Research Article
Preparation and Antibacterial Activity of New Azo-Schiff Thiazol Ligand and Some of its Metal Complexes
Eateman Salah Mahdi *, Raheem Tahir Mahdi Al-Sa'edi
Department of Chemistry, College of Education for Girls, University of K ufa, An Najaf, Iraq.
* Corresponding author. E-mail: eatemanalmaamorii93@gmail.com
Received: Jul. 7, 2018; Accepted: Jul. 23, 2018; Published: Nov. 19, 2018
Citation: Eateman Salah Mahdi, Raheem Tahir Mahdi Al-Sa'edi, Preparation and Antibacterial Activity of New Azo-Schiff Thiazol Ligand and Some of its Metal Complexes. Nano Biomed. Eng., 2018, 10(4): 369-378.
DOI: 10.5101/nbe.v10i4.p369-378.
Abstract
New chelate compounds, with general formula [M(HL1)2](H2O)2, M = Co(II), Ni(II), Cu(II)] and [M(HL1)2](H2O), M = Zn(II), Cd(II), Hg(II), were prepared by reacting chlorid salt of these metals with the new azo Schiff-base ligand 4-((E)-(3-bromo phenyl)diazenyl)-2-((E)-(thiazol-2-ylimino) methylphenol HL1 using the coupling of diazonium salt of 3-bromoaniline with (E)-2-(thiazol-2-ylimino) methyl phenol. The new azo Schiff thiazol ligand and its chelate complexes were characterized by available spectra and analytical methods such as mass spectra, proton nuclear magnetic resonance (1H-NMR), infrared (IR) spectroscopy, ultraviolet-visible spectroscopy (UV-Vis), elemental analysis (CHN), molar conductivity and magnetic susceptibility measurements. Results showed that the ratio of metal : ligand was 1 : 2, and also showed that Co(II), Ni(II), and Cu(II) chelate complexes had octahedral geometry, while Zn(II), Cd(II) and Hg(II), had tetrahedral geometry. The ligand with its complexes was tested in vintor against the sensitive organisms, including Staphylococcus aureus (gram positive) and Pseudomonas aeruginosa (gram negative). The study showed that the prepared metal complexes had more biological affectivity than the ligands themselves.
Keywords: Azo-Schiff thiazol; Chelate complexes; Biological study
Introduction
A great deal of work has been reported on the synthesis and characterization of many different types of azo Schiff-base ligands in coordination chemistry. Due to the excellent donor properties of the azo and azo methine group [1, 2], these ligands have been reported to show a wide range of varity in biological actions because of azomethine linkage which is responsible for antibacterial, antifungal, herbicidal, clinical and analytical activities [1, 4]. The presence of different atoms as the donor of electrons in the structure of azo Schiff-base ligands, such as O, N and S atoms, gives the ability of coordination between these ligands as lowes base and transition metal as lowes acid, in order to form coordination complexes with different geometrical shapes, depending on the coordination number. On the other hand, the importance of azo compounds such as thiazolyl azo dyes were found in applications such as leather, polymer, paper, paint, textiles and coating industries as a dyeing agent [5]. 1n the present study, novel thiazol Schiff-base ligand (HL1) with its metal complexes was synthesized and characterized by various spectral analysis, their biological activities having also been studied.
Experimental
Materials and instruments
Cobalt(II) chloride hexa hydrate CoCl2.6H2O (99%), glacial acetic acid CH3COOH (99.5%), hydrochloric acid HCl (99 %), m-Toluidine C7H9N (99 %), silver nitrate AgNO3 (99.5%) and Zinc(II) chloride ZnCl2 (99 %) were obtained from B.D.H. 2-aminothiazole C3H4N3S (98%), ammonium hydroxide NH4OH (98%), cadmium(II) chloride anhydrous CdCl2 (99%) and 2-hydroxybenzaldehyde C7H6O2 (96%) were obtained from Fluka. Copper(II) chloride dehydrate CuCl2.2H2O (99%) was obtained from Riedel-deHaën. Ethanol absolute C5H5OH (99.5%) was obtained from GCC. Nickel(II) chloride hexa hydrate NiCl2.6H2O 98% was obtained from Himedia. Mercuric(II) chloride HgCl2 (99%) and sodium nitrite NaNO2 (99%) were obtained from Merck. Staphylococcus aureus and Pseudomonas aeruginosawere were obtained from the Department of Biology, College of Science for Women, Babylon University, which were originally cultured in Teaching Merjan Hospital in Babylon, Iraq. Stuart melting points SMP10 was used to measure the melting point for solid prepared materials, at College of Science for Women, Kufa University. Shimadzu FTIR 8400S spectrophotometer was used to take infrared (IR) spectra, at College of Pharmacy, Kufa University. Shimadzu UV-1700 spectrophotometer was used to measure the ultraviolet (UV)-visible absorption spectra of ligand and complex solutions in ethanol as solvents for solid materials, at College of Science for Women, Kufa University. Shimadzu AA-6300 Atomic Absorption/Flame Emission Spectrophotometer was used to measure the concentration of some metal ions, in the biochemical laboratory, Kufa University. C.H.N. mth EA 99 was used to determine the percentages of carbon ©, hydrogen (H) and nitrogen (N) elements in the prepared ligand and their solid complex compounds at Ahlulbait University, Jordon. Digital Conductivity SeriesIno Lab 720 was used to measure the molar conductivity of prepared chelate complexes in dimethyl sulfoxide in the biochemical laboratory, Kufa University. Balance Magnetic Susceptibility Model-M.S.B Auto was used to measure the magnetic sensitivity of prepared chelate complexes at room temperature, at the Department of Chemistry, College of Education, Al-Mustansiriya University. Proton nuclear magnetic resonance (1HNMR) spectra were recorded with Brucker (300 MHz) using tetrametheylsilane (TMS) as an internal standard, and dimethyl sulfoxide (DMSO) as a solvent at Shahid Beheshti University of Medical Sciences and Health Services, Tehran, Iran.
Preparation of (E)-2-((thiazol-2-ylimino)methyl)phenol (SB)
Schiff base prepared by the condensation reaction of thiazol-2-amine with 2-hydroxybenzaldehyde by dissolving 1g of 0.01 mol thiazol-2-amine in 20 mL of absolute ethanol, and then being mixed with a solution of 1.06 mL of 0.01 mol 2- hydroxyl benzaldehyde dissolved in 20 mL of the same solvent with the addition of one drop of glacial acetic acid, followed by reflux for 6 h [6]. The solution was left to cool then poured over the ice; the appearance of yellow precipitate was observed. It was filtered, dried and recrystallized from hot absolute ethanol to get yellow pure crystals of Schiff base. The yield was found equal to 73% and melting point reached to 72 ℃. Scheme 1 describes preparation of the Schiff base (SB).
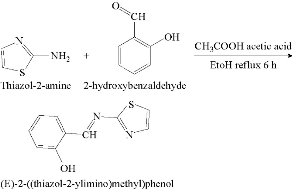
Scheme 1 Syntheses of the Schiff base.
Preparation of azo Schiff-base ligand 4-((E)-(3-bromophenyl)diazenyl)-2-((E)-(thiazol-2-ylimino)-methyl)phenol (HL1)
Azo Schiff-base ligand was prepared according to the following general procedure [7]. 1.08 mL of 0.01 mol 3- Bromoaniline was dissolved in a mixture of 3 mL hydrochloric acid and 20 mL of distilled cold water, and diazotized below 0 ℃ with 0.7 g of 0.01 mol sodium nitrite dissolved in 10 mL of distilled water. Then, the solution was filtered off, and the result was diazonium chloride. The solution was mixed with 2.04 g of 0.01 mol SB dissolved in the mixture consisting of 150 mL ethanol and 100 mL of 10% sodium hydroxide after being left in the refrigerator for 24 h. The mixture was acidified with dilute hydrochloric acid until pH = 6. The precipitate was filtered off and recrystallized twice from hot ethanol and dried. The yield was 80%; the melting point was 89-91 ℃. Table 1 shows physical and analytical data of the ligand and its starting materials, Scheme 2 describes preparation of the azo Schiff-base ligand.
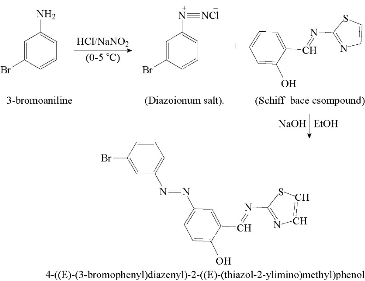
Scheme 2 Syntheses of HL1 ligand
Preparation of metal complexes
The metal complexes (1 mol) were prepared by dissolving 0.774 g 1 mol of the ligand in 50 mL ethanol, added drop by drop whilst stirring, so as to produce a stoichiometric molar ratio of metal : ligand = 1 : 2. The following salts Ni(II), Cu(II), Hg(II), Cd(II) and Zn(II) in 25 mL of absolute ethanol to the solvent and the mixture was heated for 0.5 h, and then left to cool down. A precipitate was observed, filtered off, dried and reconstituted from absolute ethanol to obtain the complex in its pure form.
Antibacterial activity
The invertor biological screening effects of the investing compound were tested against the bacteria Staphyllo coccous aureus and Pseudomonas aeruginosa by the well-diffusion method using Muller-Hinton agar as medium [8]. Wells of 6 mm in diameter were made in agar plates by using sterile cork borer; then the agar surface was inoculated with each bacterium. The tested compounds were dissolved in dimethylformamide (DMF) to obtain a solution with the concentration of 100 ppm. The plates were incubated at 37 ℃ overnight; the zones of inhibition formed were measured in millimetres. Each experiment was performed in triplicate and average of the three ages of the three determinations was recorded.
Results and Discussion
Results of the elemental analyses of the azo Schiff thiazol ligand and its metal chelates were in good agreement with the theoretical expectations. The elemental analyses of coordination compounds show that the ratio of metal : ligand = 1 : 2 existed in all the metal complexes. The molecular conductivity results confirmed for metal chelates in ethanol at 25 ℃ were in the range of 11.75-21.31 S m2 mol-1, indicating non-electrolyte properties in solution, Table 3 shows the measurements of the molar conductivity of complexes.
All the complexes and ligand HL1 were insoluble in water, but highly soluble in DMF and DMSO. The reaction of the heterocyclic azo Schiff thiazol ligand HL1 with metal ions, which was mentioned earlier presented crystals of different colors, depending on nature of the metal ion. Some physical and analytical data are given in Table 1.
Table 1 Physical and analytical data of the ligand and its complexes
|
No. |
Chemical formula |
M. wt |
MP (℃) |
Color |
Yield (%) |
% Found (Calc.) |
|||
|
C |
H |
N |
M |
||||||
|
1 |
[C16H11N4OSBr] = HL1 |
387 |
89-91 |
Brown |
80 |
49.38 (49.61) |
2.77 (2.84) |
14.62 (14.47) |
-- |
|
2 |
[(CoC16H10N4OSBr)₂](H₂O)₂ |
886.47 |
124 |
Umber |
86 |
44.08 (44.29) |
2.59 (2.77) |
12.17 (12.92) |
6.65 (6.80) |
|
3 |
[Ni(C16H10N4OSBr )2](H₂O)₂ |
866.69 |
104 |
Dark orange |
76 |
44.11 (44.31) |
2.68 (2.77) |
12.75 (12.92) |
6.89 (6.78) |
|
4 |
[Cu(C16H10N4OSBr )2](H₂O)₂ |
871.55 |
116 |
Yellow |
90 |
44.28 (44.06) |
2.69 (2.75) |
12.68 (12.85) |
7.45 (7.45) |
|
5 |
[Zn (C16H10N4OSBr )2](H₂O) |
885.39 |
155-160 |
Reddish brown |
92 |
44.62 (44.89) |
2.49 (2.57) |
12.89 (13.09) |
7.78 (7.65) |
|
6 |
[Cd(C16H10N4OSBr)2](H₂O) |
902.4 |
145-148 |
Red |
70 |
42.31 (42.55) |
2.31 (2.44) |
12.26 (12.41) |
12.71 (12.46) |
|
7 |
[Hg(C16H10N4OSBr)2](H₂O) |
990.5 |
112 |
Brown |
78 |
38.79 (38.67) |
2.09 (2.22) |
11.39 (11.28) |
--(20.21) |
Mass spectra
The mass spectra of synthesized azo Schiff thiazol ligand (HL1) were recorded at room temperature. The obtained molecular ion peaks confirmed the propsed formulae for the synthesized compounds. The mass spectrum of the ligand showed the molecular ion peak at mass-to-charge ratio (m/z) 387 +4H (7%) for compound C16H11N4OSBr confirmed the proposed formula for the synthesized product. Also, the spectrum exhibited the strongest base peak m/z 157 (37%), representing the stable species C6H5Br. Moreover, the spectrum exhibited the fragments at m/z: 170 +H, 85 -2H, 188 -H, 68, 161 +4H, and 217 -2H, corresponding to the molecular formula of (C6H4NBr)+, (C3H3NS), (C10H8N2S), (C5H8), (C9H7NS)+, (C10H7N3OS), respectively, as shown in Fig. 1 and 2.
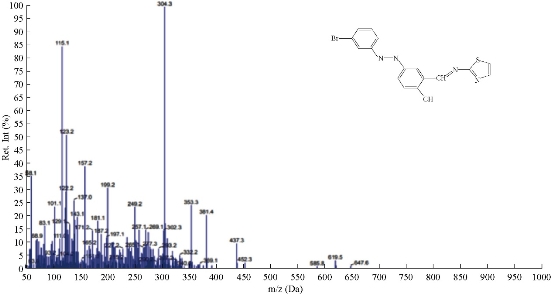
Fig. 1 The mass spectrum of ligand HL1.
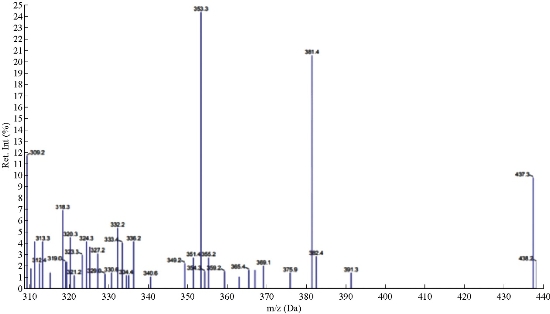
Fig. 2 Mass spectrum of ligand HL1.
The 1H-NMR spectrum of the azo Schiff thiazol ligand HL1 is shown in Fig. 3. The multiple peaks observed in the range of 7.19-8.80 ppm in 1HNMR spectrum of HL1 were attributed to the aromatic protons, while the proton of the azo-methine group produced a signal that interleaved the range; it appeared as a single signal at the value of 9.1 ppm [9]. Proton of the phenolic hydroxyl group of the salicialdehyde ring appeared as a single signal at 10.3 [10]. The absence of this peak, noted in the complexes, indicated the loss of the –OH proton due to the complex ion. There was no appreciable change in all other signals in this complexes as shown in Fig. 4.
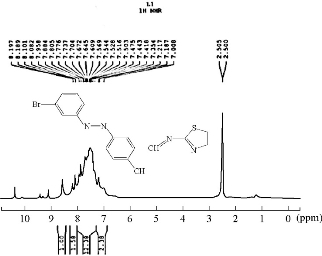
Fig. 3 1H-NMR spectrum of ligand HL1.
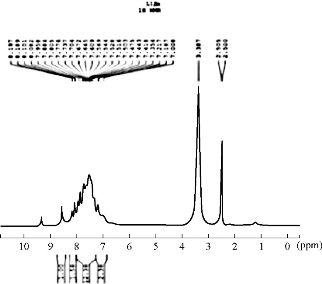
Fig. 4 1H-NMR spectrum of complex [Zn(HL1)2](H2O).
Infrared (IR) spectroscopy
The IR spectra of azo Schiff thiazol ligand (HL1) and its metal complexes were studied by using potassium bromide (KBr) disc in the range of 4000-400 cm-1. The shifts in positions or changes in the shape of metal complex bands compared with those absorption bands due to azo Schiff thiazol ligand suggested the probable modes of bonding in the metal complexes. The IR spectra of the free azo Schiff thiazol ligand (HL1) and its metal complexes with Co(III), Ni(II), Cu(II), Zn((II) , Cd(II) and Hg(II) are given in Table 2. The IR spectrum of the ligand and copper(II) complex is given in Fig. 5 and 6, respectively. The observed band at 1666 cm–1 was due to ν(C=N) group of the ligand [11]. These bands shifted to lower region during the complex formation, indicating its participation in coordination with the metal ions. The spectra of the metal complexes showed the hydroxyl group with a location, shape and intensity different from the free ligand spectrum, which was due to the association of υ(O-H) with the metal ions after the loss of proton at the formation of the complex. The sharp band at 678 cm-1 was due to ν(C-Br) [12]. In the metal complexes, the aromatic C-H stretching bands were observed between 3063 and 3061 cm-1 [13]. The IR spectra of the complex showed a weak beam to medium intensity and at frequencies of 3600-3200 cm-1, due to the asymmetric and asymmetric vibrations of the H-O-H which indicated the presence of water molecules [14]. The vibration band of the azo group both in the ligand and in the complexes was seen in the range of 1469-1463 cm-1 [15]. The constant value of its vibration both in the ligand and in the complexes indicated that there was no coordination between the azo group and the metal ions. However, there were two new bands which were not seen in the spectrum of the ligand, appearing also in the spectra of the complexes. The first one was the M-O stretching bands ranging in 513-524 cm-1, which indicated that the ligand was coordinated with the metals through the phenolic hydroxyls. And the second weak band was M-N vibrations seen in 428-44 cm-1 [16].
Table 2 IR frequencies in cm–1 of azo-Oxime ligands and their complexes
|
No. |
Compound |
υ(O-H) water |
υ(O-H) phenol |
(C-H) ar |
(C-H) alf |
υ(C=N) schiff |
υ(C=N) thiazol |
υ(N=N) |
υ(C-Br) |
υ(M-O) |
υ(M-N) |
|
1 |
HL1 |
-- |
3423 |
3059 |
2831 |
1666 |
1591 |
1469 |
677 |
-- |
-- |
|
2 |
[Co(HL1)2](H2O)2 |
3415 |
-- |
3063 |
2927 |
1627 |
1585 |
1462 |
678 |
524 |
449 |
|
3 |
[Ni(HL1)2](H2O)2 |
3387 |
-- |
3062 |
2927 |
1622 |
1583 |
1465 |
677 |
516 |
445 |
|
4 |
[Cu(HL1)2](H2O)2 |
3414 |
-- |
3061 |
2931 |
1651 |
1589 |
1465 |
678 |
520 |
451 |
|
5 |
[Zn(HL1)2]H2O |
3439 |
-- |
3061 |
2937 |
1651 |
1583 |
1463 |
677 |
514 |
447 |
|
6 |
[Cd(HL1)2]H2O |
3437 |
-- |
3061 |
2931 |
1654 |
1581 |
1465 |
678 |
513 |
428 |
|
7 |
[Hg(HL1)2]H2O |
3437 |
-- |
3062 |
2934 |
1662 |
1585 |
1465 |
675 |
514 |
443 |
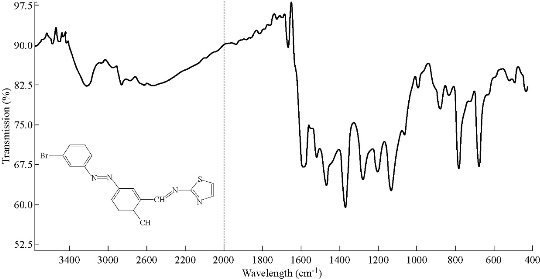
Fig. 5 IR spectroum of ligand HL1.
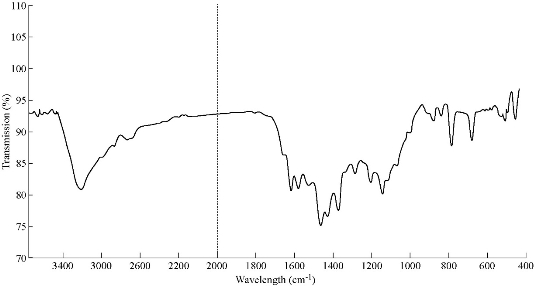
Fig. 6 IR spectroum of [Cu (HL1)2](H2O)2.
Magnetic measurements and electronic spectra
The spectral data and the magnetic moments of each complexes are listed in Table 3.
HL1 ligand
The UV-Vis spectra of azo Schiff thiazol ligand (HL1) and its metal complexes were measured in ethanol at room temperature, and the absorbance maxima are presented in Table 3. The HL1 ligand was characterized mainly by two absorption bands. The bands at 28.248 cm-1 (354 nm) and 37.174 cm-1 (269 nm) were assigned to n→π* and π→π* transitions, respectively in the azo and hydroxyimine groups in the phenyl azo-linked hydroxyimino ligand HL1. The UV-visible spectrum of the HL1 azo Schiff thiazol ligand in ethanol solution is shown in Fig. 7.
Co(II) complex
The magnetic measurement showed that Co(II) complexes exhibited magnetic moment of 4.81 B.M laying within the range of octaheadral high spin cobalt(II) complex [17]. The electronic spectrum of this complex showed an absorption band at 15384 cm-1 (650 nm); the 4T1g→4A2g transition is characteristic of octahedral stereo chemistry [18].
Ni(II) complex
The magnetic measurement showed that Ni(II) complex exhibited magnetic moment of 2.92 B.M laying within the range of octahedral high spin nickel(II) complex [17]. The electronic spectra of this complex showed two spin-allowed transitions at 23148 cm-1 (432 nm) and 13003cm-1 (769 nm) corresponding to 3A2g →3T2g and 3A2g → 3T1g, respectively [19], as shown in Fig. 8.
Cu(II) complex
The value of magnetic moment for this complex was found to be 1.72 B.M due to the presence of one unpaired electron in the compound [20]. The electronic spectra showed a broad absorption band around 11098 cm-1 (901 nm) which was assigned to the transition 2B1g→2B2g. It's reasonable to assign distorted octahedral structure [21].
Zn(II), Cd(II) and Hg(II) complexes
The magnetic susceptibility showed that all these complexes had diamagnetic moments, and the electronic spectra of these complexes did not show any d-d transition band. It's reasonable to assign tetrahedral structure.
Table 3 Spectral data, magnetic moment, conductivities and proposed structure of prepared complexes
|
No. |
Complex |
Assignment |
Absorpiton band (cm-1) |
λmax (nm) |
μeff (B.M) |
M (S m2 mol-1) in DMSO |
Proposed structure |
|
1 |
[Co(HL1)2](H2O)2 |
C.T
4T1g 4A2g |
26385 25510 15384 |
379 392 650 |
2.92 |
13.64 |
Oh |
|
2 |
[Ni(HL1)2](H2O)2 |
C.T 3A2g3T1g 3A2g3T1g |
26954 13003 23148 |
371 769 432 |
4.81 |
12.11 |
Oh |
|
3 |
[Cu(HL1)2](H2O)2 |
C.T
2B1g2A1G |
25773 22624 23474
11098 |
388 442 426
901 |
1.72 |
11.28 |
Oh |
|
4 |
[Zn(HL1)]2H2O |
C. T. |
26385 |
379 |
Dia |
12.91 |
Th |
|
5 |
[Cd(HL1)2]H2O |
C. T. |
34482 |
384 |
Dia |
11.83 |
Th |
|
6 |
[Hg(HL1)2]H2O |
C. T. |
25906 |
386 |
Dia |
14.18 |
Th |
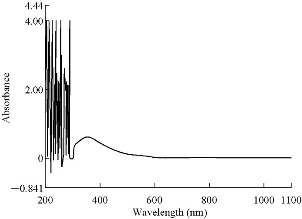
Fig. 7 ultraviolet-visible (UV) spectrum of HL1.
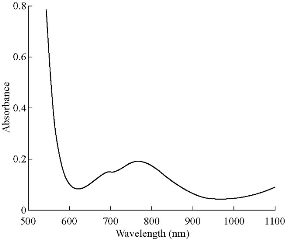
Fig. 8 ultraviolet-visible (UV) spectrum [Ni(HL1)2](H2O)2.
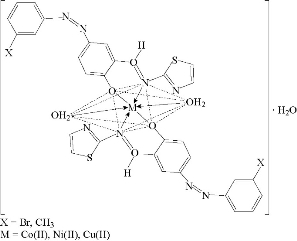
Fig. 9 Suggested geometry of Co(II), Ni(II) and Cu(II) chelate complexes.
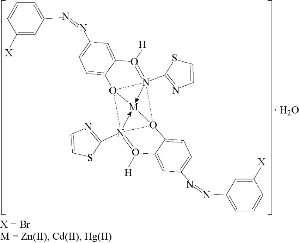
Fig. 10 Suggested geometry of Co(II), Ni(II) and Cu(II) chelate complexes.
Antibacterial activity
The effect of the ligand HL1 and its complexes were tested for invertor growth inhibitory activity against the gram-positive bacterium Staphylococcus aureus and gram-negative Pseudomonas aeruginosa by using the well-diffusion method, All the tested compounds showed a remarkable anti-bacterial activity against the tested bacteria. The result are listed in Table 4 and its statistical presentation is shown in Fig. 11. It was revealed that the metal complexes were more active than the free ligand, and such enhanced activity of metal chelates was due to the lipophilic nature of metal ions in complexes [22]. It was also suggested that the complexes possessed antibacterial activity inhibiting multiplication process of microbes by blocking their active sites [23]. The ligand showed higher effect against all the tested bacteria strains except Pseudomonas aeruginosa, Staphylococcus aureus both of the complexes were shown Co(II), Ni(II), Cu(II), Zn(II), Cd(II), Hg(II). The higher biological activity of metal complexes than the free ligand can be explained on the basis of Overtone's concept and Tweedy's chelation theory [24]. The mechanism of antibacterial drugs can be discussed under four headings: (1) inhibition of cell wall, (2) inhibition of cell membrane function, (3) inhibition of protein synthesis, and (4) inhibition of nucleic acid [25]. Finally, the toxicity of Ni(II), Cu(II), Zn(II), Cd(II) and Hg(II) complexes was found to be better than the ligand.
Table 4 Antibacterial activity data (zone of inhibition in millimetre) of the ligand and its metal complexes at the concentration of 100 ppm
|
Compound |
Bacteria |
|
|
Gram-positive: Staphylococcus aureus |
Gram-negative: Pseudomonas aeruginosa |
|
|
HL₁ |
18 |
20 |
|
[Co(HL₁)₂](H₂O)₂ |
20 |
32 |
|
[Ni(HL₁)₂](H₂O)₂ |
18 |
28 |
|
[Cu(HL₁)₂](H₂O)₂ |
22 |
38 |
|
[Zn(HL₁)₂](H₂O) |
16 |
20 |
|
[Cd(HL₁)₂](H₂O) |
20 |
32 |
|
[Hg(HL₁)₂](H₂O) |
26 |
38 |
Note: Highlyactive, Inhibition zone > 12 mm; Moderate, Inhibition zone = 9-12 mm; Silight, Inhibition zone = 7-8 mm; Inactive, Inhibition zone = 6.
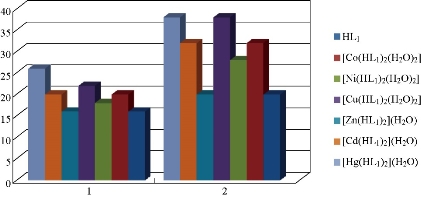
Fig. 11 Statistical representation for anti-bacterial activity of ligand (HL1) and its metal complexes at (100 ppm) conc. Inhibition zone of (a) Staphylococcus aureus and (b) Pseudomonas aeruginosa.
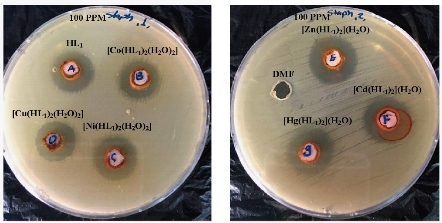
Fig. 12 Effect of ligand HL1 and its metallic complexes on the growth of bacterium Staphylococcus aureus.
Conclusions
In the present study, the preparation and characterization of Co(II), Ni(II), Cu(II), Zn(II), Cd(II) and Hg(II) complexes of 4-((E)-(3-bromo phenyl)diazenyl)-2-((E)-(thiazol-2-ylimino)methyl)phenol (HL1) were carried out and charachterized by mass spectrum, 1H-NMR , IR spectroscopy and UV-Vis studies. Electronic spectral data and magnetic susceptibility measurements supported octahedral geometry of the Co(II), Ni(II) and Cu(II) complexes, while tetrahedral geometry for Zn(II), Cd(II) and Hg(II) complexes. The complexes were found to have higher biological activities as compared to the respective ligand.
Conflict of Interests
The authors declare that no competing interest exists.
References
Copyright© Eateman Salah Mahdi, Raheem Tahir Mahdi Al-Sa'edi. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.