Aspirin in Food Samples for Separation and Micro Determination of Copper(II) Using Cloud Point Extraction/Solvation Method
Ebaa Adnan Azooz 1 *, Jihan Razzaq Moslim 2, Safa Mageed Hameed 2, Shawket Kadhem Jawad 2, Emad Abbas Jaffar Al-Mulla 3 *
1 The Gifted Students` School in Najaf. The General Directorate of Education Al-Najaf, Ministry of Education, Iraq.
2 Chemistry Department, Faculty of Education for Women, University of Kufa, Al-Najaf, Iraq.
3 Pathological Analysis Techniques, Faculty of Health and Medical Techniques, Al-Furat Al-Awsat Technical University, An-Najaf, Iraq.
* Corresponding authors. E-mail: Ebaaadnan.ed12p@uokufa.edu.iq; almullaemad@gmail.com
Received: Jun. 16, 2020; Accepted: Oct. 27, 2020; Published: Jan. 20, 2021
Citation: Ebaa Adnan Azooz, Jihan Razzaq Moslim, Safa Mageed Hameed, Shawket Kadhem Jawad, and Emad Abbas Jaffar Al-Mulla, Aspirin in Food Samples for Separation and Micro Determination of Copper(II) Using Cloud Point Extraction/Solvation Method. Nano Biomed. Eng., 2021, 13(1): 62-71.
DOI: 10.5101/nbe.v13i1.p62-71.
Abstract
In this work, sensitive method was used for the first time by joining cloud point extraction with solvation system (CPE-SS) using aspirin as the organic reagent to form cloud point layer (CPL). It had a wavelength for final absorbance under λmax = 293 nm. Different kinds and concentrations of salting out with different extraction efficiencies were used to enhance the extraction efficiency. KNO3 as a salting out under 0.1 M showed maximum extraction efficiency in the presence of 40 µg of Cu(II). The applications of extraction in this method had required the existence of 0.4 mL of Triton X-100 and heating under 85 °C for 20 minutes to form CPL quantitatively. The effect of different surfactants and various organic reagents to investigate the impact of interferences as well as spectrophotometric determination were also investigated in this study. Chicken, breast, cow meat (beef), cucumber, drainage fish, garden cress and lettuce showed low limit of detection (LOD) 0.018 ppm, limit of quantity (LOQ) 0.060 ppm and RSD% of 0.028 in 2 ppm.
Introduction
Copper is very necessary for the activity of several enzymes. Copper and iron partially require for hematopoiesis for the reason that it is required for ferroxidase (ceruloplasmin) synthesis. In biological systems, copper has an oxidation state of (I or II). The copper quantity in the adult humanoid body is about 70–100 mg. The maximum concentrations in increasing sequence are in the kidney, heart, brain, and liver. About 50% of total copper are found in Muscle. It is absorbed from food in the upper small intestine [1-4]. Aspirin (acetylsalicylic acid) is among the cheaper and most available medicines widely used over the entire world for relief of headaches, heat and muscular pains. Salicylate drug can be in effect in lessening the risk of numerous cancers, especially in lung and colon by its functions as an antioxidant and capability to extremists [5, 6]. Different techniques were examined for copper pre-concentration and separation in different environmental and organic matrices. Methods as solvent micro extraction [7,8], also used another ion [9], solid phase extraction (SPE) [10], inductively coupled plasma mass spectrometry [11], ion chromatography [12], flame and graphite furnace atomic absorbing spectrometry [13] and spectrophotometric methods [14-15] and cloud point extraction (CPE) [16], [17] were described for this purpose. Nevertheless, extraction methods such as LLME or SPE, have most amounts of biological solvent that are particularly toxic volatile, flammable and destructive to the surroundings [18], or large volumes of eluents to guarantee the whole back-extraction of the interested analytes, correspondingly. CPE has appeared as an in effect, sensitive and greener alternative to LLME and SPE [19]. CPE is in general performed by means of solutions of a solitary or a combination of surfactants beyond their critical micelle concentrations (CMC). According to the CPE process of the analytes into the micelle layer, conforming can be obtained by intensifying the temperature beyond the critical temperature (CT). This increased temperature causes the cloud point layer (CPL) formation that is the dehydration consequence of the polar surface of the micelles. It produces a good potential amid micelles. The CPL can be categorized by a smaller volume as compared with the one of the aqueous sample in which preconcentration the analytes of interest [20-22]. Many researchers have reported that successful CPE to separate and determine Cu and other elements in different real samples such as food CPE coupled with flame atomic absorption spectrometry [23] and water combined with ICP-MS [24], by using many surfactants. Amoxicillin can be applied as a ligand to the determine copper(II) by CPE joined with spectrofluorometric in the presence of Triton X-114 as the surfactant [25]. Applied to extract Cu, Co and Ni in water testers using 2-(5-bromo-2-pyridylazo)-5-(diethyl amino) phenol (5-Br-PADAP) as ligand and Triton X-114 [26]. The study indicates that the CPE has excellent potential in Nano-materials removal from wastewater Triton X-114 suitable for the recovery of Nano sized Copper oxide (NCO) from the water. Removing process of NCO has been affected by the concentrations of Triton X-114 and salt, solution pH as well as incubation temperature and time [27]. In successful studies, coupled CPE with other extraction method such as solvation method (CPE-SS) was used to extract and determine Ce (III) salting out of KNO3 by means of organic reagent 2, 4-Dimethyl pentane-3-one (2, 4-DMP) as extractant and 1% Triton X-100 [28]. Or onium system (CPE-OS) that used to separate and determine micro amount of iron and mercury by means of Nα-Benzoyl-L-arginine ethyl ester hydrochloride (BAEE) from acidic hydrochloric aqueous solution as extractant and non-ionic surfactant 1% Triton X-100. This procedure has low detection limit [29], and liquid-liquid extraction (CPE-LLE) procedure combined with CPE for the extraction trace amount of ions such Ni(II) [30] and Cu(II) in drinking water and serum samples. This method is related to ionic liquid assisted micro emulsion (IL-µE-DLLME) by means of ionic liquid 1-butyl-3-methylimidazolium hexafluorophosphate ([C4mim] [PF6]). Copper has been reacted with 8-hydroxyquinoline and Triton X-100 as the surfactant [31]. In this work, a sensitive CPE-SS technique has employed the water as the solvents without using any amounts of the organic solvents for feasible using surfactants in analytical chemistry. Instantaneous determination and preconcentration of Cu(II) using aspirin as a selective complexing agent and Triton X-100 were investigated.
Experimental
Materials
All chemicals used in this study were from Merck, Germany. Standard solution was prepared using 1 mg/mL of Cu (NO3)2 in 100 mL of distilled water. The solution aspirin ligand was prepared in distilled water with Triton X-100 as a surfactant.
Instruments
A double beam Biochrom spectrophotometer with 1.00 cm quartz cell and WNB7-45 water bath were used for spectroscopic studies and CPT heating along, Dool, CE, HR 200, Japan.
Preparation of samples
All samples under study were taken from local supermarkets and different places of Euphrates river, An-Najaf, Iraq. A 5 g of a dried sample was places in a 200 mL conical flask with 10 mL of concentrated nitric acid. The mixture was heated at 70 ᵒC for 20 min, cooled and then another of 10 mL of concentrated nitric acid, 5 mL of concentration sulpheric acid and 4 mL hydrogen peroxide were added. After that, the mixture was mixed with a magnetic stirrer and reheated at 85 ᵒC for 20 min, then 10 mL of water was added till it is colorless indicate that organic matter oxidation. A 100 mL volumetric flask was used to transfer the cooled product with adding distilled water to the mark. A 5 mL of final product was diluted with distilled water to 25 mL conical flask, then added 1 mL of 20% potassium sodium tartrate. The mixture was filtered and transferred to 10 mL volumetric flask and added distilled water for diluting to the mark.
General method
Aqueous solutions (10 mL each) in volume have the finest quantity of Cu(II) and optimal concentration of KNO3 as salting out with 1×10-4 M of aspirin in the existence suitable volume of surfactant. The solutions heated in a water bath for relevant temperature, time of heating up to cloud point layer (CPL) realization. The absorption of a solvation species is determined at λmax = 293 nm. Nonetheless, the aqueous solution has been handled along with dithiazone spectrophotometric process. Then, go back to the calibration curve with the purpose of determination the remainder amount of Cu(II) in aqueous phase next to extraction. Afterward, subtract from (40 µg) to evaluate the transferred quantity of Copper into CPL as solvation species. Subsequently, compute distribution ratio (D) in relation to Equation (1)
![]() 1)
1)
Results and Discussion
The spectrophotometric study shows that solvation species of Cu(II) with Aspirin extracted into CPL have a wavelength of maximum absorbance equal to of 293 nm (Fig. 1).
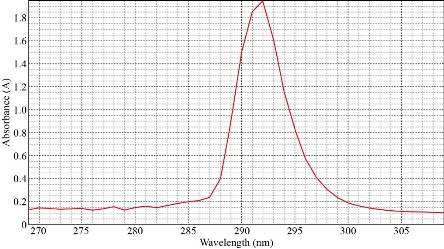
Fig. 1 UV-Vis absorption spectrum of solvation species of Cu(II) with aspirin
Factors influencing extraction efficiency
Type and concentration of salting out
Each sample of aqueous solutions (10 mL) was used in volume comprises (50 µg) of Cu(II) and increasing concentration of different salting out levels in existence of 0.5 mL of 1% Triton X-100, 1×10-4 M of aspirin, the solutions has been heated for appropriate temperature and period up to CPL formation. At that point, separated CPL and stream CPL in 5 mL of alcohol. λmax = 293 nm was appeared for alcoholic absorption against blank without Cu(II) prepared in the same manner. Aqueous phase has treated concerning dithiazone spectrophotometric method consistent with general technique. The consequences have been depicted in Fig. 2 and 3. The best concentration was evidenced at 0.1 M for all salting out levels that given higher extraction efficiency of Cu(II) as solvation species with aspirin. That emphasizes the role of kind and concentration salting out on extraction efficiency. They are considered as a thermodynamic factor and control the rate of formation of solvation species.
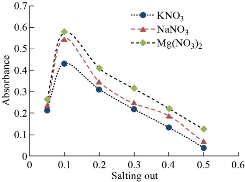
Fig. 2 Effect of type and concentration of salting out on formation solvation species of Cu(II).
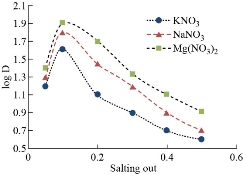
Fig. 3 Effect of type and concentration of salting out on extraction efficiency of Cu(II) and D- value.
Copper concentration
A 10 mL of each sample in Cu(II) and 0.1M of KNO3 as salting were used in the presence of 1×10-4 M of aspirin and 0.5 mL of surfactant. Electrostatic water bath was used to heat the solutions at 85 ᵒC and for 20 min. Accomplish the experiment in line with the general process. The outcomes have been clarified in Fig. 4 and 5. As shown in Fig. 4 and 5, there is a linear relationship within the concentration of Cu(II) and extraction efficiency until optimal concentration 40 µg/mL that gives greater efficiency. Increasing in concentration of Cu(II) gives an intensification in the rate of formation of solvation species. More concentration than optimal magnitude has effects of diminishing extraction efficiency because it raises the rate of backward direction of thermodynamic steadiness and lessens solvation species formation concerning mass action law.
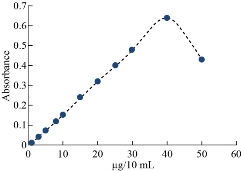
Fig. 4 Effect of Cu(II) concentration on stability of solvation species.
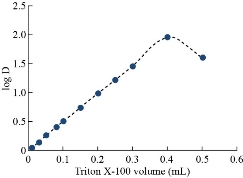
Fig. 5 Effect of Cu(II) concentration on D-values.
Surfactant volume
By following general method, the absorbance curves of 40µg of Cu(II) in 10 mL in aqueous solutions in existence of 1×10-4 M of aspirin and 0.1M of KNO3 as salting out with increasing volumes of 1% Triton X-100 as a surfactant were shown in Fig. 6 and 7. Higher extraction efficiency was observed with finest surfactant volume (0.4 mL) which accomplishes our status of analytical micelles concentration CMC to create the finest CPL with the finest features to extract solvation species quantitatively. Any volume less than the finest level was not tolerable for CMC to facilitate any volume more than optimum volume effect of dropping extraction efficiency since declining CPL properties is in effect of diffusion of micelles.
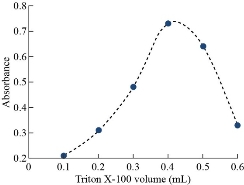
Fig. 6 Effect of surfactant volume on formation CPL with best properties.
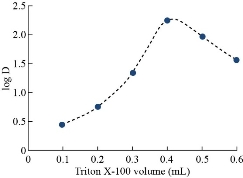
Fig. 7 Effect of surfactant volume on extraction efficiency and D-values.
Temperature
The general method was applied to determine the rising temperature for Cu(II). The absorbance consequences are shown in Figs. 8 and 9. Afterword, extraction constant (Kex) is possibly calculated in relation to equation (2). The results were as in Fig. 12.
![]()
The results of thermodynamic data for extraction Cu(II) according a straight slope line (Fig. 10) and relations of thermodynamic (Table 1). They were shown formation solvation species with endothermic reaction. The low value of enthalpy of extraction (∆Hex) with good binding for solvation species with high stability were observed. Nevertheless, the high value of entropy of extraction (∆Sex) demonstrates that the extraction method depends on entropy in the formation of solvation species.
Table 1 Thermodynamic data for extraction Cu(II) according to applied method CPE.
|
∆Hex KJ / mole |
∆Gex KJ / mole |
∆Sex J / mole. K |
|
0.1759 |
-71.625 |
200.000 |
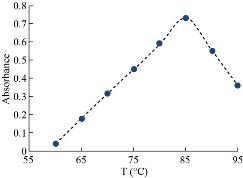
Fig. 8 Effect of temperature on formation CPL.
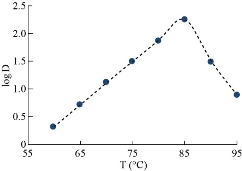
Fig. 9 Effect of temperature on extraction and D-values.
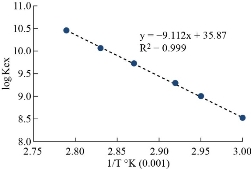
Fig. 10 Temperature based on extraction constant of Cu(II) in solvation species.
Heating time
Each sample of aqueous solutions (10 mL) was at the optimum condition of 40µg of Cu(II) in different heating times. The absorbance results were shown in Fig. 11 and 12. It was observed that the most likely heating time was 20 min. Heating period stands for the kinetic side of extraction provides the necessary energy for aggregation micelles with proper dehydration. More time in optimal value increases micelles diffusion and decreases extraction efficiency.
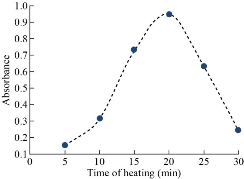
Fig. 11 Relation of heating time and formation of CPL.
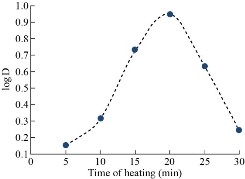
Fig. 12 Heating time with D- values.
Effect of surfactant kind
Each sample of aqueous solutions (10 mL) was at the optimum condition of 40 µg of Cu(II), 0.1 M of KNO3, 1×10-4 M of aspirin and 0.5 mL of different surfactants. The results reveal that there was a significant relationship between surfactant kind and extraction efficiency that used tweens appear lower in Distribution ratio (D). Different surfactants give different CPL with different features and various extraction efficiencies to be different in micelles structure as well as each surfactant needs special conditions to form CPL. Tween-40 gives high D value that may use in other studies after controlled conditions reaction.
Table 2 Effect of surfactant kind on extraction efficiency of Cu(II) according to solvation system
|
Surfactant |
Absorbance λmax = 293 nm |
Distribution ratio (D) |
|
Tween-40 |
0.410 |
85.670 |
|
Tween-20 |
0.310 |
72.430 |
|
Tween-80 |
0.222 |
31.57 |
Effect of organic reagent
The sample (10 mL) was treated at optimum condition of 40 µg of Cu(II), 0.1 M KNO3, 0.4 mL of surfactant Triton X-100 and 1×10-4 M of different organic reagents. The results were in Table 3. It was illustrated that organic reagent was very imperative in the formation of solvation species. Different absorbance and D- values with different organic reagents with new solvation species were observed. It has a specific wavelength for maximum absorbance with λmax =293 nm so that each organic reagent needs special conditions to form solvation species. The depictions of the spectrum for each organic reagents were shown in Fig. 13-15.
Table 3 Effect of organic reagents on extraction efficiency of Cu(II) according to solvation system
|
Organic reagent |
λ max(nm) |
Absorbance |
D |
|
Tributyle phosphate (TBP) |
291 |
1.701 |
65.160 |
|
BAEE |
292 |
1.850 |
72.720 |
|
Aspirin |
293 |
1.951 |
91.200 |
|
Rhodamine 6-G |
555 |
2.308 |
180.740 |
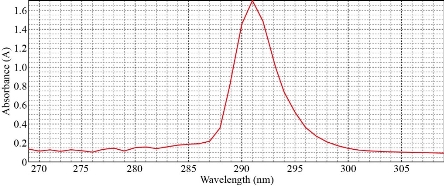
Fig. 13 Solvation method with TBP.
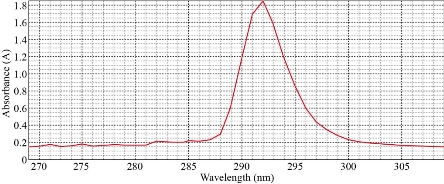
Fig. 14 Solvation method with BAEE.
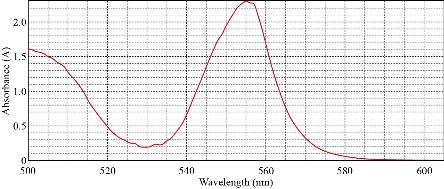
Fig. 15 Solvation method with Rhodamine 6-G.
Interferences effect
All samples contain 40 µg of Cu(II), 0.1 M of KNO3, 0.4 mL of surfactant Triton X-100 and 1×10-4 M of aspirin as organic reagents in existence of 0.01 M of different foreign metal cations in 10 mL solution. The wavelength for maximum absorbance was with λmax =293 nm. The results have been summarized in Table 4. It was shown presence of all foreign metal cations in side by side with Cu(II) to decrease extraction efficiency of Cu(II). This interferences behavior was because these metal cations participate Cu(II) in the formation of solvation species. This indicates the consumption of some salting out levels of KNO3 and aspirin. Decreases of the concentration of KNO3 and aspirin down the necessary for the formation of solvation species of Cu(II) and declines the extraction efficiency of Cu(II) [32].
Table 4 Effect of interferences on extraction efficiency of Cu(II) according to solvation system
|
Interferences |
Absorbance |
Distribution value (D) |
|
Cd(II) |
0.624 |
61.81 |
|
Zn(II) |
0.574 |
58.56 |
|
Ni(II) |
0.486 |
46.33 |
|
Co(II) |
0.741 |
64.42 |
|
Pb(II) |
0.544 |
62.61 |
|
Hg(II) |
0.422 |
38.27 |
Stoichiometry
In order to know the most likely structure of extracted solvation species into CPL, follow two types of spectrophotometric methods based on slope analysis and slope ratio. The consequences of these two methods were explained in Figs. 16-18. The results illustrated that there is a solitary molecule of aspirin binding with nitrate compound of Cu(II) [aspirin; Cu (NO3)2].
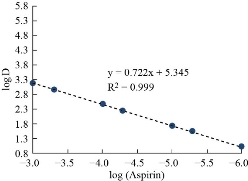
Fig. 16 Slope analysis method.
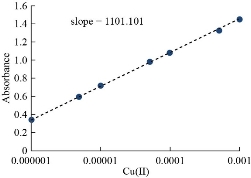
Fig. 17 Slope ratio method effect of Cu(II) Concentration.
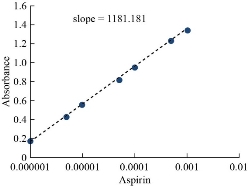
Fig. 18 Slope ratio method effect of aspirin concentration.
Analysis of food samples
The developed procedure for separation and determination of Cu(II) were from various foods to test its applicability. The masking agents (KI and KF) well be added in 0.01M. The fallouts are explained in Fig. 19. Table 5 has details of the analytical parameters of the calibration curve of this applied method (CPE-SS). Table 6 gives information regarding amount of Cu(II) by applied method (CPE-SS) in the food and water samples [33].
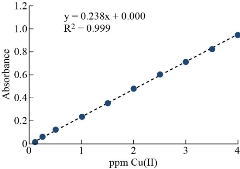
Fig. 19 Calibration curve of applied method to determine Cu(II) in real samples.
Table 5 Method validation of the determination of Cu(II) using CPE procedure according to solvation system
|
Parameter |
Cu(II) |
Parameter |
Cu(II) |
|
λmax (nm) |
293 |
LOD (ppm) |
0.0180 |
|
Degree of freedom |
8 |
LOQ (ppm) |
0.0600 |
|
Determination coefficient (R2) |
0.999 |
Molar absorptivity (L/ mol.cm) |
66306.80 |
|
RSD% (n = 6) at 2 ppm |
0.028 |
Sandell’s sensitivity (µg/cm2) |
42 × 10-10 |
|
Regression equation with extraction |
Y = 0.2382X + 0.0009 |
Slope |
0.2382 |
Table 6 Determination of Cu(II) in real samples according to applied method
|
No. |
Samples |
Dithiazone method |
Applied method* |
RSD% |
No. |
samples |
Dithiazone method |
Applied method* |
RSD% |
|
1 |
Celery |
2.00 |
1.99 |
0.31 |
7- |
Drainage fish |
1.10 |
1.20 |
0.40 |
|
2 |
Chicken (Breast) |
1.20 |
1.19 |
0.54 |
8- |
Garden Cress |
2.30 |
2.41 |
0.34 |
|
3 |
Cow meat (Beef) 1 |
0.50 |
0.51 |
0.07 |
9- |
Lettuce |
2.80 |
2.79 |
0.02 |
|
4 |
Cow meat (Beef) 2 |
0.60 |
0.56 |
0.12 |
10- |
River water 1 |
0.20 |
0.30 |
0.03 |
|
5 |
Cucumber 1 |
3.00 |
3.20 |
0.06 |
11- |
River water 2 |
3.50 |
3.20 |
0.02 |
|
6 |
Cucumber 2 |
3.100 |
3.30 |
0.07 |
12- |
Tomato |
1.60 |
1.56 |
0.03 |
* Values given represent the average of six analyses of each sample
Comparison with other studies
The applicability of the various methods coupled with CPE was successfully confirmed by separation, preconcentration of tiny Cu(II) quantity and determination it in water and food testers. Acceptable results are obtained in Table 7.
Table 7 Procedures using cloud point extraction prior copper determination in real samples
|
Ligand |
Sample volume (mL) |
RSD% |
Samples |
Applied method |
Surfactant |
LOD (ppm) |
Ref. |
|
Me-BTABr |
10 |
2.6 |
water |
CPE / FAAS |
Triton X-114 |
1.08 |
[34] |
|
TAN |
50 |
-- |
water |
CPE / FAAS |
Triton X-114 |
0.27 |
[35] |
|
O,O-DDTP |
10 |
-- |
Drinking and rainwater, serum and human hair |
CPE / FAAS |
Triton X-100 |
0.94 |
[36] |
|
PAN |
10 |
-- |
Tap water, snow water, and flavor wines |
CPE / CE |
Triton X-114 |
0.26 |
[37] |
|
A-O,O-DDTP |
40 |
-- |
Riverine water, sea water and enriched water reference materials |
CPE / ICP-MS |
Triton X-114 |
0.03 |
[38] |
|
PTU |
15 |
1.4 |
Food |
CPE / FAAS |
Triton X-114 |
1.6 ng/mL |
[39] |
|
MPMP |
25 |
1.8 |
Rice flour and water samples |
CPE-FI / FAAS |
Triton X-114 |
0.15 |
[40] |
|
MPKO |
15 |
1.2 |
Food |
CPE/ FAAS |
Triton X-114 |
1.6 ng/mL |
[41] |
|
BDAP |
10 |
-- |
Apple leaves, spinach leaves and tomato leaves
|
CPE/ FAAS |
Triton X-114 |
0.1 |
[42] |
|
IYPMI |
15 |
2.7 |
Food |
CPE/ FAAS |
Triton X-100 |
1.6 ng/mL |
[43] |
|
NDTT |
10 |
2.1 |
Water |
CPE/ FAAS |
Triton X-114 |
0.22 ng/ mL |
[44] |
|
Aspirin |
10 |
0.028 |
Food |
CPE/Uv-Vis |
Triton X-100 |
0.018 |
this work |
LOD: limit of detection; TAN: 1-(2-thiazolylazo)-2-naphthol; PAN: 1-(2-pyridylazo)-2-naphthol; O, O-DDTP: O, O-diethyldithiophosphate; A-O, O-DDTP: ammonium O, O-diethyl-dithiophosphate; Me-BTABr: 2-[2´-(6-methylbenzothiazolylazo)]-4-bromophenol; 63Cu; 65Cu; FAAS: Flame Atomic Absorption Spectrometry; ICP-MS: Inductively Coupled Plasma Mass Spectrometry; CE: Capillary Electrophoresis; PTU: 4-hydroxy-2-mercapto-6-propylpyrimidine; MPMP: 2-[(2-mercaptophenylimino) methyl]-phenol; MPKO: methyl-2-pyridylketone oxime; BDAP: 2-(2'-benzothiazolylazo)-5-(N, N-diethyl) aminophenol; IYPMI: 3-((indolin-3-yl) (phenyl) methyl) indoline; NDTT: 6-(2-naphthyl)-2, 3-dihydro-as-triazine-3-thione.
Conclusions
Aspirin medical reagent has been efficaciously employed to preconcentrate, separate, and determine Cu(II) utilizing cloud point extraction/solvation system. The method has enhanced the presentation of UV-Vis detection considerably. Aspirin reagent to extract and determinate Cu(II) through CPE-SS exhibits exciting features were used. The technique was the safest and very cost-effective since it requires lower equipment and running costs and a small amount of surfactant of about 0.4 mL. It also has uncomplicatedness in apparatus with accurateness. Aspirin proved to be encouraging to be applied in preconcentration processes including diverse methods as in solid-phase extraction, and cloud-point extraction/solvation system.
Acknowledgments
The analysis in the advanced analytical Laboratory of Chemistry Department Faculty of Education for Women University of Kufa is exceedingly acknowledged.
Conflict of Interests
The authors declare that they have no conflict of interest.
References
Copyright© Ebaa Adnan Azooz, Jihan Razzaq Moslim, Safa Mageed Hameed, Shawket Kadhem Jawad, and Emad Abbas Jaffar Al-Mulla. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.