Research Article
Design of Curcumin-Loaded Electrospun Polyhydroxybutyrate Mat as a Wound Healing Material
Reyhaneh Ghavami L 1, Esmaeil Biazar 1*, Arezoo Sodagar Taleghani 2, Saeed Heidari Keshel 3
1 Department of Biomaterials Engineering, Tonekabon Branch, Islamic Azad University, Tonekabon, Iran.
2 Department of Chemical Engineering, Science and Research Branch, Islamic Azad University, Tehran, Iran.
3 Department of Tissue Engineering and Applied Cell Sciences, School of Advanced Technologies in Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
* Corresponding author. E-mail: kia_esm@yahoo,com Tel.: +98 1924271105 Fax: +98 1924274415
Received: Oct. 16, 2019; Accepted: Feb. 7, 2020; Published: Feb. 13, 2020
Citation: Reyhaneh Ghavami L, Esmaeil Biazar, Arezoo Sodagar Taleghani, and Saeed Heidari Keshel, Design of Curcumin-Loaded Electrospun Polyhydroxybutyrate Mat as a Wound Healing Material. Nano Biomed. Eng., 2020, 12(1): 14-20.
DOI: 10.5101/nbe.v12i1.p14-20.
Abstract
Nanotechnology and tissue engineering have accelerated wound healing. Polyhydroxyalkanoates, with suitable physical, biological and mechanical properties, can be considered as a good candidate in tissue repair and regeneration. In this study, nanofibrous mats of polyhydroxybutyrate (PHB) containing curcumin as a wound healing agent, were designed by electrospinning method. The samples were evaluated by microscopic and mechanical analyses, cell assays and microbial tests. The results of microscopic images showed that the diameter of fibers increased with the increase in the curcumin concentration. The elongation and elasticity modulus of nanofibers increased and decreased respectively, with the increase in the amount of curcumin. Drug release study indicated that increasing the curcumin concentration into nanofibers accelerated rate of drug release. Cytotoxicity results of nanofibrous samples with lower curcumin showed better biocompatibility. The strongest antibacterial activity was shown by the sample with 3% curcumin. In addition, Curcumin-loaded nanofibrous PHB can be potential candidates for wound healing.
Keywords: Controlled release; Curcumin; Cytotoxicity study; Physical and mechanical properties; Polyhydroxybutyrate (PHB) nanofiber
Introduction
Tissue engineering is a multi-disciplinary field that uses the principles of biology as well as engineering to develop biological alternatives by repairing, rebuilding, and enhancing or maintaining tissues operations [1, 2]. Creation of a three-dimensional scaffold which has suitable properties such as high porosity, interconnected pores, and high degradation rate is one of the key factors of tissue engineering. Biodegradable polymeric scaffolds can typically be fabricated by different methods [3, 4]. In natural tissues, extracellular matrixes which have physical structures ranging from nanometer to micrometer scale. For this reason, a nanostructured porous material with a high surface area is as an alternative for destroyed extracellular matrixes (ECMs). Many researchers have attempted to fabricate nanofibrous structures [5] like electrospinning method [6-18] to mimic the natural ECM scaffold. A wide variety of natural and synthetic biomaterials, such as glycosaminoglycans (GAGs), collagen, hyaluronic acid (HA), fibrin, polycaprolactone (PCL) and polyglactin have been used to fabricate scaffolds for wound healing [19-22]. Polyhydroxyalkanoates (PHA) are a class of aliphatic polyesters, produced by bacteria during unbalanced growth conditions. PHAs are attractive biomaterials for tissue engineering applications due to their excellent biocompatibility and biodegradability properties [15, 23-29]. Curcumin (Cur) is the main active compound of turmeric, which is acquired from the rhizomes of the herb (Curcuma longa L.) and has been used for centuries as a natural dye in the food as well as traditional medicine, particularly in South and Southeast Asia [30]. Curcumin has antitumor, anti-inflammatory, and antioxidant properties. Moreover, due to favourable pharmacological properties of curcumin, it has a remarkable potential in biomedical applications such as wound healing, radio-sensitizing, and chemo-sensitizing [31, 32]. However, many studies have reported that the bioavailability and therapeutic efficiency of curcumin is limited due to its poor water solubility. As many studies have suggested, the efficiency of scaffolds in tissue regeneration can be increased by using curcumin as an anti-inflammatory agent and cellular stimulus [33-35]. In this study, polyhydroxybutyrate (PHB) nanofibrous mats containing curcumin with different concentrations were designed using electrospinning technique, and their suitability for wound healing was evaluated by mechanical, morphological, and microbial and cellular tests and drug release study.
Experimental
Poly-[3-hydroxybutyrate] (PHB) and solvents (chloroform, and dimethylformamide (DMF)) for preparing PHB solution were purchased from Sigma-Aldrich (St Louis, MO, USA) and used without further purification. Electrospinning apparatus used in this study prepared from Nanoazma Company (Tehran, Iran). For preparing electrospinning solutions, the PHB polymer and curcumin with different concentrations were dissolved in chloroform and DMF at the ratio of 70 : 30, by stirring for 4 hours (at 60 °C, 1250 rpm). A high-voltage power supply through a wire was used at the tip of a syringe needle. A strong electrical field was created between the polymer solution and a collector. With increasing voltage, once the electric field attained a certain critical value, the repulsive electric force overcame the surface tension of PHB solution and a charged jet of solution was ejected from the tip of the Taylor cone. Ultrafine fibres were formed as the ejected jet fluid was narrowed by increasing the net charge density due to evaporation of the solvent. An electro spun PHB nanofibrous mat was carefully detached from the collector. In order to remove solvent molecules completely, it dried in vacuum for 2 days at room temperature.
The surface morphology and the fiber diameter of electrospun PHB and curcumin-loaded PHB nanofibers were studied using a scanning electron microscope (Cambridge Stereo-scan, S-360, Wetzlar, Germany) (SEM) at various magnifications. Initially, the samples were placed on SEM specimen stubs, and then the surface of them was coated with a thin layer of Au-Pd using the sputtering device under an argon atmosphere to obtain electrically conductive surfaces. The fiber diameter was measured from different locations. The Image J software was used to analyse the average fiber diameter of PHB and curcumin-loaded PHB nanofibers.
Samples (length: 6 cm, and width: 0.3 mm) were analysed by a mechanical testing machine (Santam Co.Ltd, Iran) at a speed of 3 mm/min to investigate the mechanical properties of nanofibrous mats. Tests were conducted at 25 °C and humidity conditions of 50%. All data were expressed as the mean ± SD. The data were subjected to statistical analysis (t-test). Probability values for the significance of differences between values were calculated, and P values of less than 0.05 considered significant.
For the curcumin release study, a calibration curve for PBS was plotted. First, a known amount of curcumin in PBS was added and mixed well. Then, the solution was diluted in different proportions to obtain solutions with certain concentrations of curcumin. The maximum absorption spectrum of curcumin is 426 nm. A piece of nanofibrous samples (1 cm2) containing 1% and 3% curcumin were cut into PBS buffer in a shaker incubator (120 rpm, at 37 °C). At the predetermined time intervals, 2 mL of the solution was removed and replaced immediately with an equivalent volume of fresh medium to keep the volume constant. The amount of curcumin in the sample solution was determined spectrophotometrically using absorbance measurement at 426 nm. The drug release percentage can be obtained using the following equation:
Drug release (%) =Mt/Mn ×100 (1)
where Mt is the amount of drug released at time t, and Mn is the amount of drug loaded in polymer nanoparticles of PHB.
Cell culture
Isolation and culture protocol of the ADSCs was previously described by Kogler et al. [36]. Cell viability and proliferation were analysed with the tetrazolium salt 3-(4,5-dimethylthiazol-2-yl)-2,5diphenyltetrazolium bromide (MTT; Sigma-Aldrich). In summary, 5 × 103 human unrestricted somatic stem cells were seeded on the PHB nanofibrous mats. For analysis, MTT (20 µL, 2.5 mg/mL) was added to each well, and the plates were returned to standard tissue incubator conditions for another 4 hours at 37 °C. The culture media was then removed and the cells were solubilized in 100 mL of dimethyl sulfoxide (DMSO). The absorbance of the solution was recorded at a wavelength of 570 nm using a RAYTO microplate reader. For electron microscopic investigations, the cells (0.5 mL) were seeded into each well of 24-well culture plates at an initial density of 4 × 105 cells per mL covered with nanofibrous mats. The cells suspension was exposed to these substrates at 37 °C in a humidified atmosphere of 95% air / 5% CO2 in an incubator for 48 hours and washed twice with Hank’s Balanced Salt Solution to remove unattached cells. The cultured mats with cells were washed with PBS. They were then fixed with 2.5% glutaraldehyde at 4 °C for 2 hours. The samples were dehydrated by alcohols, and kept with tetraoxide osmium vapors at 4 °C for 2 hours. Then, the samples were kept in desiccator, coated with gold, and investigated by an SEM (Cambridge Stereoscan, S-360).
Anti-bacterial test
Anti-bacterial testing is used for measuring the efficiency of fabricated mats in killing Gram-positive and Gram-negative bacteria. Hence, the anti-bacterial activity of PHB nanofibrous mats was tested against model microbial species, including Staphylococcus aureus (as Gram-positive bacteria) and Escherichia coli (as Gram-negative bacteria), by the following method [37]. First, mats were cut into 1 × 1 cm2 and sterilized by ultraviolet radiation (the sterilization time was 10 min). Next, 5 mL of growth media (moolerhilton) for each bacterial species was taken in sample contained tube. The tubes were then seeded with 1 mL fresh culture of bacterial strains and incubated in a shaking incubator at 37 °C and for 48 hours. The turbidity of the medium was measured at 610 nm wavelength after 48 hours of incubation by a spectrophotometer (DU530; Beckman; Fullerton, CA, USA) and the numbers of survived bacteria were calculated by calibration curve.
Results and Discussion
SEM images of PHB/Cur 1%, and PHB/Cur 3% nanofibers, using different concentrations of curcumin are shown in Figure 1. To fabricate smooth and uniform nanofibers, various factors including curcumin content, polymer concentration, and electrospinning parameters were investigated. The smooth and homologous nanofibers are clearly shown in Fig. 1(a) and (b). PHB nanofibers had an average diameter of 700 nm. However, the average diameter of the PHB/Cur 1%, and PHB/Cur 3% nanofibers increased to 1-2 µm (Fig. 1(c) and (d)), and 2-4 µm, respectively (Fig. 1(e) and (f)) with the addition of curcumin. When curcumin was added to PHB solution, the viscosity of the solution increased which might have caused an increase in fiber diameter. The mechanical properties of unloaded and loaded nanofibers are summarized in Table 1. PHB nanofibrous sample displayed total elastic modulus of 1.89 MPa with an elongation of 23%, and the stress strength of this sample was 0.45 MPa. Mechanical properties of the nanofibrous samples were changed with addition of curcumin in the polymer. The yield stress reduced dramatically by increasing the curcumin percentage from 1 to 3%, however, the percent of elongation increased from 69% to 93%. It was observed a decrease in yield stress of the polymer with the addition of curcumin up to 3%, however, the elongation increased by 3 times the standard sample. Consequently, the addition of curcumin to the polymer has improved flexibility. The release of curcumin with different percentages at specified time intervals is shown in Fig. 2. A burst release for each of the two samples was observed in the first 6 hours, and curcumin was released quickly. High doses of drug release were about 37% and 46% for curcumin 1% and 3%, respectively. Then, drug release rate was reduced, and sustained release of curcumin occurred practically. Almost half of the drug was released in the first 72 hours, although this amount was about 46% and 59% for the curcumin 1% and 3%, respectively. Moreover, drug release was increased with the degradation of PHB nanofiber. The release of curcumin in the first moments was increased with an increase in curcumin concentration in the polymeric nanofiber structure of PHB. Cellular results from Adipose derived stem cells culture on the nanofibrous mats are shown in Fig. 3 and 4. The MTT assay results demonstrated good bioavailability or metabolic activity for samples with lower curcumin in nanofibrous mats (Fig. 3). With the increase of curcumin, cell viability did change significantly. As shown in Fig. 4, scanning electron microscopy images revealed appropriate adhesion of stem cells in contact with nanofibers surfaces. The cell adhesion also decreased with the increase in the amount of curcumin concentration. The anti-bacterial test was used for measuring the efficiency of PHB nanofibrous mats in curcumin release and evaluating their activity to kill microorganisms. Two bacteria of S. Aureus (Gram-positive) and E. coli (Gram-negative) were cultured on the manufactured electrospun mats. After 48 hours, the survived bacteria remaining on the culture were counted. The percentages of reduction of bacteria after 48 hours exposure against different mats are shown in Fig. 5(a) and (b). As can be seen, mats indicated the same bacterial activity against both Gram-negative and Gram-positive bacteria. Moreover, the mats with 3% curcumin revealed better anti-bacterial properties. In this work, PHB polymer was used as a biodegradable and biocompatible material with curcumin which possesses anti-inflammatory properties, anticoagulant, antibacterial, antiseptic, anti-mutation, anti-cancer, and antioxidant effects [38]. The biodegradable PHB polymer with nanofibrous structure was used to improve its release performance. Dimethylformamide and chloroform were used as an electrolytic solution. Many studies have examined the effect of different electrospinning parameters on the morphology of fibers and fiber diameter [37, 39, 40]. The node-free morphology was obtained with the polymer concentration of 15% w/v and adjustment of electrical parameters including 18 kV voltage, 13 cm distance, and the feeding rate of 1 mL/h. According to electron microscope images, the diameter of fiber was increased from 600 to 800 nm to 1-2 micrometers for curcumin 1% and about 4 microns for curcumin 3% with the addition of curcumin to polymeric structure of PHB. These results could be due to increased viscosity of samples with an increase in drug concentration. The overall morphology of the nanofibers was not changed significantly by curcumin composition. But, the fiber diameter of the samples containing drug was increased [41]. The polymeric elongation percentage for curcumin 1% was doubled compared to the unloaded sample, and for the samples containing curcumin 3% was tripled in comparison to the unloaded sample. It might be because of the presence of curcumin as a low molecular weight softener [35]. As shown by release profile of curcumin, 37% and 46% of burst release were identified, for nanofibers containing curcumin 1% and 3% respectively, in the first 6 hours of release. After that, the rate of release was reduced and release of curcumin was sustained. This prolonged release of curcumin would be beneficial to wound regeneration and repair process over an extended period of time. The maximum release of curcumin was observed in the polymeric sample containing curcumin 3%. The large surface area of nanofibers and the high curcumin content are the main reasons for it. Curcumin is a hydrophobic compound and has poor water solubility. According to previous studies, only high amounts of curcumin showed toxic effects (less than 50% of cell survival) [42]. The results showed that cell viability and survival was decreased by increasing the amount of curcumin in nanofibers. Therefore, the unloaded polymeric sample was better than the curcumin-loaded polymeric samples. There were significant changes between the three samples. Moreover, as it can be seen in SEM images, cells containing curcumin 3% on nanofibers showed less adhesion and flattening in comparison to unloaded nanofibers and 1% curcumin. This can be due to increasing of curcumin concentration on the surface [15].
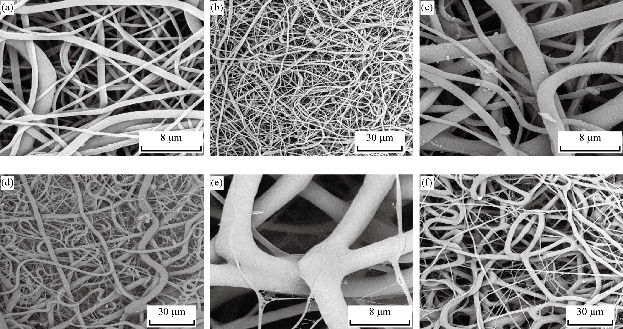
Fig. 1 SEM images of curcumin-loaded nanofibers. (a) and (b) Unloaded PHB nanofibers; (c) and (d) loaded PHB nanofibers with curcumin 1%; and (e) and (f) loaded PHB nanofibers with curcumin 3%. (Magnification: 1000 and 2000 ×)
Table 1 The mechanical properties of loaded and unloaded nanofibers with curcumin
|
Samples |
Elastic modulus (MPa) |
Yield stress (MPa) |
Elongation % |
|
PHB |
1.892 ± 0.121 |
0.445 ± 0.111 |
23.538 ± 0.124 |
|
PHB/Cur 1% |
0.091 ± 0.052 |
0.063 ± 0.005 |
69.846 ± 0.258 |
|
PHB/Cur 3% |
0.006 ± 0.002 |
0.006 ± 0.003 |
93.211 ± 0.263 |
Note: The data are presented as the mean values (p < 0.05).
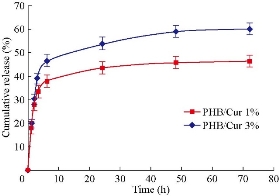
Fig. 2 The release diagram of curcumin with concentrations 1 and 3% at different times.
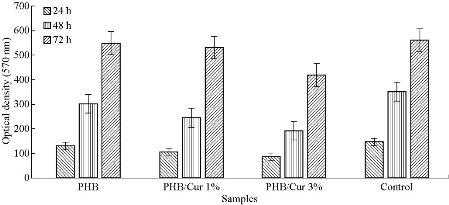
Fig. 3 Results of MTT Assay for the unloaded PHB nanofibers, Loaded PHB nanofibers with curcumin 1%, and 3% , and control (TCPS: Tissue culture polystyrene).
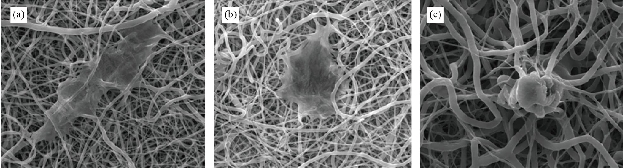
Fig. 4 SEM images of ADSCs adhesion on curcumin-loaded nanofibers. (a) Unloaded PHB nanofibers; (b) loaded PHB nanofibers with curcumin 1%; and (c) loaded PHB nanofibers with curcumin 3%. (Magnification: 2000 ×)
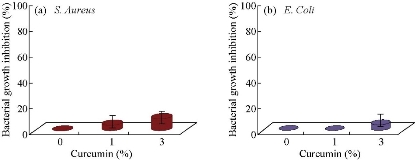
Fig. 5 Anti-bacterial properties for different CU% mats: (a) Staphylococcus Aureus and (b) Escherichia coli.
Conclusions
In summary, PHB nanofibers containing curcumin were designed and fabricated using electrospinning technique and their biological, morphological, and mechanical properties were investigated. Morphological results indicated that with increasing curcumin, fiber diameter increased significantly. The flexibility of the polymers increased significantly according to mechanical results, especially the elongation percentage of nanofibers containing curcumin. Also, in-vitro drug release study showed the appropriate rate of release for curcumin. In addition, samples with lower curcumin exhibited better cytotoxicity and cell adhesion also decreased with increasing curcumin. Due to the good properties of curcumin, this composition has the potential to be used in wound healing.
Conflict of Interests
The authors declare that no competing interest exists.
References
Copyright© Reyhaneh Ghavami L, Esmaeil Biazar, Arezoo Sodagar Taleghani, and Saeed Heidari Keshel,. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.