG CC |
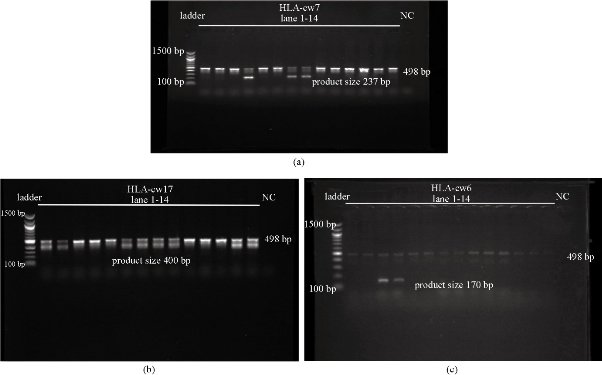
Fig. 1 Gel electrophoresis results. Each band was identified by comparing with the corresponding band of molecular ladder. (a) Result for HLA-cw7 (Lane 1-14): Product size 237 bp; the last lane is for negative control; the row of bands in the middle (498 bp) is for positive control. (b) Result for HLA-cw17 (Lane 1-14): Product size 400 bp; the last lane is for negative control; the row of bands in the middle (498 bp) is for positive control. (c) Result for HLA-cw6 (Lane 1-14): Product size 170 bp; the last lane is for negative control; the row of bands in the middle (498 bp) is for positive control.
Results and Discussion
The mean age for patients was 26.2 years. The male and female frequencies did not highly differ in psoriatic patients, accounting about 47.4% to 52.6%. About 84.2% of psoriatic patients were resident in an urban location while only 15.8% with rural residency. All the data about demographic traits of the participants are shown in Table 2. About 16% of patients were found to be smokers, and about 42% of the relatives to some psoriatic patients also smoked, in comparison to 26% smokers in non-relative group. A statistically significant differences was found between the patient and the relative groups (p-value < 0.05) (Table 3). About 18% of the psoriatic patients showed the presence of C*06 allele, compared to about 8% and 1% in relatives and non-relatives, respectively. A statistically significant difference was found between patients and non-relatives (p-value < 0.05). According to this result, C*06 increased the risk of psoriasis eleven times (OR 11.3). The C*07 allele was detected in 26% of the patients, and in lower percentages of both relatives (11%) and non-relatives (5%). C*07 increased the risk of psoriasis about five times (OR 5.7). Statistically significant difference was found between patients versus relatives and patients versus non-relatives. For C*17, there was no statistically significant difference found either between patients and relatives or between patients and non-relatives (p-value > 0.05). C*17 allele was detected in 31%, 38% and 29% of patients, relatives and non-relatives, respectively, as shown in Table 4. Psoriasis is a chronic, multisystem inflammatory disease, affecting both sexes equally. The pathogenesis is multifactorial, and has a bimodal age of onset [7]. Early onset psoriasis accounts about 75% of patients and occurs before the age of 40 years; patients have a strong family history and the majority of them are CW6 positive. Late onset psoriasis (type II) presents after the age of 40 years; patients have no family history and are Cw6 negative [8]. The current study found a higher percentage of patients were in urban than in rural residency. There are only a few studies that focus on psoriasis prevalence in association with residency. Salah and Bassam [9] found that about three quarters of psoriatic patients resided in cities in Iraq, while the rest were inhabitants of rural areas or immigrated to urban areas recently. Two explanations for this finding could be supposed. First, urban social and physical environment (e.g. traffic noise) contributed to increasing stress [10]. Second, skin microbiota alteration in psoriasis was also associated with residency, based on the facts that the skin microbiota resembles those in soil, and as the immune function in later life depends on the early cooperation between the developing immune system and microbiota [11]. This can lead to the hygiene hypothesis which postulates that reduction in microbial exposure in urban area due to improved sanitary can promote autoimmune disease development. In contrast to the rural area where microbes are specially helminth in close contact. Immune regulatory properties of microbes from environment, especially helminthes led to the therapeutic application of several immune-mediated conditions including psoriasis based on experimental models and clinical studies [12]. A lower percentage of patients were smokers compared to the relative and the non-relative groups. The association between psoriasis and smoking is bidirectional, and furthermore, the obvious effect of stress on both, make this relation become more complex, building a triangle with reversible effects with each other. Smoking can be risky for psoriasis both psychologically and physiologically. While smokers do smoke to mitigate stress, smoking itself can cause stress [13]. Furthermore, although a proven relation between smoking and both prevalence and severity of psoriasis has been existed, patients with psoriasis had a higher tendency to smoke [14]. In Iraq, Khalifa et al. [15] found that smokers with an earlier age of onset had more chronic diseases and less responses to treatment, while Ata et al. [16] found a significant association between psoriasis and smoking prior to onset of the disease while no significant association after the onset of psoriasis. Discrepancy of the current finding with several other studies that showed an association between smoking and psoriasis [17, 18] could be easily solved, because about 35% of the patients in this study were below 20 years old and female made 52% of the total patients for whom smoking is prohibited in Iraq. On the other hand, about 16% of the patients were active smokers, while about 42% of the relatives were smokers, making a high percentage of patients passive smokers (second-hand smokers). Smoking induces oxidative stress and frees radical production, increasing secretion of interleukin 2 (IL2), interleukin 12 (IL12), tumor necrotic factor (TNF) and interferon gamma (IFNγ), causing abnormal angiogenesis [19]. Free radical production during smoking activates signaling pathways implicated in psoriasis, such as nuclear factor kB, while smoking byproduct (nicotine) activates T cell to produce IL17 and IL22 major cytokines involved in the psoriasis pathogenesis [20]. Smoking also increases levels of IL-1, IL-6 and TNF-α while decreases the level of IL-10 [21]. Passive smoking as equal as active smoking risks, and can cause serious diseases in infants and children, with female more likely to be exposed [22, 23]. In the current study, the C*06 allele was significantly associated with psoriasis patients than the apparently healthy non-relative control. C*07 was also significantly associated with psoriasis, while C*17 showed no significant association with psoriasis. These three HLA types made the mainstay of this study. The frequency of C*06 antigen in Iraqi Arab Muslims and Kurd Muslims was about 2%, while the frequency of C*07 was 20% in Arab versus 9% in Kurd [24]. Another study found that from thirteen different HLA-C alleles in Iraqi Kurd, seven alleles had the frequencies higher than 4%, including HLA C*06, C*07 and C*017 [25]. Ahmed in his survey about HLA typing in UC patients in Iraq, detected C*06 and C*07 in 12% and 18% of healthy control, respectively [26]. Several other studies were conducted in Iraq that investigated the association of HLA types with psoriasis. Batool identified C*07 among others as risk allele for psoriasis [27]. Ahmed revealed that C*17 (but not C*06 or C*07) among others were significantly associated with psoriasis in Iraqi patients [5]. In neighboring countries, a study about childhood psoriasis in Kuwait by Nanda et al. showed a significant association of psoriasis with C*01 [28]. C*06 significantly increased in frequency in type I patients compared with controls in Turkish [29], while there was no statistically significant increase of C*06 allele in type I psoriatic patients compared to controls in Omanis [30]. The global association of C*06 allele with psoriasis (PSO) has been validated with different populations, including South Asians [31], East Asians [32], Europeans [33] and Egyptians [6]. The importance of C*06 allele being associated with psoriasis was not just about its role in presenting autoantigen to T-cell. C*06 positive patients tended to develop extensive and severe diseases, while C*06 negative patients suffered from dystrophic nail changes and psoriasis arthritis (PSA) [34]. HLA-C*06-positive psoriatic responded quickly to ustekinumab with almost 90% improvement within 4 weeks, compared to 60% of HLA-C*06 negative patients [35]. C*06 allele might offer protection from PSA [36].
Table 2 Distribution of patients and control according to gender and residency
|
Study sample |
||||
|
|
Patients (n =76) |
Controls (n = 87) |
Total (n = 163) |
P-value |
|
Gender (no.) % |
|
|||
|
Male |
(36) 47.4 |
(43) 49.4 |
(79) 48.5 |
0.458 |
|
Female |
(40) 52.6 |
(44) 50.6 |
(84) 51.5 |
|
|
Residency (no.) % |
|
|||
|
Urban |
(64) 84.2 |
(81) 97.2 |
(145) 89.0 |
0.06 |
|
Rural |
(12) 15.8 |
(6) 2.8 |
(18) 11.0 |
|
Table 3 Distribution of patients and controls according to smoking behavior
|
Sample |
Smoking behavior |
|
||
|
|
Smoker (no.) % |
Nonsmoker (no.) % |
OR |
Sig. |
|
Patients (Pts.) n = 76 |
(12) 15.8 |
(64) 84.2 |
Pts. vs. NR. 0.5 |
0.1 |
|
Relatives (Rel.) n = 36 |
(15) 41.7 |
(21) 58.3 |
Pts. vs. Rel. 0.2 |
0.003 |
|
Nonrelatives (NR.) n = 51 |
(13) 25.5 |
(38) 74.5 |
|
|
|
Total n = 163 |
|
|
|
|
Note: OR = Odds ratio, Chi-square test; Sig = P-value.
Table 4 Frequency of HLA-C allele among patients and control
|
C*06 |
Present (no.) % |
Absent (no.) % |
OR |
Sig |
|
Patients n=76 |
(14) 18.4 |
(62) 81.6 |
Pts. vs. NR 11.3 |
0.002 |
|
Relatives n=36 |
(3) 8.3 |
(33) 91.7 |
Pts. vs. Rel 2.5 |
0.1 |
|
Non-relatives n=51 |
(1) 1.96 |
(50) 98.03 |
|
|
|
Total n=163 |
|
|
|
|
|
C*07 |
Present (no.)% |
Absent (no.)% |
OR |
Sig |
|
Patients n=76 |
(20) 26.3 |
(56) 73.7 |
Pts. vs. NR 5.7 |
0.002 |
|
Relatives n=36 |
(4) 11.1 |
(32) 88.9 |
Pts. vs. Rel 2.8 |
0.05 |
|
Non relatives n=51 |
(3) 5.9 |
(48) 94.1 |
|
|
|
Total n=163 |
|
|
|
|
|
C*17 |
Present (no.) % |
Absent (no.) % |
OR |
Sig |
|
Patients (Pts.) n=76 |
(24) 31.6 |
(52) 68.4 |
Pts. vs. NR 1.1 |
0.5 |
|
Relatives (Rel.) n=36 |
(14) 38.9 |
(22) 61.1 |
Pts. vs. Rel. 0.7 |
0.3 |
|
Non relatives n=51 |
(15) 29.4 |
(36) 70.6 |
|
|
|
Total n=163 |
|
|
|
|
Note: OR = Odds ratio, Chi-square test; Sig = P-value.
Conclusions
In conclusion, this study showed that C*06 and C*07 alleles were associated with early-onset psoriasis, while the inheritance of C*17 was equal for patients and healthy subjects. There was also a decrease in frequency of psoriasis in rural area, which indicated that the earlier encounter with foreign environment, the better development of the immune system. And finally, avoidance of indoor smoking was crucial for precaution of psoriasis specially when there was a positive family history.
Conflict of Interests
The authors declare that no competing interest exists.
References
Copyright© Manal Mohammed Khadhim, Alaa Irhayyim Ali. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.