Research Article
Effect of Intermittent Monomode Microwave Heating on the Crystallization of Glutathione and Lysozyme on Indium Tin Oxide Films
Brittney Gordon, Aysha Zaakan, Fareeha Syed, Nishone Thompson, Edward Ned Constance, Chinenye Nwawulu, Enock Bonyi, Kadir Aslan *
Morgan State University, Department of Civil Engineering, 1700 East Cold Spring Lane, Baltimore, MD 21251, USA.
* Corresponding author. E-mail: Kadir.Aslan@morgan.edu
Received: Jul. 7, 2018; Accepted: Sep. 3, 2018; Published: Nov. 3, 2018
Citation: Brittney Gordon, Aysha Zaakan, Fareeha Syed, Nishone Thompson, Edward Ned Constance, Chinenye Nwawulu, Enock Bonyi, and Kadir Aslan, Effect of Intermittent Monomode Microwave Heating on the Crystallization of Glutathione and Lysozyme on Indium Tin Oxide Films. Nano Biomed. Eng., 2018, 10(4): 344-354.
DOI: 10.5101/nbe.v10i4.p344-354.
Abstract
Effect of intermittent monomode microwave heating on the crystallization of glutathione (GSH) and lysozyme on indium tin oxide (ITO) films using the metal-assisted and microwave-accelerated evaporative crystallization (MA-MAEC) technique was investigated. Intermittent time intervals of 5, 10, 15, 30, 40, 60, 120, 180, 240 min and 30, 40, 60, 120, 180, 240 min were employed for microwave heating of solutions of GSH (500 mg/mL) and lysozyme (40 mg/mL) using a monomode microwave source at 70 W, respectively. Optical microscopy and ImageJ software were employed to quantify and compare the size and number of GSH and lysozyme crystals grown at different microwave heating time intervals. The rate of crystallization for GSH crystals was found to be the fastest at ~ 7.52 μm/min for the 5 min interval of microwave heating and decreased to 0.57 μm/min as the time interval of microwave heating was increased to 240 min. The rate of crystallization for lysozyme crystals was found to be 0.20 ~ 0.27 μm/min for 30-120 min of microwave heating and decreased to 0.07 μm/min as the time interval of microwave heating was increased to 240 min. Intermittent microwave heating of GSH and lysozyme solutions were found to have a minimal influence on the size and count of the crystals produced. X-ray crystallography studies and Fourier transform infrared (FTIR) spectroscopic analysis of grown crystals confirmed that the duration of microwave heating have no or little effect on the crystal morphology and molecular structure of biomolecules studied.
Keywords: Indium tin oxide; Microwave heating; Monomode microwave; Lysozyme; Glutathione
Introduction
Crystallization of biomolecules is an essential process for the medical, chemical, food, and pharmaceutical fields [1]. Crystallization process affords greater purity and control over the polymorphism of drugs produced [2-4]. Within the food and chemical industry, crystallization plays a role in the preservation, processing, and manufacturing of products such as sugars, food additives and salts [5-7]. Over the past decade, researchers have incorporated materials science, nanoscience, and molecular biotechnology for greater understanding of the process of crystallization [8]. Continued interest in the field of crystallization arises from the need to monitor and regulate crystal size, shape, surface, and form to ensure the quality and purity of the reproduced biomolecules [2, 8]. Current techniques for protein crystallization mainly involve hanging drop vapor diffusion [9, 10], microbatch crystallization [11, 12] and evaporative crystallization methods [2]. X-ray diffraction (XRD) quality crystals are observed after one day with the use of hanging drop vapor diffusion method [9, 13]. However, the vapor diffusion method allows users little control over the experimental conditions following the initiation of trials [11-13]. Other techniques, such as, microbatch crystallization, which are cost effective and time efficient alternative to macrobatch crystallization, allows for greater control of the crystallization conditions. However, the typical duration of crystal growth is averaged at up to 3 weeks [13]. Subsequently, the crystallization process is further dependent upon nucleation kinetics and thermodynamic factors. Of the plethora of established crystallization methods, very few address these factors, while facilitating the appearance of crystals in less than 24 h [13]. In response to the limitations of current methods of crystallization, the Aslan Research Group has previously presented a method, called the metal-assisted and microwave-accelerated evaporative crystallization (MA-MAEC) technique, which promotes the rapid and high-throughput crystallization of amino acids (i.e., alanine, glycine, arginine) [15-17], peptides (i.e., glutathione) [18], and proteins (i.e., lysozyme) [8, 14]. The MA-MAEC technique was proven to increase the growth rate of crystallization in a shortened period while producing crystals that allow for analysis through diffraction screens [14-17]. Previous experiments utilizing the MA-MAEC technique yield fully developed crystals with the use of a conventional microwave system (multi-mode, fixed power at 900 W), industrial microwave (monomode, variable power up to 1200 W), and the iCrystal system (mono-mode, variable power up to 100 W). However, to optimize the technique, a comparative analysis of the efficiency of available microwave technology revealed that the iCrystal system rapidly produced the largest high-quality crystals with uniform size distribution within the shortest period [18]. We note that, in the MA-MAEC technique, microwave heating promotes the generation of a thermal gradient between the crystallization solution and the metal nanostructures on a planar surface. The significant difference in the temperature of the metal nanostructures on a planar surface and the solvent of the crystallization solution promotes the mass transfer of the crystallizing molecules from the warmer solution unto the cooler metal surface, as to generate thermal equilibrium [15]. The assembly of the target biomolecule onto the metal-modified surface initiates the nucleation process and subsequent formation of crystals through controlled evaporation of the solvent [15]. Also, controlled growth of XRD quality crystals of biomolecules was also studied using different types of metal surface additives. One of our recent studies demonstrated that the use of indium tin oxide (ITO) promotes the growth of sizable protein crystals in a significantly shorter time than with previously used nanostructures [8]. Through our previous work to date, we established optimal metal surface additive, type of microwave technology and microwave power level. However, the duration of microwave heating using a solid-state microwave source in the iCrystal system was yet to be studied. In our previous publications using the MA-MAEC technique, we have reported crystal formation following the first 15 min of microwave heating in the iCrystal system. However, the quality of the crystals produced after short intervals of microwave heating was not addressed [8]. In fact, the unique thermodynamic properties of proteins for crystallization raise the question of whether the duration of microwave heating affects the rate of crystal appearance and growth for different biomolecules. Therefore, tackling this issue led us to investigate the crystallization of biomolecules under varying intervals of microwave heating in the iCrystal system and use of the MA-MAEC technique. In this study, we investigated the effect of the time interval of microwave heating by evaluating the crystallization of a small tripeptide (i.e., glutathione (GSH)) and a large globular protein (i.e., lysozyme) with the use of the iCrystal microwave system and ITO films. GSH is a thiol compound abundantly present in animals and acts as an antioxidant in reducing the damaging effects of reactive oxygen species, maintaining redox equilibrium and monitoring oxidative stress within cellular environments [19-21]. The reducing capabilities of GSH are owed to its thiol group found on the cysteine amino acid. Since the cysteine is rarely found in the everyday human diet, it acts as a rate-limiting reagent for the biosynthesis of glutathione, making the in vitro synthesis of glutathione of paramount importance [22]. The most significant role of lysozyme enzymes is catalyzing the hydrolysis of peptidoglycan and other sugar molecules within bacterial cell walls. Lysozyme is also a functional component of cytoplasmic granules in specific phagocytic white blood cells. The immunological characteristics of lysozyme support interest in its crystallization [28]. In addition, lysozyme’s ease of crystallization has made the molecule a crystallization standard and model protein for analyzing the efficiency of crystallization techniques. The present study, thus, aims to investigate how the rate of protein crystallization, the size of crystals produces, and the quantity and quality of GSH and lysozyme crystals are affected by variations in the intervals of applied monomode microwave heating.
Experimental
Materials
Lysozyme and a crystallization reagent (30% w/v polyethylene glycol monomethyl ether 5,000, 1.0 M sodium chloride, and 0.05 M sodium acetate trihydrate pH 4.6) were purchased from Hampton Research (Aliso Viejo, CA, USA). L-glutathione (reduced form) and anhydrous sodium acetate were purchased from and stored as prescribed by Sigma-Aldrich (St. Louis, MO, USA). Poly(methyl methacrylate) (PMMA) disks were purchased from McMaster-Carr (Elmhurst, IL, USA) and 21 well silicone isolators (2.0 mm depth, 4.5 mm diameter) were designed by The Aslan Research Group and manufactured by and purchased from Grace BioLabs (Bend, OR, USA). ITO-coated PET films (height = 0.175 mm, width =300 mm, length = 1 mm, resistivity = <=14 ohm/sq) were purchased from MTI Corporation (Richmond, CA, USA). Deionized water (> 18.0 MΩ•cm resistivity at 25 °C) used in all prepared aqueous solutions were obtained from Millipore Direct Q3 system.
Methods
Preparation of sodium acetate solvent
Sodium acetate anhydrous powder (8.2 g) was dissolved in deionized water to make a 2.0 M sodium acetate solution (pH 4.6). Sodium hydroxide (NaOH) and hydrochloric acid (HCl) were used to achieve the desired pH of the solution. Sodium acetate was chosen as the desired solution for the dissolution of lysozyme based on the specifications given by the vendor. For GSH, of the solvents tested - dimethylformamide (DMF), dimethyl sulfoxide (DMSO), ethanol, water, phosphate buffered saline (PBS) (pH 7.4), sodium acetate (pH 4.6), and sodium phosphate dibasic (pH 9.1): only the sodium acetate solution facilitated the complete dissolution of GSH in under 60 min.
Preparation of lysozyme solution
Lysozyme (60 mg) was dissolved in 1.0 mL of sodium acetate solvent (pH 4.6) in a clean scintillation vial. Crystallization reagent (500 µL) was added to the solution and gently swirled until a colorless, transparent solution was acquired. Optical microscopy was utilized to ensure the solution does not contain any undissolved particles.
Preparation of glutathione (GSH) solution
GSH powder (500 mg) was dissolved in a 1.0 mL of sodium acetate solvent (pH 4.6) at 50 °C in a clean scintillation vial until a colorless and transparent solution is achieved. Optical microscopy was utilized to ensure the solution does not contain any undissolved particles.
Preparation of indium tin oxide (ITO) modified iCrystal plates
ITO dots, 5 mm in diameter, were punched from the ITO-coated PET films. A single dot was aligned and attached to the posterior surface of each of the 21 wells of the silicone isolator. The ITO-modified 21-well silicone isolators were then placed on blank iCrystal plates. Following the addition of the crystallization solution, a transparent polymer cover was placed atop the platform before the commencement of the crystallization experiments.
Crystallization using the iCrystal system
Crystallization experiments were carried out for the tripeptide and protein with 21-well ITO-modified iCrystal plates, using the iCrystal Microwave System. An eight (8) µL of the prepared solutions were pipetted into each well of the modified iCrystal plates, for both sets of experiments. The chosen volume was used to prevent the overflow of the solution from the wells during the experiments. Crystallization was carried out using the iCrystal system, which provided a 70 W power of continuous microwave power for each set of experiments. Crystallization experiments were conducted at different time intervals of heating. Lysosome experiments were conducted at 30, 40, 60, 120, 180 and 240 min time intervals. Similar time intervals were used, with the additional 5, 10, 15, 20 and 25 min time intervals, for the crystallization of the GSH tripeptide.
Characterization of Crystals
Optical images of lysozyme and glutathione crystals within the wells of the modified iCrystal plates were recorded at the experiment’s respective time interval, using a Swift Digital M10L monocular light microscope at 4× magnification. Measurements of crystal size in micrometers were carried out using the Motic Images 2.0 software and recorded and analyzed using the SigmaPlot 12.5 graphing software.
Fourier transform infrared spectroscopy (FTIR) analysis of crystals
The molecular structure of lysozyme and GSH crystals were analyzed using an FTIR spectrometer (Agilent Cary 630 FTIR Technologies). FTIR spectra were obtained under attenuated total reflectance (ATR) mode. GSH and lysozyme crystals were analyzed for significant observable peaks in the spectral range of 4000 to 500/cm, which coincides with previously reported findings.
X-ray diffraction (XRD) analysis of crystals
Powder XRD with Cu-K (alpha) radiation was carried out for GSH crystals with the Rigaku MiniFlex spectrometer (MicroMax 7, Rigaku/MSC, The Woodlands, TX).
Results and Discussion
Fig. 1(a) shows real color images of the iCrystal system microwave cavity (W × H: 265 mm × 50 mm) equipped with a fiber optic temperature sensor and an iCrystal plate. Fig. 1(b) shows the solid-state microwave source (8 GHz, computer-controlled variable power 0 ~ 100 W), which facilitates the transmission of monomode microwaves to the cavity holding the iCrystal plate with interest solution. ITO-modified iCrystal plates were manufactured based on the specified dimensions: 50 mm in diameter and 21 wells (each well 4.5 mm in diameter) (Fig. 1(c)). The combined use of microwave heating and iCrystal plates with 21-well capacity affords for the high throughput crystallization of glutathione and lysozyme in a rapid manner [1, 2]. It is important to note that for efficient protein crystallization, the protein solution volume inside the ITO-modified iCrystal plates was restricted to < 8 µL. Table 1 displays the results for the crystallization of GSH on the ITO-modified iCrystal plates under varying time intervals of 70 W power of monomode microwave heating. To facilitate the fastest means of GSH crystallization, 70 W power of microwave heating was utilized, as seen in the successes of previous experiments [2, 8]. It should be noted that the crystallization time refers to the time each experiment is terminated based on the total evaporation of solution (intervals: 5, 10, 120, 240 min) or the time when an increase in crystal size is no longer observable (intervals: 20, 30, 40, 60, 180 min). GSH was crystallized for the maximum time of eight hours (two intervals of 240 min) or eleven runs (each interval: 20 min). A run is defined as the period of 70 W microwave heating throughout the experiments. The maximum size of the final crystals produced for all time intervals exceeded 400 m, except for the 120 min time interval, where crystals grew to a maximum of 214 µm. Moreover, the maximum size of the crystals produced after intermittent microwave heating of 40 min, 20 min, and 60 min was 1,611, 1,050 and 993 µm, respectively. This is comparatively greater than the crystals grown under 30 min of intermittent microwave heating, which reached a maximum size of 680 µm - approximately one-third the size of crystals grown at the 40 min interval. The rate of growth of GSH crystals was calculated to be highest (7.52 µm/min) at 5 min microwave heating interval and gradually decreased to ~ 0.60 µm/min as the interval of microwave heating was increased to 240 min. Conversely, when the same experimental crystallization conditions in GSH were replicated in terms of microwave heating for lysozyme crystallization with the omission of time intervals below 30 min from the study due to our reported findings in a previous publication, we witnessed a decrease in both the crystal size and number (Table 2). GSH experiments were carried out for a minimum of 300 min (intervals: 30 and 60 min) or a maximum of 480 min (interval: 240 min) and both conditions yielded a maximum size of crystals ~ 85 and ~ 82 µm, respectively. A 120-min interval of microwave heating yielded the longest GSH crystals (i.e., 120 µm) while the shortest crystals with a maximum length of 57 µm were produced in the 60 min interval of microwave heating (Table 2). Therefore, under the same crystallization conditions (i.e., crystallization time = 480 min), the rate of GSH crystal growth was higher (0.57 µm/s) compared to that of lysozyme (0.07 µm/s). We observed the same trend in all other crystallization periods, which infers that size-dependent crystallization of molecules of interest, is possible by intermittent microwave heating. The real-time optical images obtained from light microscopy provided for visual interpretation of the rate of crystal growth. In the event of an unobservable increase in the size of crystals or growth to the point of indistinguishable individual crystals, the experiment was deemed terminable; hence, light microscopy cannot be used to distinguish crystal morphology. In that regard, Figure 2 shows comparative time-dependent optical images of glutathione crystals grown on ITO at 30, 60 and 180 min time intervals, respectively, at 70 W. Images indicate the appearance of crystals over 250 µm in length within the first run of 30 min intervals of applied microwave heating. Similar observations were made after two runs for crystals produced under 60 min intervals of applied heating. Increasing the duration of applied heat to 180 min time interval, showed crystals exceed 250 µm after three runs. However, the crystal length observed after three runs of 180 min time interval of applied heating is visibly a fraction of the sizable crystals observed at 30 min intervals of heating. Glutathione crystals produced (after 240 min of applied heating) at 30 min intervals exceeded 500 µm in length. Additional data for other time-intervals of microwave heating for GSH crystallization are shown in the supplementary information section (S1-S10). These findings show that optical images act as the first indication of glutathione crystals growing at an increased rate for shorter intervals of applied microwave heating. Also, images indicate the retention of known morphological characteristics of glutathione crystals, regardless of the interval of applied heating. Subsequently, these observations show that the MA-MAEC technique is capable of facilitating the growth of glutathione crystals over 400 µm in length, with the retention of the crystal structure. In addition, time-dependent optical images shown in Fig. 3 confirmed that protein crystallization with the use of the MA-MAEC technique, regardless of the interval of heating, retains the known morphology of lysozyme. The appearance of crystals over 100 µm was observed after two runs (for 60 min time interval) and three runs (for 30 and 180 min time intervals) of applied microwave heating. Optical images indicate the similar length of crystals grown after 240 min applied heating for 30 and 120 min. However, the observed size of lysozyme crystals grown for earlier intervals was not visible until after 360 min of applied 180 min of microwave heating. In fact, crystals were visibly larger after 360 min for experiments ran at 120 min intervals than those of 180 min intervals of heating. The same phenomenon of seeing large lysozyme crystals at advanced periods of microwave heating were observed across most of the time-intervals (Supplementary Information, S11-S16). These observations indicate that there is no consistent pattern of lysozyme crystal growth with variation in the interval of applied microwave heating. Subsequently, the sizes of GSH and lysozyme crystals were analyzed whether they followed a particular pattern upon application of 70 W microwave power at different time intervals (Fig. 4). The number of crystals produced for each time interval shows no identifiable pattern of crystal production with time interval. For example in Fig. 4(a), crystals grew to a minimum and a maximum number of 7.67± 6.24 (5 min) and 23.3 ± 2.62 for 5 min and 10 min, respectively. Thus, with only a 5 min increase in the interval of heating, there was an approximately 3-fold increase in the number of crystals produced. Conversely, increasing the interval of heating from 10 min (23.3 ± 2.62) to 20 min (6.00 ± 3.56) resulted in an approximately 4-fold decrease in crystals generated. Further increases in intervals (i.e., 60, 120, 180 and 240 min) showed no significant variations in the number of crystals produced, except the approximate 2-fold decrease in the number of crystals produced under the 120 min interval (17.3 ± 1.70) to the 240 min interval (8.33 ± 1.70) of applied microwave heating. However, large standard deviation bars were witnessed in experiments ran for 30, 40 and 60 min of applied microwave heating, which indicated variability in the number of crystals produced with reproduction of experiments. Also, in Fig. 4(b), the average number of crystals at the final run of each experiment indicated no clear relationship between the number of crystals produced and interval heating. For instance, there is a 6-fold increase in the final number of crystals, when the interval was increased from 40 min (9.33 ± 2.05) to 60 min (54.7 ± 0.94), and ~ 8-fold decrease for the 120 min interval (7.67 ± 0.94) (Table 2). The inconsistency in the relationship between the interval of heating and number of final crystals produced was again highlighted in the over 12-fold increase in the number crystals following the increase in the interval of heating from 180 (3.33 ± 1.67) to 240 min (42.7 ± 6.80). Worth noting is that if the apparent anomalous values for 60 and 240 min intervals of heating were excluded, findings would show a decline in the output with the increase in the intervals of microwave heating. The unpredictability observed with the number of crystals produced for lysozyme and glutathione crystals are indicative that the molecular size of the subject of crystallization does not influence the predictability of crystal output. Subsequently, the duration of microwave heating does not affect the number of crystals produced; a reflection of the MA-MAEC technique, which provides a systematic production of crystals. Therefore, our results prove that the MA-MAEC technique retains its process of crystallization regardless of the duration of applied heating [8]. Furthermore, the number of crystals produced are indicative of one well of a 21 well system. Therefore, assuming that the conditions of heating are the same for all wells, the average number of crystals produced is multiplied by the number of wells. However, the large crystal output values of lysozyme crystals obtained after intermittent 60 (54.7 ± 0.94) and 240 (42.7 ± 6.80) min of heating, maybe as a result of rapid and premature nucleation, which results in generally numerous smaller than average crystals [15]. These findings are subject to further investigation in future studies. To establish whether there was a correlation between microwave heating interval and the size of crystals, GSH and lysozyme solutions were subjected to microwave radiation (power = 70 W) at varied time intervals. Fig. 5(a) shows GSH crystal growth rate curves, where it was evident that crystals grown under 40 min intervals of microwave heating grew the largest of all other time intervals (60, 120, 180 and 240 min intervals) of heating. The average size of GSH crystals grown at intervals exceeding 60 min barely surpassed 400 µm in size, which is approximately twice as less than the average size of GSH crystals grown at 40 min intervals. The slopes of the individual curves representing the average size of crystals grown throughout the progression of the experiment was used to construct a curve depicting the growth rate of glutathione crystals for each time interval, where we observed a steady decline in the growth rate of glutathione crystals with an increase in the duration of heating (Fig. 5(a) Inset and Table 1). Conversely, in Fig. 5(b), lysozyme crystals grew to a maximum of ~ 100 µm, as seen for the 120 min interval of heating. There were no observable trend in the size of crystals and the duration of applied microwave heating for lysozyme crystals. All intervals experienced steady growth in crystals with the progression of the experiment. However, the crystal growth rate for the 60 min interval (0.2560 µm/min) exceeded that of all other intervals (Fig. 5(b), solid green inverted triangles). These observations were further confirmed by the rate of growth curve, which indicated a decline in the rate of lysozyme crystallization with an increase in the interval of microwave heating, excluding the growth rate associated with 60 (0.2560) and 120 min (0.2479) of heating (Fig. 5(b) Inset and Table 2). Moreover, through infrared spectroscopy, we have established that the MA-MAEC technique retains the structural integrity of both GSH and lysozyme crystals. For instance, Fig. 6(a) displays the FTIR peaks for GSH crystals grown at selected time intervals, in which identical characteristic functional group peaks (wavelengths = 3338.6, 3236.1, 2520.3, 1707.5, 1586.4, 1523.0, 1232.2 and 1073.7/cm) specific for GSH crystals were evident in all the considered experimental conditions. Subsequently, the intensity of critical structural indicating peaks indicates that crystals grown on ITO at 120 min intervals experienced the strongest bond vibrational activity (Fig. 6(a), solid blue line spectrum). Likewise, in Fig. 6(b), it is evident that lysozyme crystals produced at 70 W microwave heating and under different time intervals, retained the original molecular structure, where the characteristic peaks for lysozyme FTIR spectrum indicate all functional groups are present in the final crystal product. Therefore, both FTIR spectra for lysozyme and GSH displayed structural identifying peaks (wavelengths = 3297.8, 2883.7, 1650.9, 1534.4, 1338.7 and 1092.2/cm), which validates that the MA-MAEC technique retains the molecular integrity of crystals produced. Again, the duration of applied heating had no significant influence on the molecular structure of the crystal product. Subsequently, the MA-MAEC technique proves to provide sizable, diffraction quality crystals, regardless of the duration of microwave heating. The near identical peaks of the XRD results on GSH crystals grown for selected time intervals displayed in Fig. 7 indicate that variation in the intervals of heating does result in variability in the crystal morphology for GSH crystals. Also, the peaks obtained are reflective of previous data depicting known glutathione crystal morphology. Therefore, FTIR and XRD results confirm the morphology of lysozyme and glutathione crystals. With this, the MA-MAEC technique is shown to produce high-quality crystals regardless of the duration of applied heating for lysozyme crystals.
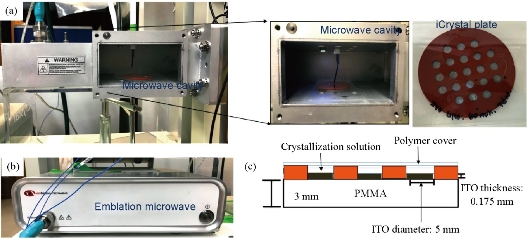
Fig. 1 Real-color photographs of the metal-assisted and microwave-accelerated evaporative crystallization (MA-MAEC) iCrystal system: (a) Monomode microwave cavity with iCrystal plate and fiber optic temperature sensor; (b) Monomode microwave source. And (c) Schematic depiction of the ITO-modified iCrystal Plates with crystallization solution.
Table 1 Summary of results obtained from the crystallization of glutathione from 500 mg/mL solution with the use of indium tin oxide (ITO)-modified iCrystal plates at 70 W of microwave power. Results show at least 3 trials for varied intervals of microwave heating
|
Microwave heating interval a (min) |
Crystallization time b (min) |
Size range, min ~ max (µm) |
Average number of crystals |
Rate of growth (µm/min) |
|
5 |
40 |
98.5 ~ 454 |
7.67 ± 6.24 |
7.52 |
|
10 |
60 |
118 ~ 437 |
23.3 ± 2.62 |
2.90 |
|
20 |
220 |
440 ~ 1,050 |
6.00 ± 3.56 |
2.21 |
|
30 |
240 |
217 ~ 680 |
13.7 ± 5.43 |
2.11 |
|
40 |
240 |
112 ~ 1,611 |
11.3 ± 9.53 |
2.81 |
|
60 |
240 |
177 ~ 993 |
17.3 ± 13.3 |
2.04 |
|
120 |
240 |
64.4 ~ 214 |
17.3 ± 1.70 |
0.54 |
|
180 |
360 |
180 ~ 669 |
10.0 ± 3.74 |
0.67 |
|
240 |
480 |
69.7 ~ 474 |
8.33 ± 1.70 |
0.57 |
Note: a Crystallization interval refers to the time interval of microwave heating applied. b Crystallization time refers to the time of experiment termination.
Table 2 Summary of results obtained from the crystallization of lysozyme from 65 mg/mL solution with the use of indium tin oxide (ITO)-modified iCrystal plates at 70 W of microwave power. Results show at least 3 trials for varied intervals of heating
|
Microwave heating interval a (min) |
Crystallization time b (min) |
Size range, min ~ max (µm) |
Average number of crystals |
Rate of growth (µm/s) |
|
30 |
300 |
29.6 ~ 85.8 |
11.7 ± 5.44 |
0.22 |
|
40 |
320 |
44.1 ~ 88.3 |
9.33 ± 2.05 |
0.19 |
|
60 |
300 |
20.2 ~ 57.0 |
54.7 ± 0.94 |
0.27 |
|
120 |
360 |
62.5 ~ 120 |
7.67 ± 0.94 |
0.25 |
|
180 |
360 |
52.8 ~ 102 |
3.33 ± 1.67 |
0.10 |
|
240 |
480 |
20.8 ~ 82.1 |
42.7 ± 6.80 |
0.07 |
Note: a Crystallization interval refers to the time interval of microwave heating applied. b Crystallization time refers to the time of experiment termination.
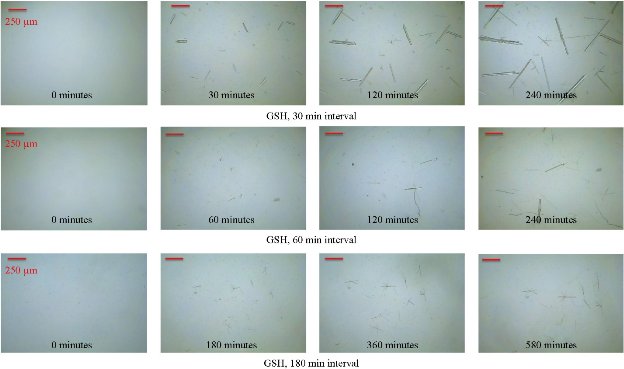
Fig. 2 Time dependent optical images of glutathione crystals grown on indium tin oxide (ITO) using the iCrystal system at 70 W (time intervals = 30, 60 and 180 min).
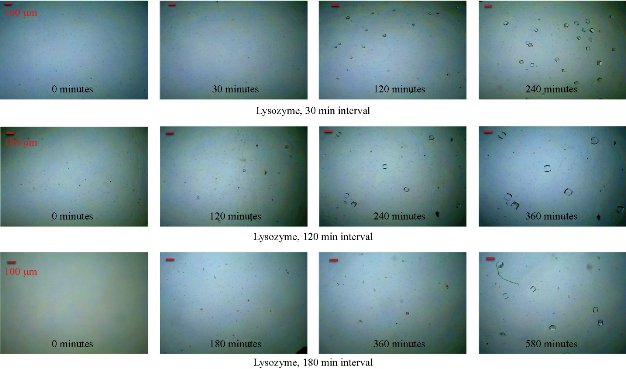
Fig. 3 Time dependent optical images of lysozyme crystals grown on indium tin oxide (ITO) using the iCrystal system at 70 W (time interval = 30, 120 and 180 min).
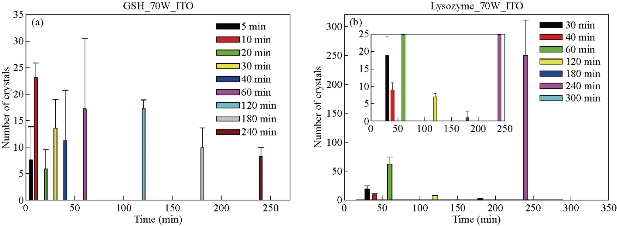
Fig. 4 Bar graphs depicting the average number of crystals produced at 70 W power on indium tin oxide (ITO)-modified iCrystal plates for (a) glutathione and (b) lysozyme. Results show at least 3-trial experimentation for varied intervals of heating.
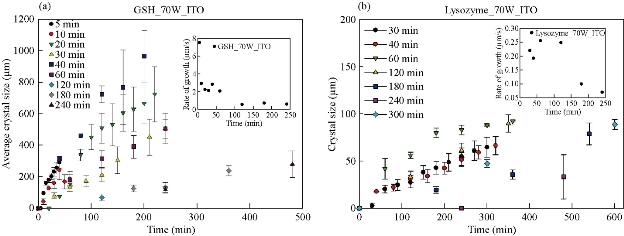
Fig. 5 Crystal size of (a) glutathione (GSH) and (b) lysozyme for different time intervals and their respective rate of growth (Inset).
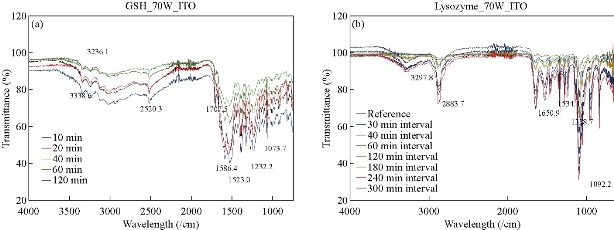
Fig. 6 Fourier-transform infrared spectroscopy (FTIR) spectra for (a) glutathione (GSH) and (b) lysozyme crystals for select time intervals.
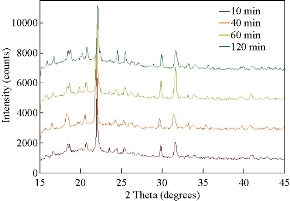
Fig. 7 X-ray diffraction (XRD) results of glutathione (GSH) crystals for select time intervals completed at 70 W of microwave power on indium tin oxide (ITO)-modified iCrystal platforms.
Conclusions
Analysis of crystals for GSH and lysozyme demonstrate that the MA-MAEC technique does not affect crystal morphology, making it a cost-efficient substitute for more time-consuming methods of crystallization. The comparative study on GSH and lysozyme crystallization proved the effectiveness of the MA-MAEC technique, while also highlighting the effect of different intervals of heating on the biomolecular crystallization. Optical images provide visual evidence that crystals with retained morphological structure were produced with the use of metal (i.e., ITO) additives and microwave heating. Crystals were observed for both biomolecules and all intervals of applied heating. Also, the number of crystals produced was shown to be independent of increases in the period of microwave heating. Furthermore, there is a noticeable lack of predictability in the quantity of crystal output. Conversely, the duration of microwave heating is seen to affect the rate of crystal growth for the tripeptide. The growth rate of crystals grown at 5 min intervals was calculated to be 7.52 µm/min, followed by a 2.5-fold decrease with a 5-min increase in the interval of 10 min: 2.90 µm/min. The decrease in the rate progressed with the increase in the interval of heating. This finding was not reflective in the crystallization of lysozyme, demonstrating that the duration of microwave heating may not influence lysozyme crystal growth rate. Morphological and molecular structure analysis indicated that the duration of heating does not distort the crystals produced. FTIR and XRD data confirm that the MA-MAEC technique is a cost-effective technique which produces quality crystals, regardless of time of applied microwave radiation.
Conflicts of Interests
The authors declare no competing interest.
Supporting Information
Additional information not shown in the main text are provided.
References
Copyright© Brittney Gordon, Aysha Zaakan, Fareeha Syed, Nishone Thompson, Edward Ned Constance, Chinenye Nwawulu, Enock Bonyi, and Kadir Aslan. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.