Research Article
Microwave-assisted and Ultrasonic Phyto-synthesis of Copper Nanoparticles: A Comparison Study
Farinaz Hadinejad 1, Mohsen Jahanshahi 1, Hamed Morad 2, 3 *
1 Nanotechnology Research Institute, Faculty of Chemical Engineering Babol Noushirvani University of Technology, Babol, Iran.
2 Department of Pharmaceutics, Faculty of Pharmacy, Mazandaran University of Medical Sciences, Sari, Iran.
3 Student research committee, Mazandaran University of Medical Sciences, Sari, Iran.
* Corresponding author. E-mail: h.morad@mazums.ac.ir
Received: Mar. 21, 2020; Accepted: Aug. 21, 2020; Published: Dec. 28, 2020
Citation: Farinaz Hadinejad, Mohsen Jahanshahi, and Hamed Morad, Microwave-assisted and Ultrasonic Phyto-synthesis of Copper Nanoparticles: A Comparison Study. Nano Biomed. Eng., 2021, 13(1): 6-19.
DOI: 10.5101/nbe.v13i1.p6-19.
Abstract
Elimination of hazardous chemicals in the process of synthesis, which guarantees the safety of the nanoparticles (NPs) for therapeutic utilization, could be obtained by using the phyto-synthesis method. The present project is a multidimensional survey that aimed to optimize the phyto-synthesis conditions of copper nanoparticles (Cu NPs) using the microwave and ultrasound-assisted methods and facilitate approaching the dilemma of choosing between these two methods by characterizing the final products of each method. Based on the transmission electron microscopy (TEM), the obtained NPs were sub 10 nm in both methods. The optimized NPs were achieved in 5 min using 6 mL of phytoextract at 95 °C in a microwave oven, and amplitude 100% and cycle 0.8 in an ultrasonic processor. In addition to the antibacterial property and molecular wound healing stimulation of Cu NPs, these amorphous nanoscale particles could provide desirable absorption and distribution over the wounds to be suggested as an effective transdermal drug delivery system. The ultrasound-assisted method was the most appropriate way to obtain an amorphous mixture of Cu NPs with a majority of copper oxide while the microwave-assisted method was more suitable for synthesis procedures using plant extracts with heat-sensitive and volatile components.
Keywords: Phytonanotechnology, Phyto-synthesis, Copper nanoparticles, Fraxinus excelsior, Regenerative medicine, Drug delivery system
Introduction
Nature-based products are severe rivals for chemicals in a wide range of applications. Recently, the phyto-synthesis method, as a subset of green synthesis, has become the preferred approach for the fabrication of metal and metal oxide NPs because of its feasibility and safety to the environment and human health when compared with other chemical or physical methods. Phyto-synthesis also surpasses the other member of the green synthesis family, microorganism-mediated synthesis of NPs, because plant materials are versatile and readily available, using them does not require sophisticated laboratory facilities of working on unicellular organisms and their production rate is much faster, so they can become an economic candidate for the large-scale synthesis of NPs [1].In the phyto-synthesis, pharmacologically important compounds of plant extracts used for the reduction of metal NPs. They also surround metal ions and act as a stabilizer, which improves their biocompatibility and inherent antimicrobial properties that make them appropriate choices for biomedical applications. Since the characteristics of the NPs influenced by the source of the plant extract, selection of plants carries out based on the content of phytochemicals such as flavonoids, alkaloids, phenolic compounds, and polysaccharides that exhibits inherent biological properties [2]. The miraculous extract of Fraxinus excelsior, which belongs to the Oleaceae family, possesses a diversity of biological activities such as anti-inflammatory, antiallergic, antioxidative, hepatoprotective, diuretic, and skin regenerating. These bioactive components also demonstrated antiproliferative properties against human cancerous cells, besides exhibiting considerable antibacterial activities. The presence of coumarins (Fraxetin, Fraxin, Esculin), secoiridoids (Oleoside-7, 11-dimethyl ester, Oleuropein), Flavonoids (Quercetin, Kaempferol, Catechin, and epicatechin), Simple phenolic compounds (benzoic acid and cinnamic acid derivatives), phenylethanoids (Phenylethanoid glycosides) is a characteristic feature of Fraxinus species that makes this plant a great choice for synthesis of NPs [3]. Since Cu NPs represent an ideal compromise between cost and remarkable properties, they find applications as catalysts [4], electrochemical [5] and photonic [6] devices, sensors [7], optical [8] and magnetic [9] devices, environmental remediation [10], heat exchangers [11], and heat transfer fluids [12]. Recent works have been reported superior antibacterial activity of Cu NPs over Ag NPs using single representative strains of Escherichia coli and Bacillus subtilis. That’s because of their mutation-resistant characteristics [13, 14]. Cu NPs also have considerable potency to enhance cutaneous wound healing by modulation of various cells, cytokines and growth factors during different phases of the healing process, which makes them a great choice for fabrication of wound healing ointments and bandages [15]. Moreover, the small size of these particles in Nano scale with high surface area provides them great properties to be easily spread over the surface of the wound and increase the chance of delivering to the area and subsequently increase absorption and bioavailability [16, 17]. One common approach in green chemistry is replacing the conventional synthesis methods with greener ones like sonochemical synthesis, microwave synthesis, hydrothermal synthesis, solvothermal synthesis, biosynthesis, etc [1]. Ultrasound is the part of the sonic spectrum with frequencies between 20 kHz to 10 MHz, which range from 20 kHz to around 1 MHz is commonly used in Sonochemistry [18]. The ultrasonic technique accelerates and facilitates physical and chemical reactions through efficient agitation, dissolution, heat and mass transfers, and sonolysis of the reagents, which all arise from the acoustic cavitation phenomenon due to the continuous formation, growth and vigorous collapse of microbubbles in solution accompanying with the intense shock. During the bubble implosion, the concentrated energy stored in the bubble is released within a very short duration of time producing a localized region of extremely high pressures(~1000 bar) and temperatures(~5000 K), with heating and cooling rates greater than 1010 Ks-1 [19]. In certain solvents, the ultrasonic energy can create free radicals that participate in chemical reactions, for example, the homolytic cleavage of the water molecules to H and OH radicals in aqueous solutions thereupon accelerates the overall reaction rate [18]. Microwaves are a subset of the electromagnetic spectrum in the frequency range from 300 MHz to 300 GHz (most commonly used frequency is 2.45 GHz). When microwaves penetrate and pervade through a dielectric solution or suspension, an internal electric field is generated, which makes charged complexes to rotate such as dipoles [20]. The main advantages of microwave-assisted reactions in comparison with conventional heating methods of synthesis are: (a) increasing in the kinetics of the reaction by one to two orders of magnitude, (b) new phases are formed, and (c) rapidity of initial heating can lead to energy saving (up to 90%) [21]. Compared to ultrasonic irradiation, there is no similar bubble formation during microwave heating, although analogously, superheating occurs in localized spots where both high temperatures and pressures enhance reaction rates [20]. Recently, in scant projects, researchers implemented ultrasonic or microwave-assisted techniques along with the usage of the natural polymers and phytoextracts for the fabrication of nanomaterials. For instance, chitosan capped copper oxide nano leaves were successfully obtained using high-intensity ultrasound (30 kHz) and also without further calcination [22]. In another study, glycerol was used as a solvent, reducing agent and capping agent for the formation of Cu2O NPs under sonochemical irradiation by the ultrasonic horn with a 20 kHz frequency and the output power of 750 W. Crystalline-natured Cu2O NPs obtained in the range size of 80–150 nm [23]. Terminalia arjuna bark extract was used as a reducing/stabilizing agent for Cu2+ → Cu0 under 8 min microwave irradiation, and bio-capped Cu NPs were near-spherical shaped with crystallite size about 23 nm and antioxidant properties [24]. Fabrication of bio-conjugated silver NPs was also carried out using Fraxinus excelsior aqueous extract by a microwave-assisted method. They reported that synthesized NPs were polydisperse with diameters in the range of 25–40 nm and desirably stabilized by capping organic materials, thereby preventing their agglomeration [25].
The present investigation aims to optimize the conditions for the synthesis of Cu NPs using Fraxinus excelsior methanolic extract by both: ultrasonic and microwave-assisted techniques and compare the characteristics of their products to be suggested as a wound healing formulation.
Experimental
Materials
Methanol gradient grade 99.8% (Merck, Germany) was used as a solvent. Copper (II) sulfate pentahydrate extra pure was purchased from Sigma-Aldrich (Germany) as a precursor salt and also the leaves of Fraxinus excelsior were collected from Isfahan, Iran.
Preparation of methanolic leaf extract
The percolation method [26] was used to extract phytochemical compounds of plant leaves. 500 g of the crushed dried leaves of Fraxinus excelsior (washed thrice with double distilled water) were poured into a separatory funnel and macerated for 48 hours with absolute methanol. Then the valve was opened to let out the extract and simultaneously from above fresh solvent was added drop wise to the system. Subsequently, the extract was filtered with Whatman No.1 filter paper to remove impurities and condensed with a rotary evaporator and freeze-dried for 48 hours afterward to obtain the extract powder which refrigerated for further use.
Green synthesis of Cu NPs
Microwave-assisted method
In the first method[25], Cu NPs were synthesized using a microwave digestion system (WX-4000, Preekem, China). In a typical reaction procedure, 6 mL of Fraxinus excelsior methanolic extract solution (1 wt %) was added to 10 mL of 3×10-3 M CuSO4.5H2O solution in methanol. The solution mixture was then exposed to microwave irradiation at a temperature of 95 °C with a power of 1000 W for 5 min. After microwave irradiation treatment, the resultant colloidal solution was naturally cooled to room temperature to be ready for the ultraviolet-visible (UV–vis) spectroscopy test. The Cu NPs obtained by leaf extract were centrifuged at 10000 rpm for 20 min and subsequently three times dispersed in double distilled water to get rid of any uncoordinated biological materials. After that, they were placed in a vacuum oven at 75 °C for 12 hours. The dried powders then used for further characterization.
Ultrasound-assisted method
In the second method [27], ultrasound technology was employed to synthesize Cu NPs. In this synthesis process, 10 mL of 3×10-3 M CuSO4.5H2O solution in methanol was mixed with 4 mL of Fraxinus excelsior methanolic extract solution (1 wt %) in a beaker. Then the beaker was transferred into an ultrasonic processor (UP400S, Hielscher company, Germany) [400 W, 24 kHz, H22 Sonotrode (22 mm dia.)] for 5 min in optimum amplitude and cycle, 100% and 0.8 respectively. Other steps were carried out similar to the microwave-assisted method to isolate the NPs and convert them to powder.
Optimization of green synthesis of Cu NPs
The exact position, as well as the intensity of the SPR (Surface Plasmon Resonance) band, may change depending on the individual particle characteristics and properties including size, shape, capping agents and also their surrounding environments [28, 29] .
So, in this project, UV-vis analysis was utilized as a mean of optimization to investigate synthesis parameters in both microwave and ultrasound-assisted methods. The optical spectra of colloid solution samples, achieved at different synthesis temperatures (25 °C, 45 °C, 65 °C, 80 °C and 95 °C), irradiation times (2.5, 5 and 7 min), volumes of phytoextract (2 mL, 4 mL, 6 mL and 8 mL), sonication times (5, 10 and 15 min), amplitudes (80% and 100%), and cycles (0.6 and 0.8). Subsequently, statistical analysis were employed to investigate the differences and find the best method with optimum experimental conditions.
Statistical analysis
Statistical analysis was performed by using SPSS software, version 21.0 (IBM Corp., USA). Values obtained as the mean ± standard deviation (continuous variables) or percentages of the group (categorical variables). One-way ANOVA and then Tukey (as post hoc analysis) were employed to estimate the statistical associations. Statistical significance was regarded as P-values less than 0.05.
Characterization of Cu NPs
Ultraviolet-visible spectroscopy
Along with visual identification, UV-vis spectroscopy is generally used to confirm the synthesis of Cu NPs. SPR is observed due to the combined vibration of the abundant free electrons of metallic NPs, with the light wave and for Cu NPs has been reported to appear at around 570 nm [29]. The absorption maxima determined by scanning the colloid solution samples by UV-vis Spectrophotometer (UV-2450, Shimadzu, Japan).
Morphological characteristics and zeta potential analysis
Atomic force microscopy (AFM), along with two electron microscopy methods for scanning the surface of NPs: scanning electron microscopy (SEM FEI Quanta 200, USA) and transmission electron microscopy (TEM Philips EM 208S, USA) were employed to determine the height and diameter of the NPs and their appearance. The topology of the surfaces was studied using a JPK atomic force microscope (BioAFM, NanoWizard® ॥, Germany). AFM parameters were evaluated using the Nanosurf Easyscan 2 software (Nanosurf, Switzerland), and the ImageJ software was used to process the obtained micrographs of the SEM and TEM analysis.
With the help of dynamic light scattering, zeta potential of Cu NPs which is a key characteristic for determination of their stability was determined by using a zeta seizer (Nano ZS, Malvern Instruments, Malvern UK). The value of zeta potential gives the degree of electrostatic repulsion between adjacent, similarly charged particles in the dispersion.
Energy dispersive X-ray spectroscopy (EDX)
To detection the traces of elements in the structure of NPs, energy dispersive (EDX) spectra were taken by an EDAX Element detector (US) of an SEM device (SNE-4500M, SEC, South Korea) and the results analyzed by EDAX APEX software.
Fourier transform infrared (FTIR) analysis
The achieved Cu NPs from each method and also the active components in the extract which are responsible for the reduction of precursor and also act as capping agents were analyzed by using FTIR Spectrophotometer (Beijing Rayleigh Analytical Instrument Corp. (BRAIC), WQF-510A, China) in the spectral range of 4500 to 500 cm -1 using KBr pellets.
X-ray diffraction (XRD) study
The crystallinity studies were performed employing the X-ray diffraction (XRD) patterns recorded by an X-ray diffractometer (X'Pert Pro, Philips, Nederland) with X'Pert HighScore software using Ni-filtered Cu Kα radiation (=1.54 Å).
Differential scanning calorimetry (DSC)
The thermal properties measurements of finely powdered samples were carried out using a DSC calorimeter (S.DS.T, Sanaf, Iran) at a heating rate of 10 °C·min−1 in the temperature range 30-320 °C using alumina pans.
Results and Discussion
Ultraviolet-visible spectroscopy
Fig. 1 shows the optical spectra of colloid solution samples in different operating conditions. These graphs obviously illuminate that the surface plasmon peak for Cu NPs synthesized with both the microwave (Fig.1 a-c) and the ultrasound-assisted method (Fig. 2 (c)-(f)) appeared at around 570 nm [29] which confirms that Cu NPs were successfully synthesized.
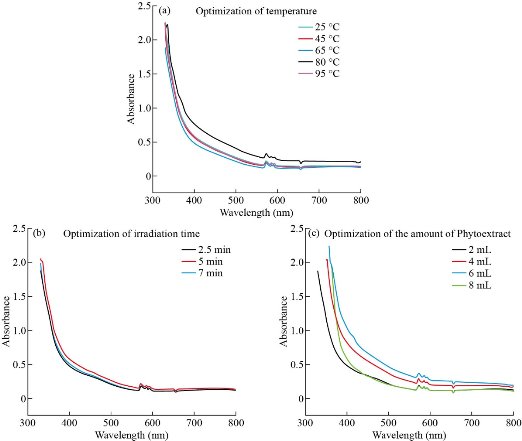
Fig. 1 UV-vis spectra of Cu NPs based on optimization parameters in microwave- assisted method.
Optimization of phyto-synthesis of Cu NPs
Optimization of temperature in microwave-assisted method
The optical spectra of colloid solution samples, achieved at different synthesis temperatures is shown in Fig. 1(a). The one way ANOVA test showed significant difference (P≈0) between the five temperature groups (25 °C, 45 °C, 65 °C, 80 °C, and 95 °C). Tukey post hoc test elucidated that the average absorption at temperature 95 °C (0.86 ± 0.85)was significantly higher than the temperature 45 °C (0.77 ± 0.88), 25 °C (0.75 ± 0.87), 65 °C (0.73 ± 0.89), and 80 °C (0.77 ± 0.89), (P < 0.05). But other groups didn’t show any obvious difference in the average absorption. As the temperature rises, an enhancement in the kinetics of the reaction occurs, which leads to enhancing the nucleation process. As a result, the absorbance of the synthesized NPs increased, indicating an increase in the concentration of Cu NPs [29]. According to the above results, temperature 95 °C was chosen as the optimum temperature for synthesis of NPs by microwave irradiation.
Optimization of irradiation time
One-way ANOVA test for three different holding times (2.5, 5 and 7 min) indicated that there is no significant statistical difference between the mean absorption of these three groups (P>0.05). No significant shift in SPR observed in the optical spectra (Fig. 1(b)) which also confirms the above result. So the formation process of the NPs could be performed at all three times mentioned above. But as the figure demonstrates, the highest mean absorbance of the colloid solution that is attributed to the concentration of Cu NPs [29] is observed at 5 min irradiation time (0.76 ± 0.87). Also considering that the UV–vis spectrum of Cu NPs demonstrates shifting of the λmax to shorter wavelength when the size decreases [30], NPs with desired size characteristics should be produced in 5 min holding time which had the most proper peak position(λmax = 572.6nm).
Optimization of the amount of phytoextract
The difference between groups (2 mL, 4 mL, 6 mL, and 8 mL) was significant (P≈0) in both methods. From the results of the Tukey post hoc test for microwave- assisted method it was observed that the average absorption in the volume of 6 mL (0.98 ± 0.92) was significantly higher than 4 mL (0.88 ± 0.91) and for the volume of 8 mL (0.81 ± 0.93) was more than 2 mL (0.72 ± 0.92), (P < 0.05)(Fig. 1(c)). However, the mentioned difference was not statistically significant for other groups. Tukey post hoc test for ultrasonic method showed that the volume of 4 mL had greater average absorption (1.02 ± 0.86) in comparison with 2 mL(0.91 ± 0.89), 6 mL(0.96 ± 0.85) and 8 mL(0.81 ± 0.88),(P < 0.05)(Fig. 2(c)). Presence of excess biomolecules in higher amounts (8 mL and above) ends up agglomeration of zerovalent Cu NPs rather than nucleation, which leads to the generation of less number of particles [31]. Thus the addition of 6 and 4 mL phytoextract had the most effect on average absorption in the microwave-assisted and ultrasonic method, respectively. So these amounts would be the optimum volume of phytoextract for the synthesis of Cu NPs. Since the cavitation phenomenon in the ultrasonic method leads to stimulation of the nucleation process [19] and generation of more H• radicals than normal, thereby enhancing the reduction of Cu2+ ions under the sonochemical conditions [32], the ultrasonic method requires a lower amount of capping and reducing agents (phytoextract) for the synthesis of the NPs.
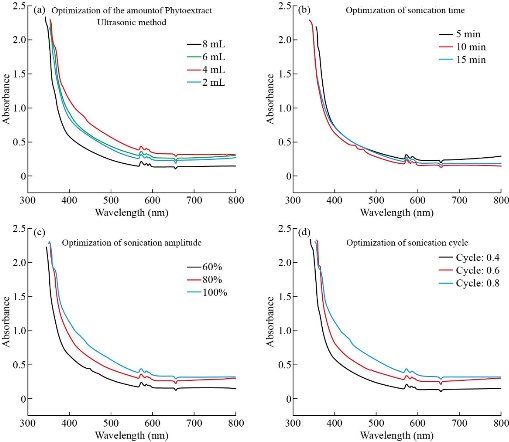
Fig. 2 UV-vis spectra of Cu NPs based on optimization parameters in ultrasonic method.
Optimization of sonication time
The output of the ANOVA test shows a significant statistical difference between the three time groups (5, 10, and 15 min) (P < 0.05). Tukey post hoc test, which performed afterward, revealed that the average absorption of the first group (5 min) (0.91 ± 0.93) is statistically more than 10 (0.81 ± 0.92) and 15 min (0.84 ± 0.89), (P < 0.05), which means that the concentration of the synthesized NPs is higher in 5 min sonication time. Therefore, we considered five min as the optimal time for the synthesis of NPs. In the previous study [27], chemical reductants were employed to ultrasound synthesis of copper oxide NPs. It was reported that the process of synthesis takes at least fifteen min and no NPs were formed within five min. This point approves the rapidity of the process as an advantage of the phyto-synthesis method. Besides, during long sonication times, bubbles produced by cavitation increase, which leads to the reduction of heat transfer rate and thus, the reaction rate decreases [19].
Optimization of sonication amplitude and cycle:
The ANOVA test results, which performed for three sonication amplitude (60%, 80% and 100%) showed that the average absorption in 60% (240 W) (0.81 ± 0.92) and 80% (320 W) (0.96 ± 0.91) was significantly lower than 100% (400 W) (1.02 ± 0.86). The previous study [27] revealed that increasing the level of ultrasonic power used during the treatment tended to reduce the size of the NPs and reached its minimum in 100% (400 W). In the pulsed mode ultrasound operation, the wave is generated in a cycle of “on” and “off” periods that usually last a few seconds. The ANOVA test results showed that the difference in mean absorption between three wave cycles (0.4 (0.81 ± 0.95), 0.6(0.93 ± 0.89) and 0.8(0.99± 0.86)) was statistically significant and was higher in 0.8. Because of the limitations in the operation of the ultrasonic processor, more than 0.8 cycles were not considered. So the parameters of the ultrasound processor, including intensity and cycle, were adjusted to 100% and 0.8, respectively.
Comparison of the two phyto-synthesis methods
Fig. 3 demonstrates a visual comparison between the two methods in case of employing the same amount of phytoextract (4 mL) and holding time (5 min). The statistical comparison between the two methods carried out based on the results of T- test between the data of the average absorption of the two groups ((0.89 ± 0.91) for microwave-assisted method and (0.92 ± 0.89) for ultrasound-assisted method) and this test revealed that there is no significant statistical difference between the mean absorption of these two groups (P>0.05) in the same amount of phytoextract. In case of the same holding time, the average absorption in ultrasound method (0.91 ± 0.93) was significantly higher than microwave method (0.76 ± 0.87) (P0.05), which means that the concentration of the Cu NPs in the colloid solution in this method is higher than the microwave-assisted technique. Besides, by looking closer to the curves in the same holding time, it can be noticed that the ultrasound-assisted method has the most proper peak position, which means that in this method the size of the Cu NPs is smaller than the microwave-assisted method.
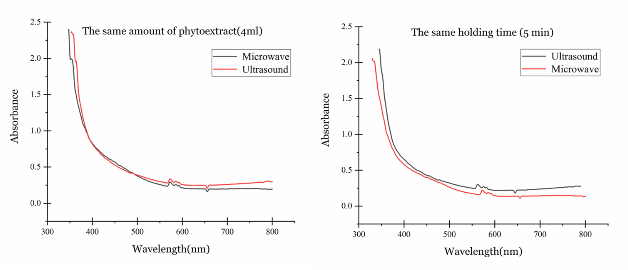
Fig. 3 Comparison of the two phyto-synthesis methods in the same amount of the phytoextract (4 mL) and holding time (5 min).
Morphology and stability study
Atomic force microscopy (AFM) was used to investigate the shape and height distribution of Cu NPs. Images show the presence of densely packed small semi-spherical particles over the entire surface of the scanned area. At some places, small NPs mingled all together, which observed as enlarged particles (Fig. 4(a) and (b)). Fig. 4(c) and (d) demonstrates the SEM micrographs of Cu NPs. The prominent performance of the capping agent is responsible for the interparticle binding of several individual small NPs. Transmission electron microscopy was employed to provide further insight into the morphology, shape, and size of the synthesized Cu NPs. The TEM images reveal that they are spherical, with the polydispersity of about 22% in microwave-assisted (Fig. 5. (a)) and 21% in ultrasound-assisted method (Fig. 5. (b)). The histograms were obtained by analyzing the images using ImageJ software are exhibiting the size distribution of the synthesized NPs. The Gaussian function was used for fitting the data of the TEM micrographs, and the mean particle size and standard deviation were respectively extracted from the center position and the width of the Gaussian. The results showed that the size of ultrasound-assisted synthesized Cu NPs is significantly lower (P < 0.05) than the microwave-assisted synthesized Cu NPs (Table1). Besides, the mean value of zeta potential of Cu NPs (Table 1) was higher in ultrasound-assisted method, but this difference was not significant. As the TEM image obviously shows, the Cu NPs are surrounded by a faint thin layer of other material, which is believed to be the organic bio components from the methanolic leaf extract acting as the capping agent that provides the stability of NPs [25].
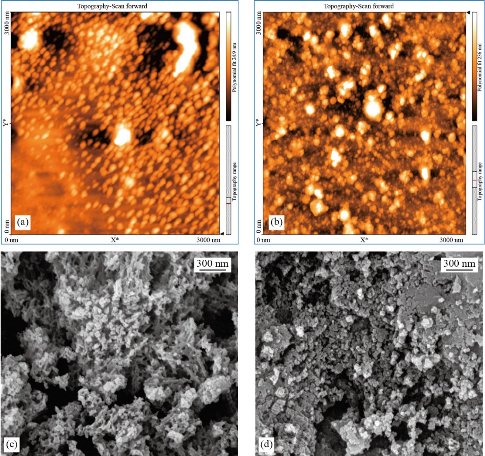
Fig. 4 (a) and (b) AFM, and (c) and (d) SEM images of Cu NPs, synthesized in the working parameters of (a) and (c) 95 °C, 5 min, 6 mL of phytoextract by microwave, and (b) and (d) amplitude:100%, cycle: 0.8, 5 min, 4 mL of phytoextract by ultrasound method.
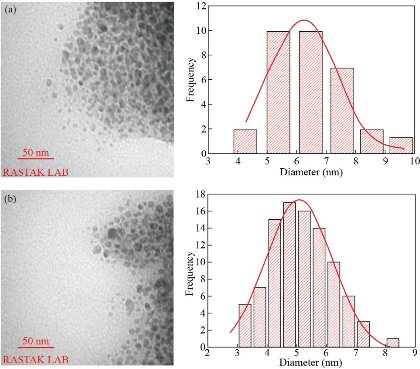
Fig. 5 TEM images and the histograms of the size distribution data that was fitted by Gaussian function (red curve): In working parameters of (a) 95 °C, 5 min, 6 mL of phytoextract by microwave, and (b) amplitude: 100%, cycle: 0.8, 5 min, 4 mL of phytoextract by ultrasound method (b).
Table 1 Characterization of Cu NPs synthesized by Fraxinus excelsior.
|
Method of the synthesis |
Average particle size (nm) |
Zeta potential (mV) |
|
Microwave |
6.30 ± 1.12 |
-27.9 ± 8.16 |
|
Ultrasound |
5.07 ± 1.13 |
-33.7 ± 7.78 |
Energy dispersive X-ray spectroscopy (EDX)
The EDX spectrum for microwave-assisted method shows Cu alone as the major element (Fig. 6(a)). While in the ultrasound-assisted method, as could be seen in Fig. 6(b), O is the most abundant element. The spectral sharp peak at around 8 keV indicates that the Cu has been correctly identified.Observation of two other sharp peaks for the element of C and O must be due to phytochemicals present in plant extract [33], which elucidates that the organic substances attached to the Cu NPs. Furthermore, we see a small peak at about 2.0 keV for P in both spectra, which may have originated from the polysaccharides. Also, two weak peaks for Al and Cl were observed, which could be a part of the biomolecules bounded to the surface of the Cu NPs [25].
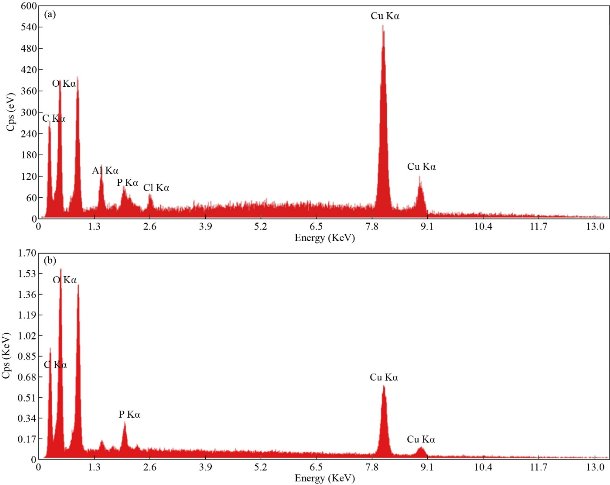
Fig. 6 EDX spectra of Cu NPs: In working parameters of (a) 95 °C, 5 min, 6 mL of phytoextract (a) by microwave and (b) amplitude: 100%, cycle: 0.8, 5 min, 4 mL of phytoextract by ultrasound method.
Table 2 The quantities of elements obtained by the EDX test
|
Method |
Element |
C |
O |
Al |
P |
Cl |
Cu |
|
Microwave |
Weight % |
24.09 |
19.58 |
3.38 |
1.88 |
0.96 |
50.11 |
|
Atomic % |
47.40 |
28.92 |
2.96 |
1.43 |
0.64 |
18.64 |
|
|
Ultrasound |
Weight % |
30.43 |
39.82 |
-- |
2.17 |
-- |
27.58 |
|
Atomic % |
45.85 |
45.03 |
-- |
1.27 |
-- |
7.85 |
Fourier transform infrared (FTIR) analysis
Fig. 7 illustrates the FTIR spectra of the microwave and ultrasound-assisted synthesized Cu NPs and Fraxinus excelsior extract. The comparison of three spectra demonstrated the similarities between them. Table 3 shows frequencies of the major peaks, band name, type of vibration, and chemical compounds that each one of them represents. The amide band (N-H), hydroxyl functional group (O-H) and carbonyl group (C=O) are respectively representatives of the proteins, alcohols, phenolic compounds, and flavonoids released by the Fraxinus leaves. The small decrease in the intensity of two bands from 1739 to 1718 and 1728 cm-1 is believed to be due to the reduction of CuSO4, which signifies the involvement of the C=O group in the reduction process. On the other hand, a band at around 1620 cm-1 is assigned to the stretching vibration of CO-(NH) group [25], which indicates the bond formation between the phyto-components and the surface of the Cu NPs as a mean of stabilization. Besides, the absorption peak below 700 cm-1, at around 609 cm-1and 611 cm-1 in the Cu NPs spectra of both methods are due to Cu-O vibrations, which indicates that Cu NPs are partially oxidized and formed CuO phase [34], that confirmed the presence of oxygen in EDX results.
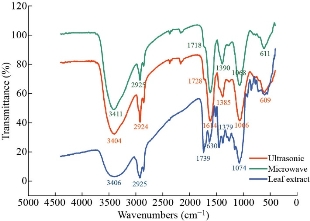
Fig. 7 FTIR spectra of Cu NPs synthesized in working parameters of 95 °C, 5 min, 6 mL of phytoextract) by microwave and amplitude:100%, cycle: 0.8, 5 min, 4 mL of phytoextract by ultrasound method and Fraxinus excelsior methanolic extract.
Table 3 The extracted data from FTIR spectra and the corresponding bands and chemical compounds.
|
Frequency (cm-1) |
Bond name, type of vibration |
Representative of |
Ref. |
||
|
Fraxinus excelsior extract |
M.Sa Cu NPs |
U.Sb Cu NPs |
|||
|
3406 |
3411 |
3404 |
O-H (Stretch) |
Carboxylic acids |
[25, 27, 35] |
|
2925 |
2925 |
2924 |
C-H Aliphatic |
Aliphatic compounds |
[25, 27, 35] |
|
1739 |
1718 |
1728 |
C=O(Stretch) |
Carboxylic acids |
[25, 27, 35] |
|
1630 |
1616 |
1614 |
N-H (Bend) |
Amide |
[25-35] |
|
1379 |
1390 |
1385 |
CH3 (Bend) |
Alkane |
[35, 36] |
|
1074 |
1068 |
1066 |
C-O (Stretch) |
Ether (C–O–C) linkage |
[25, 35, 37, 38] |
|
- |
611 |
609 |
Cu–O |
CuO |
[34, 35] |
a Microwave- assisted synthesized; b Ultrasound-assisted synthesized.
X-ray diffraction (XRD) study
The XRD pattern of Cu NPs depicted in Fig. 8. With a glance at the broad pattern of XRD, no obvious crystal peak was observed. Therefore, this pattern is ascribed to highly amorphous and bio-capped NPs and could not be indexed with any of the existing PCPDF files of Cu metal [24].
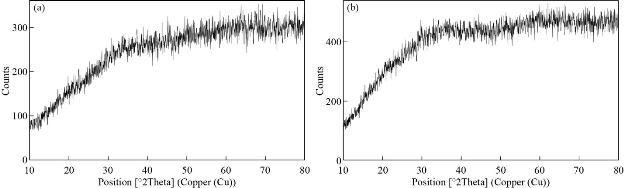
Fig. 8 XRD pattern of Cu NPs: In working parameters of (a) 95 °C, 5 min, 6 mL of phytoextract by microwave and (b) amplitude: 100%, cycle: 0.8, 5 min, 4 mL of phytoextract by ultrasound method.
Differential scanning calorimetry (DSC)
The DSC curves of the Cu NPs along with the precursor salt and Fraxinus excelsior leaf extract are shown in the Fig. 9, while the extrapolated data of melting peak temperatures (Tm), enthalpy of melting (ΔHm), crystallization peak temperature (Tc), enthalpy of crystallization (ΔHc), and phase transition temperature (Tg) are listed in Table 4 [39]. Fig. 9 elucidates that, with increasing the temperature, both groups of NPs remained stable. At around 350◦C, a fracture line was seen in the graph related to the thermal decomposition of samples [40].
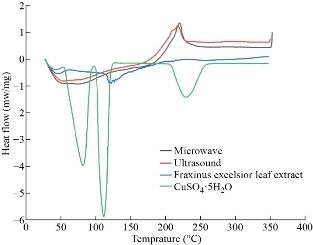
Fig. 9 The DSC curves of the Cu NPs in working parameters of (a) 95 °C, 5 min, 6 mL of phytoextract by microwave and (b) amplitude:100%, cycle: 0.8, 5 min, 4 mL of phytoextract by ultrasound method, precursor salt, and Fraxinus excelsior leaf extract.
Table 4 The extrapolated data from the DSC curves
|
Sample name |
Glass transition temperature, Tg (◦C) |
Crystallization temperature, Tc (◦C) |
Crystallization latent heat, ΔHc (mj) |
Melting temperature, Tm (◦C) |
Melting latent heat, ΔHm (mj) |
Other peaks |
|
M.Sa Cu NPs |
37 |
220 |
560 |
-- |
-- |
-- |
|
U.Sb Cu NPs |
41 |
220 |
510 |
-- |
-- |
-- |
|
Cu.SO4.5H2O |
-- |
-- |
-- |
116 |
135 |
Thermal degradation peak: 235 ◦C |
|
Fraxinus excelsior leaf extract |
-- |
-- |
-- |
127 |
40 |
Hysteresis peak: 51 ◦C |
a Microwave-assisted synthesized; b Ultrasound-assisted synthesized.
Discussion
The similarity in the shape and location of the peaks in the UV–vis spectra of both methods corroborated that the size distribution of NPs is approximately the same in two methods which is also confirmed by the results of TEM size analysis. In comparison with the previous studies, size distribution of the green synthesized Cu NPs by sonochemical method using glycerol [23] and microwave assisted method by T. arjuna bark extract [24] was respectively in the range of 80 t0 150 nm and around 18 to 23 nm, which elucidate that employment of the introduced methods in this article leads to reduction of the size of the NPs. The Cu NPs produced by ultrasonic were more uniform in size and had smaller particle size distributions, which might be due to cavitation stress in the ultrasonic process that inhibits the growth of the NPs and intensifies the nucleation [19]. The values of average roughness (Ra), provided by the AFM, showed that this parameter was 18.06 nm and 17.87nm for microwave and ultrasound methods, respectively, determining that the NPs were smooth and semi-spherical in shape. No visible cracks or pinholes were visualized in the NPs. Spherical NPs, which synthesized in this article, have relatively less toxicity and also have more convenient and faster endocytosis when compared with NPs in the shape of rod or fibers, which is beneficial for biological applications [41]. They also had more suitable zeta potential, which makes them more stable in the colloidal environment. Recent studies have reported that NPs produced by phyto-synthesis method are generally more stable than the microorganism-mediated method [1], that’s because of the presence of surrounding phytocomponents that act as the capping agents. They also have smaller diameter than some crystalline NPs generated by bacterial species [42, 43]. Analysis of the constituent elements of the NPs by the EDX method shows that the weight percent of oxygen in the ultrasound-assisted synthesized NPs is almost twice that of the microwave-assisted ones and makes up about two-fifths of the corresponding sample’s weight. The EDX spectra of the similar study using ultrasound and Brown Alga extract demonstrated the same results [44]. This finding is in line with the fact that the sonochemical method yields a mixture of metallic copper and copper oxide NPs which can be attributed to the partial oxidation of copper by in situ generated H2O2 under the sonochemical conditions, which leads to the generation of H• and OH• radicals [32]. The FTIR spectra indicated that there is an appropriate interaction between the capping agents and synthesized Cu NPs and they were interestingly stabilized by biomolecules of the leaf extract including coumarins, flavonoids, phenolic compounds, and polysaccharides, which leads to acceptable stability of NPs in colloidal solution. Zeta potential outputs also confirmed these results. XRD test demonstrated that the NPs synthesized by both microwave and ultrasound methods are structurally amorphous (even though they were subjected in 75 °C for 12 hours as a calcination post-treatment) and this characteristic makes them interestingly suitable for transdermal applications. Since NPs with amorphous structure often dissolve more rapidly than their crystalline counterparts [45, 46] and the amorphous state could provide better diffusion through the skin surface [47]. DSC test outputs also confirm the above results. Charts of differential scanning calorimetry represent that unlike the crystallinity of the precursor salt (intrinsic property) and Fraxinus excelsior leaf extract (as a result of the lyophilization process), NPs were amorphous which supports the results of the XRD test. We also tracked down the reasons of this amorphous structure focusing on the precursor, solvent, and employed methods in the process of the synthesis. It is generally observed that using volatile precursors in the sonochemical synthesis of NPs yields amorphous products because in this condition decomposition of the volatile precursor (and reduction of Cu2+ ions) occurs inside the bubble (generated through the cavitation) and quenching rate experienced by the products is very high (108 K/s) which makes ultrasound technology a great method for synthesis of amorphous metal NPs [32]. The solvent selection is also an important factor defining the structural features of the product. For example, Suslick and co-workers [48] have prepared amorphous iron by the sonochemical decomposition of metal carbonyls in an alkane solvent. Kumar and co-workers [49] also used an organic solvent (aniline) along with the ultrasound method to produce amorphous Cu. Furthermore, in the microwave-assisted method, organic chemicals like polyols are desirable solvents because a relatively high dipole moment makes them very proper susceptors of microwave radiation. Previous works reported that employing inorganic chemicals along with microwave irradiation has mostly led to crystalline materials [50]. Bio capping potential of phytoextract, which performs as a stabilizing and protective agent for NPs could also be another determining factor in achieving amorphous structure. In most cases, appending a calcination stage to the drying procedure leads to the elimination of the biomolecules and increases the crystallinity of the NPs [24]. Since both of these methods have their advantages, the appropriate method can be selected according to the expected characteristics of the target product, its subsequent applications, and the properties of the used solvent, capping and reducing agents (phytoextract). The synthesis process takes about the same time, and due to proper heat generation through both of these methods, the reduction process of copper ions by the phytomolecules of the extract could be interestingly accelerated. But because of the more accurate temperature control mechanism of the microwave method, this method is more suitable for plant extracts with a majority of heat-sensitive and volatile components such as terpenoids, phenolic compounds, and aldehydes. In the case of using volatile solvents, ultrasonic is the most appropriate way to obtain amorphous NPs. Also based on the EDX outputs, because of the oxidation followed by the cavitation phenomenon which leads to partial oxidation of copper, more copper oxide NPs are produced in the ultrasonic method which is a favorite product for Biomedical, antimicrobial, solar sell, optical sensor, and water purification applications. The optimum conditions for phyto-synthesis of Cu NPs, was obtained to be 5 min microwave irradiation in 95◦C with addition of 6 mL phytoextract in the first method, that in comparison with the similar study, our method is relatively faster and requires less amount of phytoextract to complete the reduction process in the same temperature [24]. In the second method, phyto-synthesis of Cu NPs carried out by sonication in 0.8 cycle and 100% amplitude by adding 4 mL of phytoextract in only 5 min, which is far less time consuming in comparison with similar methods using chemical reagents[22,27] and alga extract[44] in the same amplitude.
Conclusions
The process of choosing the appropriate auxiliary method for the phyto-synthesis of NPs is usually performed based on the expected characteristics of the final products, and therefore, a comparison between the properties of the products gained from each method can be beneficial. Documented facts were reported to facilitate selection procedure between the two methods. In this project, amorphous sub 10 nm Cu NPs with proper colloidal stability were synthesized in only 5 min without using of any crystallization inhibitors with microwave and ultrasound-assisted technique. In conclusion, the microwave-assisted method can be used as a suggested method for synthesis in polyol solvents or employing phytoextracts containing heat-sensitive materials. In the ultrasonic method, the required amount of the phytoextract is relatively lower than microwave method and also, because of the oxidation conditions, the most common way to obtain amorphous metal oxide NPs is the sonication method. Besides, Cu NPs produced by the ultrasound-assisted method had smaller size in comparison with the microwave-assisted method. Therefore, the ultrasonic could be suggested as the more efficient method for phytosynthesis of Cu NPs. Cu NPs will have greater dermal absorption in the amorphous state, which will ultimately make them an eligible wound healing agent. This conclusion can facilitate the selection of the most appropriate method for the synthesis of NPs according to the type of desired product, as well as introducing the two facile methods along with the optimum conditions to synthesis amorphous Cu NPs.
Conflict of Interests
The authors declare that no competing interest exists.
References
1. M. Nasrollahzadeh, M. Atarod, M. Sajjadi, et al., Plant-mediated green synthesis of nanostructures: mechanisms, characterization, and applications. Interface Sci. Technol., 1st edition. Elsevier Ltd., 2019:199-322.
2. P. Singh, Y.J. Kim, D. Zhang, et al., Biological synthesis of nanoparticles from plants and microorganisms. Trends Biotechnol, 2016, 34(7): 588-599.
3. I. Kostova, T. Iossifova, Chemical components of Fraxinus species. Fitoterapia, 2007, 78(2): 85-106.
4. J.N. Solanki, R. Sengupta, Z.V.P. Murthy, Synthesis of copper sulphide and copper nanoparticles with microemulsion method. Solid State Sci, 2010, 12(9): 1560-1566.
5. S. Singh, N. Kumar, M. Kumar, et al., Electrochemical sensing and remediation of 4-nitrophenol using bio-synthesized copper oxide nanoparticles. Chem Eng J, 2017, 313: 283-292.
6. G. Polino, R. Abbel, S. Shanmugam, et al., A benchmark study of commercially available copper nanoparticle inks for application in organic electronic devices. Org Electron physics, Mater Appl, 2016, 34: 130-138.
7. M. Ge, R. Gondosiswanto, C. Zhao, Electrodeposited copper nanoparticles in ionic liquid microchannels electrode for carbon dioxide sensor. Inorg Chem Commun, 2019, 107: 107458.
8. D. Gupta, S.R. Meher, N. Illyaskutty, et al., Facile synthesis of Cu2O and CuO nanoparticles and study of their structural, optical and electronic properties. J Alloys Compd, 2018, 743: 737-745.
9. M.A. Qader, A. Vishina, L. Yu, et al., The magnetic, electrical and structural properties of copper-permalloy alloys. J Magn Magn Mater., 2017, 442: 45-52.
10. E.M. Bakhsh, F. Ali, S.B. Khan, et al., Copper nanoparticles embedded chitosan for efficient detection and reduction of nitroaniline. Int J Biol Macromol, 2019, 131: 666-675.
11. P.J. Fule, B.A. Bhanvase, and S.H. Sonawane, Experimental investigation of heat transfer enhancement in helical coil heat exchangers using water based CuO nanofluid. Adv Powder Technol, 2017, 28(9): 2288-2294.
12. J. John, R.M. Mathew, I. Rejeena, et al., Nonlinear optical limiting and dual beam mode matched thermal lensing of nano fluids containing green synthesized copper nanoparticles. J Mol Liq, 2019, 279: 63-66.
13. N. Cioffi, L. Torsi, N. Ditaranto, et al., Copper nanoparticle/polymer composites with antifungal and bacteriostatic properties. Chem Mater, 2005, 17(21): 5255-5262.
14. K.Y. Yoon, J. Hoon Byeon, J.H. Park, et al., Susceptibility constants of Escherichia coli and Bacillus subtilis to silver and copper nanoparticles. Sci Total Environ, 2007, 373(2-3): 572-575.
15. A. Gopal, V. Kant, A. Gopalakrishnan, et al., Chitosan-based copper nanocomposite accelerates healing in excision wound model in rats. Eur J Pharmacol, 2014, 731: 8-19.
16. S. Hamdan, I. Pastar, S. Drakulich, et al., Nanotechnology-Driven Therapeutic Interventions in Wound Healing: Potential Uses and Applications. ACS Cent Sci, 2017, 3(3): 163-175.
17. A. Enayati-Fard, R. Akbari, J. Saeedi, et al., Preparation and Characterization of Atenolol Microparticles Developed by Emulsification and Solvent Evaporation. Lat Am J Pharm, 2019, 38(7): 1342-1349.
18. J.J. Hinman, K.S. Suslick, Nanostructured Materials Synthesis Using Ultrasound. Top Curr Chem, 2017, 375(1): 59-94.
19. J. Cheeke, Fundamentals and applications of ultrasonic waves, 2nd edition. CRC Press, 2016.
20. R.R. Mishra, A.K. Sharma, Microwave-material interaction phenomena: Heating mechanisms, challenges and opportunities in material processing. Compos Part A Appl Sci Manuf, 2016, 81: 78-97.
21. J. Sun, W. Wang, and Q. Yue, Review on microwave-matter interaction fundamentals and efficient microwave-associated heating strategies. Materials (Basel), 2016, 9(4).
22. T. Abiraman, E. Ramanathan, G. Kavitha, et al., Synthesis of chitosan capped copper oxide nanoleaves using high intensity (30 kHz) ultrasound sonication and their application in antifouling coatings. Ultrason Sonochem, 2017, 34: 781-791.
23. M.A. Bhosale, B.M. Bhanage, A simple approach for sonochemical synthesis of Cu2O nanoparticles with high catalytic properties. Adv Powder Technol, 2015, 2(3).
24. S. Yallappa, J. Manjanna, M.A. Sindhe, et al., Microwave assisted rapid synthesis and biological evaluation of stable copper nanoparticles using T . arjuna bark extract. Spectrochim Acta Part A Mol Biomol Spectrosc, 2013, 110: 108-115.
25. M. Parveen, F. Ahmad, A. Mohammed, et al., Microwave-assisted green synthesis of silver nanoparticles from Fraxinus excelsior leaf extract and its antioxidant assay. Appl Nanosci, 2015.
26. S. Jain. Bentley ’ s Textbook of pharmaceutics, 1st edition. Elsevier, 2011: 776.
27. H. Dang, R.K. Brundavanam, G.E. Jai, et al., Rapid sonochemical synthesis and characterisation of copper oxide nanoparticles from Schweizer’s reagent. Alkhaer Pub, 2015.
28. E. Hutter, J.H. Fendler, Exploitation of localized surface plasmon resonance. Adv Mater, 2004, 16(19): 1685-1706.
29. P.M. Lisiecki I, Synthesis of copper metallic clusters using reverse micelles as microreactors. Am Chem Soc, 1993, 115(4): 3887-3896.
30. S. Peng, J.M. McMahon, G.C. Schatz, et al., Reversing the size-dependence of surface plasmon resonances. Proc Natl Acad Sci USA, 2010, 107(33): 14530-14534.
31. N. Sreeju, A. Rufus, and D. Philip, Microwave-assisted rapid synthesis of copper nanoparticles with exceptional stability and their multifaceted applications. J Mol Liq, 2016.
32. N.A. Dhas, C.P. Raj, and A. Gedanken, Synthesis, characterization, and properties of metallic copper nanoparticles. Chem Mater, 1998, 10(5): 1446-1452.
33. M. Valodkar, R.N. Jadeja, M.C. Thounaojam, et al., Biocompatible synthesis of peptide capped copper nanoparticles and their biological effect on tumor cells. Mater Chem Phys, 2011, 128(1-2): 83-89.
34. M.U. Anu Prathap, B. Kaur, and R. Srivastava, Hydrothermal synthesis of CuO micro-/nanostructures and their applications in the oxidative degradation of methylene blue and non-enzymatic sensing of glucose/H2O2. J Colloid Interface Sci, 2012, 370(1): 144-154.
35. D.L.Pavia, G.M. Lampman, and G.S.K. Jr, Introduction to spectroscopy, 2001: 680.
36. J. Xiong, Y. Wang, Q. Xue, et al., Synthesis of highly stable dispersions of nanosized copper particles using l-ascorbic acid. Green Chem, 2011, 13(4): 900-904.
37. S. Jain, A. Jain, P. Kachhawah, et al., Synthesis and size control of copper nanoparticles and their catalytic application. Trans Nonferrous Met Soc China (English Ed), 2015, 25(12): 3995-4000.
38. A. Rajan, V. Vilas, and D. Philip, Studies on catalytic, antioxidant, antibacterial and anticancer activities of biogenic gold nanoparticles. J Mol Liq, 2015, 212: 331-339.
39. M. Genc, B. Inci, Z.K. Genc, et al., Preparation and investigations of thermal properties of copper oxide, aluminium oxide and graphite based on new organic phase change material for thermal energy storage. Bull Mater Sci., 2015, 38(2): 343-350.
40. A.R. Barron, Physical methods in chemistry and nano science. Connexions, Rice University, 2012: 702.
41. M.A. Gatoo, S. Naseem, M.Y. Arfat, et al., Physicochemical properties of nanomaterials : Implication in associated toxic manifestations. 2014, 2014.
42. M. Rad, M. Taran, and M. Alavi, Effect of incubation time, CuSO4 and glucose concentrations on biosynthesis of copper oxide (cuo) nanoparticles with rectangular shape and antibacterial activity: taguchi method approach. Nano Biomed Eng, 2018, 10(1): 25-33.
43. M.I. Nabila, K. Kannabiran, Biosynthesis, characterization and antibacterial activity of copper oxide nanoparticles (CuO NPs) from actinomycetes. Biocatal Agric Biotechnol, 2018, 15: 56-62.
44. H. Gu, X. Chen, F. Chen, et al., Ultrasound-assisted biosynthesis of CuO-NPs using brown alga Cystoseira trinodis: Characterization, photocatalytic AOP, DPPH scavenging and antibacterial investigations. Ultrason Sonochem, 2018, 41: 109-119.
45. Y. Kawabata, K. Wada, M. Nakatani, et al., Formulation design for poorly water-soluble drugs based on biopharmaceutics classification system: Basic approaches and practical applications. Int J Pharm, 2011, 420(1): 1-10.
46. R. Jog, D.J. Burgess, Pharmaceutical amorphous nanoparticles. J Pharm Sci, 2017, 106(1): 39-65.
47. G.B. Romero, A. Arntjen, C.M. Keck, et al., Amorphous cyclosporin A nanoparticles for enhanced dermal bioavailability. Int J Pharm, 2016, 498(1-2): 217-224.
48. K.S. Suslick, S.B. Choe, A.A. Cichowlas, et al., Sonochemical synthesis of amorphous iron. Nature, 1991, 353(6343): 414-416.
49. R.V. Kumar, Y. Mastai, Y. Diamant, et al., Sonochemical synthesis of amorphous Cu and nanocrystalline Cu2O embedded in a polyaniline matrix. J. Mater. Chem., 2001: 1209-1213.
50. S. Makhluf, R. Dror, Y. Nitzan, et al., Microwave-assisted synthesis of nanocrystalline MgO and its use as a bacteriocide. Adv Funct Mater, 2005, 15(10): 1708-1715.
Copyright© Farinaz Hadinejad, Mohsen Jahanshahi, and Hamed Morad. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.