Research Article
Electrochemical Determination of Japanese Encephalitis Virus Antigen Using Silver Nanoparticles Modified Screen-Printed Carbon Electrode
Suk Fun Chin 1, Lih Shan Lim 1, Huat Choi Lai 1, Suh Cem Pang 1, Magdline Sia Henry Sum 2, David Perera 2
1 Faculty of Resource Science and Technology, 2 Institute of Health and Community Medicine, Universiti Malaysia Sarawak, 94300 Kota Samarahan, Sarawak, Malaysia.
* Corresponding author. E-mail: sukfunchin@gmail.com
Received: Sep. 12, 2019; Accepted: Nov. 2, 2019; Published: Nov. 2, 2019
Citation: Suk Fun Chin, Lih Shan Lim, Huat Choi Lai, Suh Cem Pang, Magdline Sia Henry Sum, and David Perera, Electrochemical Determination of Japanese Encephalitis Virus Antigen Using Silver Nanoparticles Modified Screen-Printed Carbon Electrode. Nano Biomed. Eng., 2019, 11(4): 333-339.
DOI: 10.5101/nbe.v11i4.p333-339.
In this study, we aim to fabricate a silver nanoparticles (Ag NPs) based electrochemical biosensor for Japanese Encephalitis virus antigen detection. Ag NPs were deposited onto screen-printed carbon electrode (SPCE) and the electrochemical properties of the Ag NPs modified SPCE were investigated via electrochemical impedance spectroscopy (EIS) and cyclic voltammetry (CV). It was observed that the deposition of Ag NPs onto electrode surface has significantly enhanced the conductivity up to 20.5% than that of bare SPCE. The EIS data indicated limit of detection (LOD) of 2.60 ng/mL (at S/N = 3) towards JEV antigen, with an analysis assay time of 20 min. This presented Ag NPs modified SPCE has demonstrated a promising and rapid alternative to conventional biosensing techniques towards JEV antigen.
Keywords: Silver nanoparticle; Screen printed carbon electrode; Electrochemical impedance spectroscopy; Japanese encephalitis virus antigen
Introduction
Japanese encephalitis virus (JEV) is a type of flaviviruses that is transmitted by Culex tritaeniorhynchus mosquito to human. This disease has an incubation period of two weeks but could cause mortality rate as high as 40%, and nearly 50% chance of permanent neurologic damage after recovery [1]. As such, early and rapid detection of JEV disease is crucial for timely diagnosis and treatment of JEV patients, as well as for effective control of JEV outbreak. JEV detection methods reported in clinical assays include enzyme-linked immunoglobulin assay (ELISA) [2], reverse transcriptase droplet digital PCR (RT-ddPCR) [3], plaque reduction neutralization test (PRNT) [4] and virus isolation [5]. However, these methods have the drawback of being time-consuming, tedious and complicated analyses procedures [6]. The costs of specific instruments and reagents also limit their wide application in clinical laboratories. Therefore, there is a need to develop an alternative diagnostic method which offers low-cost instrumentation, rapid and portable for on-site testing. Biosensors are gaining great attention in clinical diagnosis as they are highly sensitive and have excellent selectivity towards targeted antigen or antibody. Among all types of biosensors, the electrochemical transducer is the most commonly available. An electrochemical biosensor has a wide detection limit, requires an only a small amount of analyte, simple operational procedure and cost-effective. Advance in screen printing technology enables low cost disposable electrochemical biosensor to be manufactured, where three different electrodes were printed on the same substrate [7]. A screen-printed carbon electrode (SPCE) is simple, user-friendly, compact, disposable, cost-effective and capable of mass production. Single usage based SPCE avoids the needs of cleaning process before analysis, unlike glassy carbon electrode and thus cross-contamination from previous analysis can be avoided [8, 9]. Numerous researches and developments in nanotechnology have further enhanced the performance of SPCE. By the incorporation of nanoparticles onto electrode surfaces, the high surface to volume ratio significantly increase the loading of recognition elements on the electrode surface, improve the sensitivity and lower the detection limit of SPCE [10]. Silver nanoparticles (Ag NPs) are selected for the fabrication and modification of SPCE as they can be easily prepared by chemical reduction and are cost effective as compared to gold nanoparticles. Ag NPs exhibited excellent conductivity property and is used to enhance the performance of a glucose biosensor [11]. Our research group has attempted to fabricate an optical JEV biosensor by coating of silver nanoparticles onto glass substrates [12]. There are several electrochemical-based biosensors for the detection of JEV antigen reported in the literature. Li et al. utilized gold coated-magnetite for immobilization of biomolecules and carbon nanotubes for electrochemical signal enhancement. This reported method has the disadvantage of expensive raw materials and electrode regeneration process was needed prior to every detection [13]. As for Huy and the team, they had developed interdigitated electrode for JEV antigen detection. However, the detection limit reported of only 0.75 micrograms per milliliter was insufficient and unsatisfactory for clinical diagnosis [14, 15]. In this work, we report an Ag NPs-modified SPCE by deposition of Ag NPs onto the SPCE working electrode surface. Deposition of Ag NPs onto SPCE was observed to amplify the electrochemical signal of Ag NPs modified SPCE.
Experimental
Materials
Trisodium citrate (Na3C6H5O7), phosphate-buffered saline (PBS, pH ~7.0) consisting of 10 mM monosodium phosphate (NaH2PO4) and disodium phosphate (Na2HPO4) were obtained from Sigma-Aldrich. Bovine serum albumin (BSA), potassium chloride (KCl) and silver nitrate (AgNO3) were obtained from Merck. Potassium ferricyanide {K3[Fe(CN)6]} and potassium ferrocyanide {K4[Fe(CN)6]} were obtained from Bendosen. Screen printed carbon electrodes (SPCE) were purchased from Rapid Genesis Sdn Bhd. JEV E specific monoclonal antibody, MV12/1/C2-2/1 and JEV antigen [16] were provided by the Institute of Health & Community Medicine, Universiti Malaysia Sarawak. All other chemicals were of reagent grade and were used without further purification. Ultrapure water (18.2 MΩ.cm, 25 ºC) was obtained from a Millipore Mili Q-system and used in all synthesis.
Characterization
The morphology and particle size of samples were characterized using scanning electron microscope (SEM) (JEOL JSM 6390LA). Energy dispersive X-ray (EDX) (JOEL 6390LA, Japan) was used to confirm the presence of silver nanoparticles. Cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS) measurements were performed on a Potentiostat (Princeton Applied Research, PARSTAT 2263) using an electrolyte containing 5.0 mM [Fe(CN)6]3/4 and 0.1 M KCl. For CV characterization, the potential was cycled from 1.0 to 1.0 V at a scan rate of 50.0 mVs-1. The EIS measurements were recorded within the frequency range of 0.01 Hz to 100.0 kHz.
Methods
Ag NPs were prepared based on the method reported by Pillai and Kamat [17]. Fig. 1 shows the schematic representation of the fabrication process for Ag NPs-modified SPCE. First, 10 µL of as-prepared Ag NPs suspension was deposited on the working electrode of bare SPCE and air-dried. Followed by the addition of 8 µL of 50 µg/mL of JEV antibodies in PBS for 60 min and rinsed with PBS. Non-specific sites of working electrode were blocked with 1% (w/v) BSA. 5 µL of JEV antigen was then dropped onto the working electrode of SPCE, incubated for 20 min, rinsed and dried at room temperature. For the detection of JEV antigen, JEV antigen with concentrations ranged from 0.1 to 20.0 ng/mL was dropped onto the SPCE working electrode surface, incubated for 20 min at 37 oC, rinsed and air-dried. The interaction of JEV antigen immobilized on the Ag NPs-modified SPCE working electrode surface was studied using CV and EIS, with an electrolyte containing a mixture of 5.0 mM [Fe(CN)6]3/4 and 0.1 M KCl. Uncertainties were estimated based on standard deviation of the samples data.
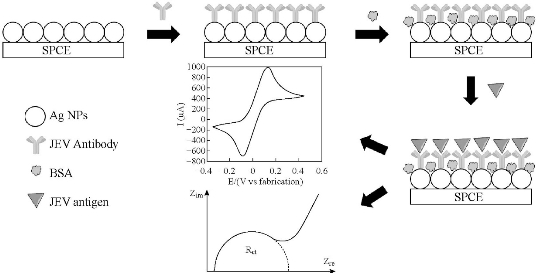
Fig. 1 Schematic representation of the fabrication process for Ag NPs-modified SPCE.
Results and Discussion
Characterization of Ag NPs modified SPCE strip
The surface morphologies of bare and Ag NPs modified SPCE was observed by using SEM and the micrographs were shown in Fig. 2(A) and (B), respectively. The morphology of the working electrode surface of bare SPCE (Fig. 2(A)) revealed roughed and crumpled structure. Deposited Ag NPs were of spherical in shape with a size range of 50 – 90 nm and some Ag NPs clusters of 135 – 147 µm were observed on the surface (Fig. 2(B)) of SPCE. The presence of Ag NPs on the electrode surface was also supported by the EDX spectrum in Fig. 2(C). There were two peaks of 59.24% carbon and 40.76% silver elements detected. As for the high peak of aluminum content, it was from the aluminum plate substrate which was used as the sample holder. CV with [Fe(CN)6]3/4 redox pair is informative in characterizing the electrochemical property of the electrode surface. Therefore, the electrochemical responses of different preparation phases were evaluated as shown in Fig. 3(A) and (B). Compared to bare SPCE (Fig. 3(i)), Ag NPs modified SPCE (Fig. 3(ii)) exhibited increment of cathodic peak current up to 20.5%, implying enhancement of electron transfer rates by Ag NPs deposited on the SPCE electrode surface. Ag NPs possessed excellent conductivity and can provide a significant effective surface area to the electrode surface [18]. From Fig. 3(iii), upon immobilization of JEV antibody onto Ag NPs, there was a decrease in the cathodic peak current, which is the evidence for successful immobilization where non-conductive antibody hindered [Fe(CN)6]3-/4- from reaching the electrode surface, led to decrease in conductivity. Further reduction of the cathodic peak current was observed after the introduction of BSA and antigen to the electrode surface (Fig. 3(iv) and (v)). The results were in concurrence with the one reported in the literature [19]. The resistance of the electrode surface was evaluated via the EIS technique. The real (Z’) and imaginary impedance (Z’’) are the components of the complex impedance (Z). Randle’s circuit (inset of Fig. 4(A)) was implemented to simulate the impedimetric results obtained (Fig. 4(A)). Regarding the Randle’s circuit, Rs is the resistance of electrolyte, Cdl is the constant phase element, ZW is the Warburg impedance due to diffusion of ions to the electrode surface and Rct is the charge transfer resistance related to the dielectric and insulating characteristics between the electrode surface and electrolyte [20]. The impedance plot of Ag NPs modified SPCE exhibited a much smaller semicircular diameter than bare SPCE (Fig. 4(ii)) due to the faster electron transfer kinetics of [Fe(CN)6]3-/4- on Ag NPs modified SPCE. Fig. 4(B) showed that Rct value of Ag NPs modified SPCE was 853 ± 143 Ω (plot (b)), which was 65.8% lower than the one obtained with bare SPCE (2497 ± 54 Ω) (plot (a)). The semicircular diameter was observed to gradually enlarged with the increase of immobilized JEV antibody (1401 ± 200 Ω), BSA (1843 ± 342 Ω) and JEV antigen (3946 ± 585 Ω) (Fig. 4(A), curve (iii)-(v)). This is because as the insulating layer thickness on the electrode surface increase the electron transfer resistance also increased [21].
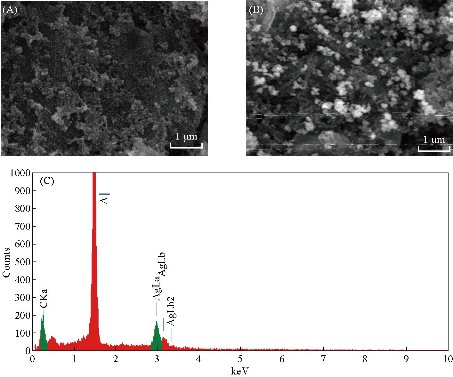
Fig. 2 SEM images of working electrode of (A) bare SPCE, (B) Ag NPs-modified SPCE, and (C) energy dispersive X-ray (EDX) spectrum of working electrode surface of Ag NPs-modified SPCE.
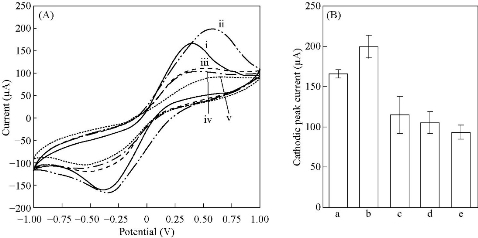
Fig. 3 (A) Cyclic voltammograms (CV) and (B) histogram of cathodic peak current of CV for different preparation phases of Ag NPs-modified SPCE in 5.0 mM [Fe(CN)6]3/4 and 0.1 M KCl; (i) bare SPCE, (ii) Ag NPs-modified SPCE, (iii) JEV antibodies immobilized AgNPs-modified SPCE, (iv) BSA blocked SPCE and (v) JEV antigens immobilized AgNPs-modified SPCE. (Error bars were calculated from the mean value, s/n = 3).
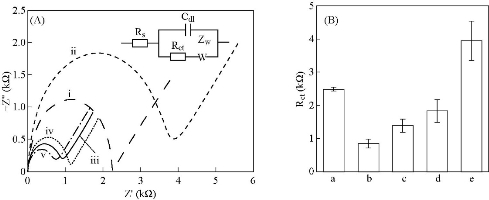
Fig. 4 (A) Nyquist plots of impedance spectra and (B) histogram of Rct of Ag NPs-modified SPCE at different preparation phases in 5.0 mM [Fe(CN)6]3/4 and 0.1 M KCl; (i) bare SPCE, (ii) Ag NPs-modified SPCE, (iii) JEV antibody immobilized SPCE, (iv) BSA blocked SPCE, (v) JEV antigen immobilized SPCE. (Inset of Fig. 4(A) Randle’s circuit). (Error bars were calculated from the mean value, s/n = 3).
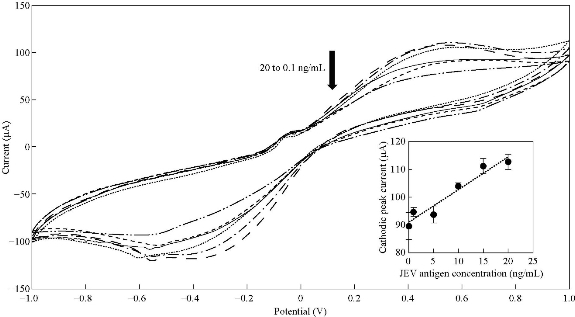
Fig. 5 CV spectra of Ag NPs-modified SPCE at different JEV antigens concentration; 0.1 ng/mL, 1.0 ng/mL, 5.0 ng/mL, 10.0 ng/mL and 20.0 ng/mL in 5.0 mM [Fe(CN)6]3/4 and 0.1 M KCl. (Inset: Relationship between cathodic peak current and JEV antigen concentration). (Error bars were calculated from the mean value, s/n = 3).
Electrochemical response of JEV antigen on Ag NPs modified SPCE
Fig. 5 shows the CV spectra of optimized Ag NPs modified SPCE incubated with different JEV antigen concentration. The cathodic peak currents were observed to decrease with an increase concentration of JEV antigen. A linear correlation between the cathodic peak current of CV and JEV antigen concentration (ng/mL) was obtained (inset of Fig. 5) based on Equation (1):
![]() , (1)
, (1)
where CJEV is the concentration of JEV antigen in ng/mL. The value of the regression coefficient (R2) and standard deviation (σ) were 0.9376 and 2.6993, respectively. Higher standard deviation values were obtained and could be due to higher concentrations of JEV antigen had resulted in turbidity of solution. From Equation (1), the detection sensitivity of Ag NPs modified SPCE was 1.1781 µA.mL/ng. Limit of detection (LOD) of Ag NPs modified SPCE which was defined as 3σ/sensitivity was 6.87 ng/mL. Nyquist plots in Fig. 6 shows that with the increase of JEV antigen concentration immobilized onto the working electrode surface, thicker insulating layers were formed and resulted in Rct increment. The relative resistance was calculated using Equation (2):
![]() , (2)
, (2)
where Rct (i) is the value of Rct after the binding of JEV antigen to JEV antibodies and Rct (0) is the value of Rct after the immobilization of JEV antibodies and BSA onto Ag NPs-deposited SPCE working electrode surface. Inset of Fig. 6 exhibited the correlation of relative resistance and JEV antigen concentration, which is represented by Equation (3). The value of R2 and σ were 0.9906 and 49.0341, respectively. The detection sensitivity of Ag NPs-modified SPCE was 56.789 mL/ng and LOD calculated was 2.60 ng/mL.
![]() . (3)
. (3)
EIS technique has demonstrated better reliability in quantifying the interaction of different amount of JEV antigen and the electrode surface. Referred to Equation (2) and (3), the sensitivity of Rct generated from EIS (56.789 mL/ng) is higher than the sensitivity of cathodic peak currents from CV (1.1781 µA.mL/ng) as EIS has higher sensitivity towards the changes of resistance of electrode surface. As only small amplitude perturbation applied, EIS test minimized the destructive of the electrode surface, which can cause inaccurate result generated [20]. Apart from that, LOD obtained from EIS (Equation 3) is 62.30% lower than LOD obtained from CV (Equation 2) Hence, EIS technique is recommended as the transducer method for Ag NPs-modified SPCE for the detection of JEV antigens.
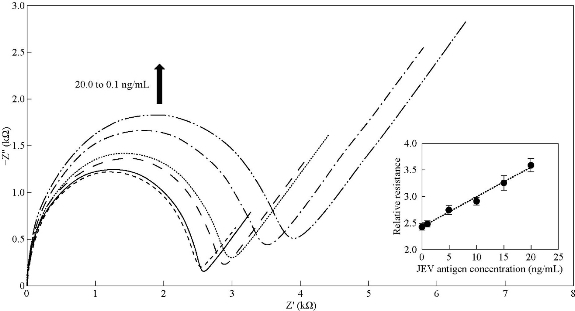
Fig. 6 Nyquist plots of Ag NPs-modified SPCE at different JEV antigens concentration; 0.1 ng/mL, 1.0 ng/mL, 5.0 ng/mL, 10.0 ng/mL and 20.0 ng/mL in 5.0 mM [Fe(CN)6]3/4 and 0.1 M KCl. (Inset: Relationship between relative resistance and JEV antigen concentration). (Error bars were calculated from the mean value, s/n = 3).
Conclusions
A disposable biosensor strip was successfully fabricated by deposition of Ag NPs onto SPCE. CV studies showed that the incorporation of Ag NPs has significantly enhanced the conductivity of SPCE by facilitating the electron transfer kinetic on the electrode surface. Under optimum conditions, namely 20 minutes of incubation time and 50 µg/mL JEV antibody was used, Ag NPs-modified SPCE biosensor strips exhibited LOD of 2.60 ng/mL to JEV antigen, with an analysis assay time of 20 min. The LOD of 2.60 ng/mL to JEV antigen of this work is much lower as compared to the Ag NPs sensing probe with LOD of 12.8 ng/mL reported previously [12].
Acknowledgements
Financial support by the Ministry of Education (MOE) through the Exploratory Research Grant Scheme (ERGS), Grant no. ERGS / STG05 (01) / 1005/2013 (02), F07 / (DPP01) / 1121 / 2014 and graduate scholarships from MyBrain15 (MyMaster) Programme, Universiti Malaysia Sarawak Zamalah, MyBrain15 (MyPhD) Programme are gratefully acknowledged.
Conflict of Interests
The authors declare that no competing interest exists.
References
Copyright© Suk Fun Chin, Lih Shan Lim, Huat Choi Lai, Suh Cem Pang, Magdline Sia Henry Sum, and David Perera. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.