Research Article
A Magnetic Nanoparticle Labeled Immunochromatography Kit for SARS-CoV-2 Infection Diagnosis
Qi Shen 1 †, Hui Liang 1 †, Jing Tian 1, Cheng Zhou 1, Ang Gao 2, Dan Wang 1, Jian Ni 2 *, Daxiang Cui 1, 2 *
1 National Engineering Research Center for Nanotechnology, 28 East Jiangchuan Road, Shanghai 200241, P. R. China.
2 Institute of Nano Biomedicine and Engineering, Shanghai Engineering Research Centre for Intelligent Diagnosis and Treatment Instrument, Department of Instrument Science and Engineering, School of Electronic Information and Electrical Engineering, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai 200240, PR China.
* Corresponding author. E-mail: 13818096617@139.com; dxcui@sjtu.edu.cn;
† These authors contributed equally to this work.
Received: Aug. 24, 2020; Accepted: Oct. 27, 2020; Published: Oct. 29, 2020
Citation: Qi Shen, Hui Liang, Jing Tian, Cheng Zhou, Ang Gao, Dan Wang, Jian Ni, and Daxiang Cui. A Magnetic Nanoparticle Labeled Immunochromatography Kit for SARS-CoV-2 Infection Diagnosis. Nano Biomed. Eng., 2020, 12(4): 325-330.
DOI: 10.5101/nbe.v12i4.p325-330.
Abstract
The novel coronavirus disease (COVID-19) is breaking out and spreading rapidly around the world. There is an urgent need for an accurate and rapid detection method to quickly find infected patients and asymptomatic carriers in order to prevent the spread of the severe acute respiratory syndrome coronavirus [SARS‐Cov‐2]. In this paper, we designed a test strip which used the principle of double antigen sandwich. Fe3O4 magnetic nanobeads are firstly coupled with specific antibodies, and the S protein of the new coronavirus is used as the coating antigen to capture specific antibodies against the new coronavirus, which is used to detect the virus nucleoprotein of specific antibodies in clinical samples. At the same time, Fe3O4 magnetic nanobeads have unique magnetic properties, which can be used to generate different types of detection signals and simplify the detection process. These results can be judged by color changes and magnetic changes at the test and control lines. Compared with the traditional method, this test strip of Fe3O4 magnetic nanobeads has high sensitivity and can qualitatively detect samples within 15 minutes. The magnetic performance of the magnetic nanobeads can be used to improve the sensitivity of the strip in our further research and product development.
Keywords: SARS-CoV-2, Magnetic Nanoparticle, Fe3O4 nanobeads, Immunochromatography
Introduction
The outbreak and rapid spread of the novel coronavirus disease (COVID-19) has had a huge impact on the whole world [1]. Although RT-PCR method has become the standard method for diagnosing SARS-CoV-2 infection [1], these methods have many limitations, such as the high false negative rate [2]. So, there is an urgent need for a fast and precise detection methods to screen out people infected with the virus and asymptomatic carriers. At present, the diagnosis of SARS-COV-2 at the molecular level includes nucleic acid detection methods and antigen/antibody immune detection methods [1, 4]. Nucleic acid-based detection methods include specific gene detection of viral nucleic acid and viral genome sequencing [4]. The nucleic acid detection kits have been approved for clinical use now, but there still has some limitations, such as cumbersome operation, long time consuming, need for centralized inspection and professional equipment, which greatly restricts the use and convenience of detection due to its high technical threshold [5]. And the false negative rate of nucleic acid testing is relatively high in clinical. The antibody detection method are mainly rapid diagnostic kits (colloidal gold method) for antibody IgG and IgM, using the S protein of new coronavirus as the coating antigen and labeling antigen to detect the specific antibodies of virus nucleoprotein in clinical samples [6]. This method does not need complicated equipment and it can qualitatively test samples in a very short time, so it is suitable for preliminary screening in primary hospitals. Further, we can use the magnetism of magnetic nanoparticles for quantitative detection instead of colloidal gold. There is no relevant quantitative detection product on the market. Magnetic nanoparticles are a kind of magnetic material with a size of 1-100 nm [7]. It has unique optics, magnetism, electricity, thermal, mechanical and chemical activities, so it has a broad prospect in the diagnostic technology based on magnetic nanoparticles [8]. There are several kinds magnetic nanoparticles, such as cobalt oxide, nickel oxide, and iron oxide [9]. Among them, iron oxide nanoparticles have been widely studied in the field of biomedicine due to their good biocompatibility, biodegradability and superparamagnetic [10]. In this work, we have developed a specific test strips that can qualitatively and quantitatively detect the antibody (IgG and IgM) of new coronavirus (2019-nCoV) in serum by immunochromatography, and the high sensitivity and color rendering ability of Fe3O4 magnetic nanobeads gives the test strip a unique performance. IgM antibody positive means recently infected, IgG antibody positive means longer infection time or previously infected. The clinical test results show that the accuracy rate of the test strip we developed is over 90%, and the result can be received within 5 minutes at the fastest. The structure of the test strip is shown in Fig. 1.
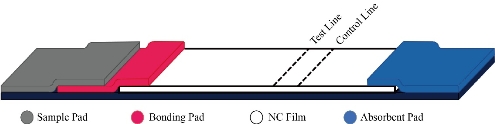
Fig. 1 The structure of the magnetic nanoparticle-based test strip.
Experimental
Materials
Ferric trichloride (FeCl3, 99%), Trisodium citrate (Na3Cit, 99%), Sodium acetate (NaAc, 99%), Ethanol (99.5%) were purchased from Sigma-Aldrich. 2-Morpholinoethanesulfonic acid (MES, 99%), n-(3-dimethylaminopropyl)-n’-ethylcarbodiimide hydrochloride (EDC, 99%), N-Hydroxysuccinimide (NHS, 99%), Tween-20 (99%), Boric acid (99%), Sodium tetraborate (99%) were purchased from Sinopharm Chemical Reagent Co., LTD. IgG and IgM antibody were purchased from Abcam. The positive and negative samples were purchased from National Institutes of Food and Drug Control.
Preparation of Fe3O4 magnetic nanobeads
Add 0.65 g FeCl3 and 0.2 g Na3Cit to 20 ml of ethanol, stir until dissolved. Then add 1.2 g of NaAc and stir for 30 minutes. The mixed solution was added to a 50 ml hydrothermal kettle and reacted at 200℃ for 10 hours. After being cooled to room temperature, the nanoparticles were aged and washed with 80% ethanol solution. Finally, put the precipitate in an oven at 50℃ for drying to obtain the magnetic bead powder.
Preparation of immunomagnetic beads
The whole preparation process includes four steps of activation, coupling, sealing and preservation. (1) Activation: Use pH 6.0, 0.01M MES solution as the activation buffer solution. Took 10 mg of carboxyl Fe3O4 magnetic nanobeads into a 2 mL centrifuge tube, added 1000 μL activation buffer, mixed well on the vortex shaker, and then placed the centrifuge tube on the magnetic separation rack, when the Fe3O4 magnetic nanobeads were completely adsorbed, removed the supernatant. Then, added 1000 μL activation buffer into the centrifuge tube to wash the magnetic beads twice. Added 200 μL 5 mg/mL EDC and 200 μL 5mg/mL NHS into the Fe3O4 magnetic nanobeads. Mixed well on a vortex shaker, then activate the carboxyl groups on the surface of Fe3O4 magnetic nanobeads at 37°C for 30 minutes. After activation, wash the Fe3O4 magnetic nanobeads with MES solution which was added 0.05% TWEEN-20 twice to remove extra EDC and NHS. (2) Coupling: use pH9.0, 0.02M borate Tween buffer (0.05% Tween-20) solution as coupling buffer, washed the Fe3O4 magnetic nanobeads twice with 995 μL coupling buffer and then added 5 μL of 20 μg/μL Goat-anti-human IgG Fc and Goat-anti-human IgM mu chain antibody. The mixed solution was reacted at 37°C for 3 hours, and then subjected to shake at room temperature overnight to prepare antibody modified Fe3O4 magnetic nanobeads. After the coupling was completed, the reaction supernatant was collected and the amount of coupled protein was detected with the BCA kit. (3) Blocking: Added 1000 μL of 1% BSA to block the activated groups on the surface of the immunomagnetic nanobeads that have not fully reacted for 30 minutes at room temperature to reduce the non-specific combine that may occur in the future test. (4) Storage: Washed the blocked immunomagnetic beads with BST buffer for four times, and finally resuspend the magnetic beads in 1000 μL preservation solution and stored in a refrigerator at 4℃.
BCA method to detect the amount of coupling protein
Dilute the standards with PBS to a final concentration of 0.5 mg/mL, and take 0, 1, 2, 4, 8, 12, 16, 20 μL standard solution in the enzyme reaction well, each standard solution is repeated in parallel. Then add PBS to each well to a total volume of 20 μL, add 200 μL of BCA reaction solution to all wells with standard solution or supernatant, gently mixed at 60℃ and react for 30 minutes. Finally use a microplate reader to detect the absorbance value of each well. Make a standard curve based on the concentration and absorbance of the standard solution, and then obtain the amount of antibody based on the absorbance of the supernatant, thereby obtaining the coupling amount of the antibody on the magnetic nanobeads surface.
Preparation of test strips
The test strip sample pad and the bonding pad are processed as follows: (1) Cut the glass fiber membrane into a size of 25 mm×5 mm. In order to optimize the performance of the test strip, the sample pad needs to be pre-treated before construction. Soak the cut glass fiber membrane in PBST for half an hour. Then put the soaked sample pad in an oven at 37°C for 2 hours and then take it out for use. (2) Cut the glass fiber membrane into a size of 5 mm×5 mm. Soak the cut glass fiber membrane with the binding pad treatment solution for 30 minutes. Sonicate the diluted immunomagnetic nanobeads solution for 5 minutes. Use a pipette to draw 15 μL of the immunomagnetic bead solution, and evenly place it on the binding pad. Then put the bonding pad in an oven at 37°C for 2 hours and then take it out for use. (3) The T line (SARS-CoV-2 spike antigen and the N protein epitope peptide), the C line (Rabbit anti-goat IgG HL) have been draw on the NC film. Finally, the processed sample pad, binding pad, NC film and absorbent pad have been assembled and the test strips are cut into 4 mm wide test strips with a cutting machine.
Sensitivity of test strips
The positive standards P1-P10 and the negative standards N1-N7 are all national drug standards purchased from China Food and Drug Control Institute. Then use three batches of test strips to test the negative and positive standards to explore the sensitivity of test strips.
Specificity of test strips
Negative standard N1, positive standard P1, and positive standard P5 were selected as negative, weakly positive and positive samples. Interfering samples (hemoglobin, bilirubin, triglycerides) were prepared by artificial addition, and three batches of kits were used to test the influence of adding different concentrations of interfering samples to the experimental groups.
Results and Discussion
Characterizations of Fe3O4 magnetic nanobeads
As showed in Fig. 2, the preparation of Fe3O4 magnetic nanobeads had the uniform shape and the average size was 220 nm.

Fig. 2 The SEM image of Fe3O4 magnetic nanobeads.
Amount of coupled protein
According to the absorbance value of the standard solution and the standard protein concentration, the standard curve of the standard protein concentration and absorbance can be obtained. As shown in the Fig. 2, substitute the average value of three wells (0.156 a.u.) into the formula Y=0.6697X+0.1383 (Y is the absorbance, X is the protein concentration) to calculate the sample supernatant with antigen concentration of 26.4 μg/mL, so the coupling rate is 73.6%.
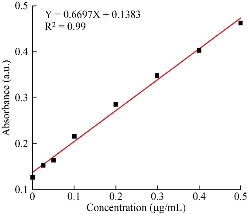
Fig. 3 The standard curve of protein concentration and absorbance.
Sensitivity of test strips
The experimental results are shown in Table 1. Three batches of kits were used to test 10 positive standards (P1-P10), all results were positive, and the positive coincidence rate was 100%. The results of 7 negative standards (N1-N7) are all negative with a specificity of 100%.
Table 1 The coincidence rate of positive and negative samples
|
Batch |
Lot1 |
Lot2 |
Lot3 |
|
|
Sample |
Results |
|||
|
Positive Standards |
P1 |
P |
P |
P |
|
P2 |
P |
P |
P |
|
|
P3 |
N |
P |
P |
|
|
P4 |
P |
P |
P |
|
|
P5 |
P |
P |
P |
|
|
P6 |
P |
P |
P |
|
|
P7 |
P |
P |
P |
|
|
P8 |
P |
P |
P |
|
|
P9 |
P |
P |
P |
|
|
P10 |
P |
P |
P |
|
|
Negative Standards |
N1 |
N |
N |
N |
|
N2 |
N |
N |
N |
|
|
N3 |
N |
N |
N |
|
|
N4 |
N |
N |
N |
|
|
N5 |
N |
N |
N |
|
|
N6 |
N |
N |
N |
|
|
N7 |
N |
N |
N |
|
Specificity of test strips
The experimental results are shown in Table 2. The test results showed that when negative samples, weak positive samples and positive samples were added with hemoglobin concentration of 5.0 mg/mL, triglyceride concentration of 25 mg/mL, and bilirubin concentration of 0.2 mg/mL, the test result after adding interference sample is consistent with the test result without adding interference samples. Although low-concentration interfering agents have no effect on the test results of the samples, red backgrounds may appear in hyperlipidemia, soluble samples and hemolytic samples, which will still affect the test results. It is still necessary to avoid the use of special samples.
Table 2. The influence of different endogenous interferences on sample detection
|
Batch |
Lot1 |
||||||||||
|
Interfering Substances |
None |
Hemoglobin (mg/mL) |
Triglycerides (mg/mL) |
Bilirubin (mg/mL) |
|||||||
|
Samples |
1.25 |
2.5 |
5.0 |
6.25 |
12.5 |
25 |
0.05 |
0.1 |
0.2 |
||
|
Negative Samples |
1 |
N |
N |
N |
N |
N |
N |
N |
N |
N |
N |
|
2 |
N |
N |
N |
N |
N |
N |
N |
N |
N |
N |
|
|
3 |
N |
N |
N |
N |
N |
N |
N |
N |
N |
N |
|
|
Weak Positive Samples |
1 |
P |
P |
P |
P |
P |
P |
P |
P |
P |
P |
|
2 |
P |
P |
P |
P |
P |
P |
P |
P |
P |
P |
|
|
3 |
P |
P |
P |
P |
P |
P |
P |
P |
P |
P |
|
|
Positive Samples |
1 |
P |
P |
P |
P |
P |
P |
P |
P |
P |
P |
|
2 |
P |
P |
P |
P |
P |
P |
P |
P |
P |
P |
|
|
3 |
P |
P |
P |
P |
P |
P |
P |
P |
P |
P |
|
|
Batch |
Lot2 |
||||||||||
|
Interfering Substances |
None |
Hemoglobin (mg/mL) |
Triglycerides (mg/mL) |
Bilirubin (mg/mL) |
|||||||
|
Samples |
1.25 |
2.5 |
5.0 |
6.25 |
12.5 |
25 |
0.05 |
0.1 |
0.2 |
||
|
Negative Samples |
1 |
N |
N |
N |
N |
N |
N |
N |
N |
N |
N |
|
2 |
N |
N |
N |
N |
N |
N |
N |
N |
N |
N |
|
|
3 |
N |
N |
N |
N |
N |
N |
N |
N |
N |
N |
|
|
Weak Positive Samples |
1 |
P |
P |
P |
P |
P |
P |
P |
P |
P |
P |
|
2 |
P |
P |
P |
P |
P |
P |
P |
P |
P |
P |
|
|
3 |
P |
P |
P |
P |
P |
P |
P |
P |
P |
P |
|
|
Positive Samples |
1 |
P |
P |
P |
P |
P |
P |
P |
P |
P |
P |
|
2 |
P |
P |
P |
P |
P |
P |
P |
P |
P |
P |
|
|
3 |
P |
P |
P |
P |
P |
P |
P |
P |
P |
P |
|
|
Batch |
Lot3 |
||||||||||
|
Interfering Substances |
None |
Hemoglobin (mg/mL) |
Triglycerides (mg/mL) |
Bilirubin (mg/mL) |
|||||||
|
Samples |
1.25 |
2.5 |
5.0 |
6.25 |
12.5 |
25 |
0.05 |
0.1 |
0.2 |
||
|
Negative Samples |
1 |
N |
N |
N |
N |
N |
N |
N |
N |
N |
N |
|
2 |
N |
N |
N |
N |
N |
N |
N |
N |
N |
N |
|
|
3 |
N |
N |
N |
N |
N |
N |
N |
N |
N |
N |
|
|
Weak Positive Samples |
1 |
P |
P |
P |
P |
P |
P |
P |
P |
P |
P |
|
2 |
P |
P |
P |
P |
P |
P |
P |
P |
P |
P |
|
|
3 |
P |
P |
P |
P |
P |
P |
P |
P |
P |
P |
|
|
Positive Samples |
1 |
P |
P |
P |
P |
P |
P |
P |
P |
P |
P |
|
2 |
P |
P |
P |
P |
P |
P |
P |
P |
P |
P |
|
|
3 |
P |
P |
P |
P |
P |
P |
P |
P |
P |
P |
|
Clinical sample tests
We have received 20 clinical samples of patients who have recovered from COVID-19 with anti-SARS-CoV-2 IgM/IgG antibodies, dilute the serum according to the ratio required in the kit instructions, and the positive detection rate is 100%.
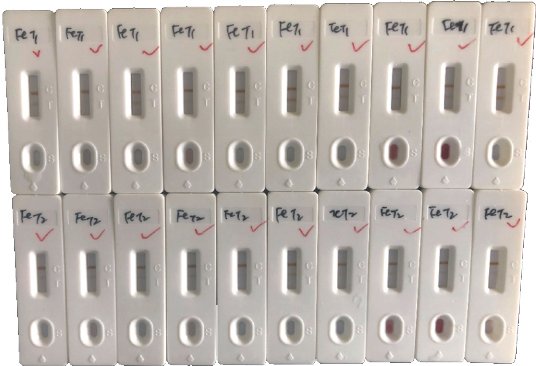
Fig. 4 Clinical results of the test strips.
Conclusions
In summary, the Fe3O4 magnetic nanobeads show the great potential to be the nanomaterial for the detection of SARS-CoV-2 instead of Nano-Au. The test strip of Fe3O4 magnetic nanobeads has high sensitivity and can qualitatively detect samples within 15 minutes according to the experiment. Also, the Fe3O4 magnetic nanobeads have unique magnetic properties, so that can be used to generate different types of detection signals such as color signal and magnetic signal. We will further explore its magnetic signal response in immunochromatographic detection.
Acknowledgements
This work was supported by Key Basic Research Program of China (No.2017YFA0205304), Nature Scientific Foundation of China (No.81602184), and Medical Engineering Cross Project of Shanghai Jiao Tong University (YG2017ZD12). This work was also supported by “the Belt and Road” young scientist exchange program of the Science and Technology Commission of Shanghai (Grant No.18410741600).
References
Copyright© Qi Shen, Hui Liang, Jing Tian, Cheng Zhou, Ang Gao, Dan Wang, Jian Ni, and Daxiang Cui. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.