Research Article
Biosynthesis of Silver Nanoparticles from Streptomyces Spp., Characterization and Evaluating of Its Efficacy against Phomopsis theae and Poria hypolateria in Tea Plants (Camellia sinensis)
Karthik Natesan 1, Ponnusamy Ponmurugan 1*, Balasubramanian Mythili Gnanamangai 2, Mani Suganya 2, Shivasji Kavitha
1 Department of Botany,Bharathiar University, Coimbatore - 641 046, Tamil Nadu, India.
2 Department of Biotechnology, K. S. Rangasamy College of Technology, Tiruchengode –637 215, Tamil Nadu, India.
* Corresponding author. E-mail: drponmurugan@gmail.com
Received: Feb. 17, 2020; Accepted: Jul. 14, 2020; Published: Sep. 23, 2020
Citation: Karthik Natesan, Ponnusamy Ponmurugan, Balasubramanian Mythili Gnanamangai, Mani Suganya, and Shivasji Kavitha, Biosynthesis of Silver Nanoparticles from Streptomyces Spp., Characterization and Evaluating of Its Efficacy against Phomopsis theae and Poria hypolateria in Tea Plants (Camellia sinensis). Nano Biomed. Eng., 2020, 12(3): 272-280.
DOI: 10.5101/nbe.v12i3.p272-280.
The biosynthesis of metal nanoparticles is an emerging area of advanced research in plant protection. In this study, an eco-friendly and rapid protocol was developed where silver nanoparticles were biosynthesized by using Streptomyces spp. The biocontrol agent was isolated from different tea soils and the efficient strain (VASC201) was identified by dual culture and antibiosis. Nanoparticles were successfully synthesized from this efficient strain. The silver nanoparticles exhibited their resonance peak at 448 nm under ultraviolet-visible spectroscopy (UV-Vis). The structural analysis of the silver nanoparticles synthesized by the Streptomyces spp. exhibited a strong crystalline structure. The morphology and chemical composition of the silver nanoparticle were examined by high-resolution transmission electron microscopy (HR-TEM) equipped with energy-dispersive X-ray spectrometer (EDX) and were predominantly spherical in shape and uniformly distributed without significant agglomeration. The average crystalline size of the prepared silver nanoparticle was found to be 51.2 nm. The stretching vibrational peak at 3420, 2920 and 2350 cm-1 in Fourier-transform infrared spectroscopy (FTIR) were attributed to various functional group of metal nanoparticle synthesis. We assessed the effectiveness of silver nanoparticles against Poria hypolateritia and Phomopsis theae. The results showed that the nanoparticles showed very good inhibitory effect of about 69.90% inhibition of Poria at 5 ppm concentration on the 15th day. Phomopsis was inhibited to 67.47% at 5 ppm of silver nanoparticles at the same time interval.
Keywords:Streptomyces; Biosynthesis of nanoparticles; Phomopsis; Poria; Silver nanoparticles
Introduction
Tea is the inexpensive and popular beverage produced from the young shoots of the tea plant [1]. Tea plant (Camellia sinensis (L.) O. Kuntze) is a perennial woody plant that is widespread throughout subtropical areas and tropical areas such as India, China, Sri Lanka and Kenya. In more than 50 countries, tea is being cultivated and it prefers a suitable warm, humid climate conditions with well distributed rainfall and long time sunshine hours [2]. The fresh shoots of tea plant provides a wide variety of nutrition to the human body, including polyphenols, alkaloids, flavonoids, thearubigin, catechins and theanine. Different diseases can be protected by long-term tea consumption; therefore, tea has become the most commonly accepted non-alcoholic beverage in the world for its medicinal properties [3].Tea, is exposed to many pests and diseases and is a monocultural crop; it provides a micro-climatic conditions and a supply of food for pests and diseases. In the tea growing countries Phomopsis theae (P. theae) Petch, a Deuteromycetes fungus which causes Collar canker is one of the major growing concern [4-6]. Collar canker caused by fungus P. theae, is considered as the major problem in tea plants cultivations [7]. Young tea plantations are prevalently affected by Phomopsis and to certain extend in mature tea plantations [8], Infection is sporadic in nature [1]. Among the many diseases reported in tea plants, red root- rot disease which infects roots of the tea plants and causes severe damage to the tea plants, results in death of the bush and leads to capital loss [9, 1]. Red root rot is caused by Poria hypolateritia (P. hypolateritia) [10]. Nanobiotechnology is used in many disciplines due to its environmental benign nature. Nanoparticles have unique chemical and physical properties and the nanoscale (1 - 100 nm) size plays an important role in various fields of pharmaceutical, medicine, and environmental technologies. Now daysnanoparticles are biosynthesized by biological systems like fungi, bacteria, algae, yeast, and plants [11]. The nanoparticles synthesized by physical method yields product with complication of consuming high energy and generation of heat, while the chemically synthesized nanoparticles production causes toxic effects with production of hazardous by-products [12]. Alternatively the biological synthesis of nanoparticles is eco-friendly, economic and appreciable [13]. The Cu NPs and Ag NPs biosynthesized extracellularly from the antagonists were characterized and its biocontrol activities was determined against P. theae which emphasizes and recommends the use of Cu NPs synthesized by indigenous biocontrol agent S. sannanensis for the control of bird's eye spot disease [14]. It has been reported that many kinds of metallic nanoparticles such as copper, silver, and gold show strong antimicrobial activities [15]. Silver nanoparticles exhibit excellent bactericidal properties against microorganisms. In extracellular synthesis of Ag NPs, trapping of metal ions occurs on the outer surface of cells and reduces them in the presence of enzymes [16]. The present report describes the synthesis of silver nanoparticles using Streptomyces. Prevalent actinomycetes were isolated from the rhizosphere soil of tea plants in Valparai, Coorg, Munnar and Vandiperiyar. Efficient strains were screened via antibiosis and dual culture methods against Poria and Phomopsis. In this study, we reported that Streptomyces biomass treated with silver nitrate mediated the synthesis of silver nanoparticles extracellularly, under aerobic conditions. The nanoparticles synthesis was attributed to the secreted proteins by bacteria that converted bulk metal to nanoparticles. The antifungal activity of nanoparticles was tested for fungicidal efficacy against red root-rot and collar canker pathogens.
Experimental
Isolation of indigenous biocontrol agents from tea soil
Serial dilutions of the processed soil samples were plated to isolate the biocontrol agents Streptomyces spp. The procedure was performed for all the tea soils collected from different regions [17]. Based on dual culture and antibiosis method, efficient biocontrol agents were selected.
Tea plant pathogen
The fungus P. hypolateritia causing red root rot and P. theae causing collar cankerdiseases were obtained from MTCC, Chandigarh and used for the present study. They were sub-cultured and maintained in PDA for further experiments.
Dual culture test
Dual culture was performed with slight modification of Dennis and Webster [18]. PDA plates were plated and inoculated with 6mm mycelia discs of P. theae at the centre point of one-half of the plate and the other half was streaked with Streptomyces spp. at the centre. Linear growth of the pathogen was measured in both control and biocontrol agent treated plates. Similarly, the same technique was followed in the case of P. hypolateritia.
Antibiosis test
Antagonist test was performed by following Dennis and Webster [19]. Isolates were grown in their SCN media for the production of toxic metabolites. The sterilized culture filtrate was bioassayed at 10% on PDA against P. theae and P. hypolateritia and the percentage inhibition on linear growth was calculated.
Biological synthesis of silver nanoparticles using Streptomyces spp. obtained from teasoils
Streptomyces spp. isolated and screened as potential antagonist from soil samples were inoculated in SCN broth and incubated at 25 ºC on a shaker (150 rpm) for 72 h. The biomass were filtered using Whatman No. 1 filter paper and the cell free filtrate was separated. The cell free filtrate thus obtained was used in the nanoparticle production. The AgNO3 (1 mM) solution was mixed with cell free filtrate and agitated at 25 ºC in the dark [17, 20].
Characterization of silver nanoparticle
The primary confirmation of silver nanoparticle synthesis was confirmed by the UV-visible spectroscopy in the range of 200 - 900 nm. The size of the particles was measured by using zeta potential analysis. The structural and crystalline phase of the silver nanoparticle was characterized by X-ray diffractometer (Rigaku). Particle size was analyzed by Nanophox. The morphology and chemical composition of the silver nanoparticles were examined by JEOL JEM 2100 high resolution transmission electron microscopy (HR-TEM) equipped with energy-dispersive X-ray spectrometer (EDX). The functional group present in the silver nanoparticles was examined by Fourier-transform infrared spectrometer (Bruker).
Evaluation of efficacy of synthesized nanoparticles against tea plantation pathogens
The antagonistic activity of biocontrol mediated nanoparticles was evaluated against P. theae and P. hypolateritia at different concentrations. The most common method of food poisoned technique [21] was followed under in-vitro condition against the pathogensat different concentrations of silver nanoparticles. Percentage of inhibition was calculated by screening the platesfor the period 15 days in daily interval.
Nanotoxicity analysis of biosynthesized silver nanoparticles in zebrafish embryos
Zebrafish (Danio rerio), weighing between 0.7 - 1.3 gm were maintained in the lab. They were kept into water tank and proper aeration was provided with 12 h light and 12 h dark light cycles. The zebrafish is a hardy fish and can withstand a pH ranging between 7.2 and 7.5. The temperature of the water was maintained at 26 ± 1 °C throughout the study. During the acclimation period, the adult fish was fed twice per day with readily available fish food. The care and husbandry of the zebrafish used in this study were in conformity with the guidelines that regulated the care and use of laboratory animals by humans for research purposes. Total 24 fishes were divided in to four different groups with 6 fishes per group. The three different concentrations of test compound were prepared according to LC 50 values. The concentrations treated were 0.15, 0.30 and 0.45 µg. Group 1 served as a control group. Group 2 - 4 received 0.15, 0.30 and 0.45 µg of test sample respectively. The drug was prepared as extract prior to addition in to respective fish tank. The fishes were exposed to test compounds for a period of 15 days. After the 15th day, the fishes were taken for the respective analysis. Each fish was slowly anesthetized by adding ice chips into the water, until the temperature reached 12 oC. When the fish remained still to any external stimuli, they were taken for dissection. The muscle tissue was removed carefully and stored. The dissected muscle tissue was stored in formalin for histopathology analysis [30].
Statistical analysis
The statistical analysis was performed using SPPS (Version 20). The experiments were carried out in triplicates and the results obtained were expressed as mean ± standard deviation. Histogram of TEM analysis for size distribution using MATLAB® was used [31].
Results and Discussion
For the screening of the best efficient strain shown in Table 1, dual culture described by Sariah [22] was done followed by antibiosis. Overall percentage inhibition of P. hypolateritia and P.theae was superior for strain VASC201 as determined from dual culture and antibiosis (Fig. 1) as compared to the dual culture method done by Rahman et al. [23]. Out of the 76 isolated bacteria, 27 were inhibitory to C. gloeosporioides on PDA. Out of the 27 bacteria, 4 isolates namely B23, B19, B04 and B15 had a significantly (p < 0.05) higher inhibitory effect than the others. The antagonistic activity of biocontrol mediated nanoparticles was evaluated against P. theae and P. hypolateritia at different concentrations. The most common methods like food poisoned technique was followed under in-vitro condition against the pathogens. Various concentrations of silver nanoparticles (1, 3 and 5 ppm) were used against tea pathogens. Antagonistic activity of biocontrol mediated silver nanoparticles was evaluated against P. hypolateritia and P. theae. The results showed that the nanoparticles showed very good inhibitory effect. 69.90% was the highest percentage inhibition found against Poria on the 15th day of incubation by silver NPs at 5 ppm concentration in comparision with Carbendazim which showed 90.12% inhibiton of Poria at the same stage (Table 2). The percentage inhibition of P. theae isolates which was high at initial stage for Ag Nps synthesized culture filtrate was in the range from 59.42 to 79.76%, followed by Cu NPs (43.66 - 76.19%) [14]. Against Phomopsis, 67.47% was the highest percentage inhibition found by silver NPs on the 15th day at 5 ppm (Table 3), as compared with the green synthesized silver nanoparticles which were found highly toxic against pathogenic bacteria and fungi at a concentration of 30 ppm [24].
Table 1 Microbes screened against Poria hypolateritia and Phomopsis theae
|
S. No. |
Microbial group |
Genus |
Strain name |
|
1. |
Actinomycetes |
Streptomyces |
VASC201 |
|
2. |
Streptomyces |
COSC301 |
|
|
3. |
Streptomyces |
VPSC401 |
|
|
4. |
Streptomyces |
CGSC201 |
|
|
5. |
Streptomyces |
CGSC501 |
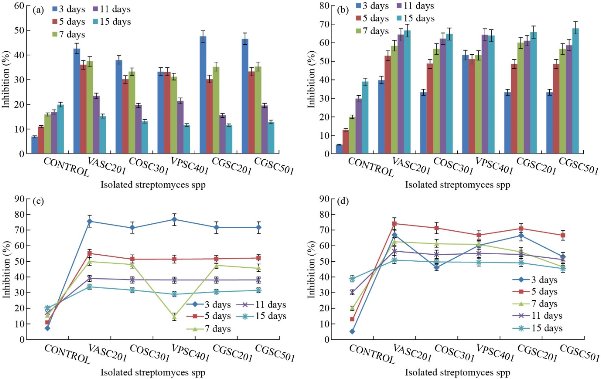
Fig. 1 (a) Percentage inhibition of Poria hypolateritia using indigenous Streptomyces spp. via dual culture. (b) Percentage inhibition of Phomopsis theae using indigenous Streptomyces spp.via dual culture. (c) Percentage inhibition of Poria hypolateritia using indigenous Streptomyces spp. via antibiosis. (d) Percentage inhibition of Phomopsis theae using indigenous Streptomyces spp. via antibiosis.
Table 2 Antagonistic activity of silver nanoparticles against Poria hypolateritia using food poisoned technique
|
Concentration (ppm) |
Day |
Silver nanoparticles |
Carbendazim |
|
1 |
3 |
73.73 ± 0.8 |
87.36 ± 0.5 |
|
9 |
60.30 ± 0.3 |
82.01 ± 0.2 |
|
|
15 |
64.90 ± 0.2 |
79.03 ± 0.4 |
|
|
3 |
3 |
79.30 ± 0.8 |
88.01 ± 0.2 |
|
9 |
62.30 ± 0.5 |
86.05 ± 0.5 |
|
|
15 |
67.00 ± 0.3 |
84.00 ± 0.3 |
|
|
5 |
3 |
84.03 ± 1.3 |
98.23 ± 0.8 |
|
9 |
65.93 ± 0.3 |
96.52 ± 0.3 |
|
|
15 |
69.90 ± 0.2 |
90.12 ± 0.2 |
Table 3 Antagonistic activity of silver nanoparticles against Phomopsis theae using food poisoned technique
|
Concentration (ppm) |
Day |
Silver nanoparticles |
Carbendazim |
|
1 |
3 |
48.53 ± 0.7 |
80.01 ± 0.3 |
|
9 |
56.73 ± 0.3 |
82.0.3 ± 0.2 |
|
|
15 |
61.17 ± 0.1 |
81.02 ± 0.5 |
|
|
3 |
3 |
48.47 ± 0.7 |
90.02 ± 0.2 |
|
9 |
57.80 ± 0.3 |
90.05 ± 0.5 |
|
|
15 |
66.77 ± 0.1 |
91.01 ± 0.2 |
|
|
5 |
3 |
62.27 ± 0.7 |
98.02 ± 0.7 |
|
9 |
60.43 ± 0.3 |
97..01 ± 0.3 |
|
|
15 |
67.47 ± 0.1 |
98.01 ± 0.0 |
UV-visible absorptions study
The UV-visible absorption spectroscopy is one of the most broadly used analytical techniques for the structural characterization of the synthesized NPs [25]. The change in the colour of the silver nitrate solution due to reduction of Ag+ ions was well observed from colourless to brown (Fig. 2(a)). The absorption spectra of silver at 448 nm nanoparticles are shown in Fig. 2(b). The extracellular reduction of silver ions in the aqueous solution of silver nitrate by the Pseudomonas extract was visualized by the color change from yellow to brown [26]. After the addition of cell free supernatant in the aqueous solution of silver ions, the solution started to change in color from yellow to brown in 1 h. The intensity of color increased with increase in incubation time up to 6 h. After the incubation period of 6 h there was no significant change in color of the nanoparticles, indicating the completion of reduction reaction. Nanoparticle synthesis is due to the excitation of free electron in conduction band, leading to in-plane oscillation, known as surface plasmon resonance. It was obviously noted that the prepared silver nanoparticles showed a single, broad peak at 448 nm, which was due to the excitation of surface plasmon vibration. The SPR band appeared in the visible region arecharacteristic of noble metal nanoparticles [27]. In the extracellular synthesis of Ag NPs, trapping of metal ions occurred on the outer surface of cells and reduced them in the presence of enzymes [16].
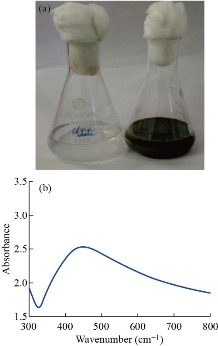
Fig. 2 (a) Colorless silver nitrate solution turned into brown color in Streptomyces extract, (b) UV of silver nanoparticle synthesized from Streptomyces spp.
Functional group analysis by FTIR
Fig. 3 shows the FTIR spectrum of silver nanoparticle synthesized by Streptomyces. The absorption peak at 3420 cm-1 was due to the stretching vibration of –OH molecules. The peak at 2920 and 2350 cm-1 was assigned to the C-H stretching of aromatic compounds and O=C=O stretching respectively. The peaks at 1634 and 1422 cm-1 represented the bending vibration of N-H group which indicated the presence of protein. Earlier studies reported that protein could bind to nanoparticles and therefore provided stabilization of Ag NPs [26]. The peaks at 1342 and 1150 cm-1 represented the C-N stretching of aromatic amine and C-O stretching vibration respectively. The peak at 1010 cm-1 corresponded to C-N stretching vibrationamines. The band at 583 cm-1 could be assigned to C-Br stretching, which was a characteristic features of alkyl halides. The presence of various functional groups revealed the successful bio-reduction of Ag+ and helped the stabilization of silver.
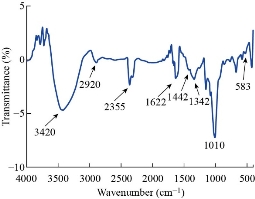
Fig. 3 FTIR of silver nanoparticle synthesized from Streptomyces spp.
Zetapotential
The magnitude of electrostatic charge potential at the electrical double layer surrounding Ag NPs was measured by zeta potential. The zeta potential data specifies the stability of material in aqueous solution [28]. The result revealed that the synthesized silver nanoparticles had zeta potential of -15.9 mV, which indicated the high stability of silver nanoparticles in aqueous environment Fig. 4(a).
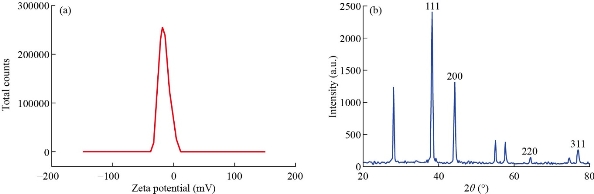
Fig. 4 (a) Zeta potential analysis. (b) XRD of Silver nanoparticle synthesized from Streptomyces.
Structural characterization of silver nanoparticle
The XRD diffraction pattern of synthesized silver nanoparticles is shown in Fig. 4(b). The silver nanoparticles prepared by Streptomyces spp. was crystalline in nature. The diffraction peak of the prepared silver nanoparticles was observed at 2θ = 38.11, 44.21, 64.42, 77.42 which corresponded to the lattice plane of (1 1 1), (2 0 0), (2 2 0) and (3 1 1). The XRD pattern revealed the metallic silver with FCC (face centered cubic) crystal structure of silver (JCPDS file no. 04-0783). By using scherrer formula, the average crystalline size of the prepared silver nanoparticle was found to be 51.2 nm. The XRD result was in agreement with the recently reported work by Singh et al. [29]. In that investigation, four peaks corresponding to (111), (200), (220) and (331) planes were observed, which emphasized the diffraction pattern of silver.
TEM with EDX
The TEM images of bio-reduced silver nanoparticles with different magnification are shown in Fig. 5 (a)-(c). The image clearly revealed the synthesized silver nanoparticles were predominantly spherical in shape without significant agglomeration. The EDX spectroscopy was applied to quantify the elemental composition of the synthesized silver nanoparticle. In Fig. 5 (d), strong signal for silver in the EDX spectrum at 3 Kev confirmed the formation of Ag NPs. Silver (60.61%) was the major constituent element followed by oxygen (52.33%) and chlorine (23.73%). In the EDX profile, oxygen peak might correspond to biomolecules that were bound to the surface of Ag NPs.
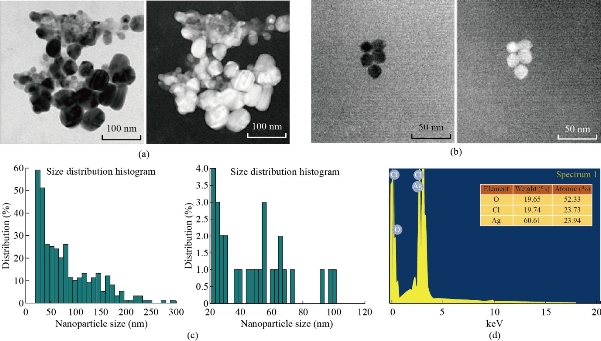
Fig. 5 (a)-(c) TEM images of silver nanoparticles synthesized from Streptomyces spp.: (a) Original TEM images; (b) Complemented image of (a); (c) Particle size distribution histogram of respective TEM Images. (d) EDX spectrum of synthesized silver nanoparticle.
Particle size analysis
The distributions of particle size of biosynthesised Ag NPs was determined using PS analyser. Size distributions of silver nanoparticles was determined by (DLS) dynamic light scattering and is indicated in Fig. 6. PS distribution curve revealed that Ag NPs obtained were monodispersed in nature. The average size was determined to be around 50 nm in size, similar to the analysis in which the biosynthesised Ag NPs and Cu Nps were determined using PS analyser. The curve reveals that Ag NPs were polydispersed in natue, with average size ∼250 nm [14].
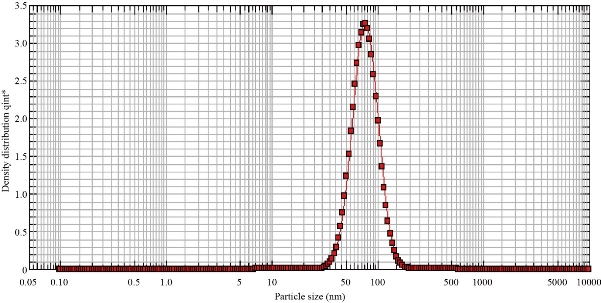
Fig. 6 Particle size analysis of biosynthesized silver nanoparticles.
Nanotoxicity analysis in adult zebrafish
In Fig. 7, control, 0.15, 0.30 and 0.45 µg respectively results were shown. At the concentration 0.150 µg shows the normal muscle tissue anatomy. Zebrafish muscle displayed widespread severe atrophy; muscle fibres were disorganized. A large increase in nuclei was observed in 0.30 and 0.45 µg, which showed thatmuscle fibre size and their myofibres were too disorganised to discern individual myotomes. As hepatic architecture of control group had no changes in comparision with the ketoprofen treated zebrafish, ketoprofen exposed to three different concentrations (1, 10, and 100 µg/L) showed changes in histoarchitectures, such as necrosis, nuclear degeneration, cytoplasmic degeneration, nuclear degeneration, and cytoplasmic vacuolation, sinusoid and pyknotic nuclei when compared with the control group [32].
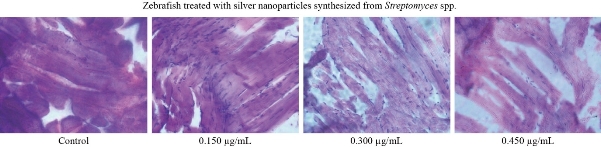
Fig. 7 Toxicity effect of different concentrations of silver nanoparticles synthesized from Streptomyces spp. in zebrafish model.
Conclusions
The Ag NPs biosynthesized extracellularly from the efficient biocontrol agent were characterized, and their activities were determined against P. hypolateritia and P. theae. The average crystalline size of the prepared silver nanoparticles was found to be 51.2 nm.. Owing to the stability aroused by Ag Nps, high antagonistic activity was detected under in-vitro conditions. This paper recommended the use of Ag NPs synthesised by Streptomyces sp. for the control Poria and Phomopsisinfected tea plants. This results of antagonistic activity can leap forward for the scaled-up application of biosynthesized silver nanoparticles for greenhouse and field studies to control fungal diseases. Toxicity analysis revealed that silver nanoparticles below 0.150 µg might be safe for adult zrbrafish.
Acknowledgements
The authors are thankful to the National Tea Research Foundation (NTRF), Kolkata, for financial support.
Conflict of Intrerests
We authors cordially declare that we have no conflict of interest.
References
Copyright© Karthik Natesan, Ponnusamy Ponmurugan, Balasubramanian Mythili Gnanamangai, Mani Suganya, and Shivasji Kavitha. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.