Research Article
Structural, Optical and Antibacterial Response of CaO Nanoparticles Synthesized via Direct Precipitation Technique
Suresh Kumar 1, 2, Vandana Sharma 1, Jatindra Kumar Pradhan 3, Sanjay Kumar Sharma 4, Prem Singh 5, Jatinder Kumar Sharma 1 *
1 Department of Physics, MMEC, Maharishi Markandeshwar (Deemed to be University), Mullana – 133207, Haryana, India.
2 Department of Physics, Maharishi Markandeshwar University - Sadopur, Ambala –134007, Haryana, India.
3 Department of Zoology, Department of Zoology, Kalahandi University, Kalahandi –766001, Odisha, India.
4 School of ICT, Gautam Buddha University, Greater Noida – 201308, Uttar Pradesh, India.
5 S.D. College, Ambala Cantt. – 133001, Haryana, India.
* Corresponding author. E-mail: sureshlakhanpal@gmail.com; sharmajk.69@gmail.com
Received: Dec. 14, 2020; Accepted: Mar. 2, 2021; Published: May 18, 2021
Citation: Suresh Kumar, Vandana Sharma, Jatindra Kumar Pradhan, Sanjay Kumar Sharma, Prem Singh, and Jatinder Kumar Sharma, Structural, Optical and Antibacterial Response of CaO Nanoparticles Synthesized via Direct Precipitation Technique. Nano Biomed. Eng., 2021, 13(2): 172-178.
DOI: 10.5101/nbe.v13i2.p172-178.
Abstract
Calcium oxide (CaO) is a promising material that works as a glass constituent, catalyst, toxic-waste remediation, poisonous gas absorbent, industrial refectory element for metal smelting, paper bleaching, sulfur neutralization in sugar, cosmetics and drug delivery mediator. This work proposes the synthesis of CaO nanoparticles by direct precipitation technique using an aqueous solution of calcium chloride and sodium hydroxide precursors. The synthesized nanoparticles are characterized for the structure, morphology, chemical composition and optical behavior using Fourier transform infrared (FTIR) spectroscopy, X-ray diffraction (XRD), field effect scanning electron microscopy (FE-SEM), energy dispersive X-ray spectroscopy (EDS), and diffuse reflectance spectroscopy. The XRD results show that the CaO nanoparticles have been synthesized in a cubic crystalline structure with the average crystallite size of about 13 nm and display aggregated morphology of nanoclusters. The presence of distinct peaks in the FTIR and EDS spectra reveal that the nanoparticles are successfully formed from the chemical recipe of the precipitation process. The optical bandgap has been estimated from the Kubelka-Munk plot and found to be 3.48 eV. The antibacterial activity of CaO nanoparticles has been tested by the well diffusion technique and observed that it shows good antibacterial action against the gram-negative bacterial strains.
Keywords: CaO nanoparticles, FTIR, XRD, Optical properties, Antibacterial activity
Introduction
The materials research in the past few decades dedicated to the metal oxide semiconductors (MOS) because of their enormous applications in many fields including photocatalysts, sensors, photonics, agriculture, electronics, optoelectronics, magnetism, superconductivity, environmental remediation, energy storage and conversation, biomedical materials, etc. [1-8]. MOS are generally distinguished by their wide band gap behavior (> 3.0 eV) and most of them are inexpensive, safe, chemically stable and earth-abundant. The emerging science of nanomaterials shows a new way of material expansion where size ranging from 1 nm – 100 nm exhibits unique physicochemical properties. Thus, the MOS nanoparticles with excellent features are expected to be a good choice for futuristic multifunctional materials. Calcium oxide (CaO), also known as quicklime, is a caustic, alkaline, whitish chemical and the oldest known material that is used since the medieval age. It is crystalline solid at room temperature having oxidation number +2 and belongs to II-VI group of the periodic table. It is formed by an array of cations (Ca2+ ions) and anions (O2– ions) having strong ionic bonding between them. However, the presence of moisture and water content in CaO converts it into slaked lime i.e. Ca(OH)2 [9]. It exhibits semiconducting properties and remains chemically stable at very high temperatures [9]. It is widely used in various fields as an efficient agent for ceramic, catalyst, cosmetic, waste remediation, destructive adsorbent for toxic chemicals and exhaust gases, targeted drug delivery and refectory additive in the paints [9–11]. It is also used to remove SO2 or other sulfur containing pollutants from the exhaust gases [12]. Recently, the ultrafine particles of CaO are used as bactericide and antibacterial agents for controlling the microorganisms and absorbent [8, 13-15]. The common human diseases like typhoid, diarrhea, cholera, intestinal infections, etc., are due to the infection from water born bacterial pathogens. The vast use of antibiotics builds up antibacterial resistant characteristics in the microorganisms that easily deny medical treatment. Hence, the search for safe and alternate antibacterial materials to fight infectious organisms creates research interest toward MOS nanoparticles. Moreover, the controlled production of MOS nanoparticles is essential for effective and successful applications. It has been reported that solution based chemical methods has excellent tendency to control the size, shape, distribution and other properties of the end products [4]. CaO and Ca(OH)2 nanoparticles are reported to synthesized by sol-gel method, thermal decomposition, precipitation, hydrothermal, microwave-assisted method, etc., [3, 9, 11-19]. Among all these methods, direct precipitation is simple, cost-effective, reproductive and rapid technique for nanoparticles synthesis [20-22]. Therefore, in this work direct precipitation technique was used to synthesize CaO nanoparticles and characterize them using Fourier transform infrared (FTIR) spectroscopy, X-ray diffraction (XRD), field effect scanning electron microscopy (FE-SEM), energy dispersive X-ray spectroscopy (EDS) and diffuse reflectance spectroscopy for different properties. Moreover, the antibacterial activity of these nanoparticles was also studied against two gram negative pathogen bacteria, Escherichia coli (E. coli) and Vibrio cholerae (V. cholerae). As per our knowledge, this is the first report that has focused on the antibacterial response of CaO nanoparticles on V. cholerae bacteria.
Experimental
Chemicals
The analytical grade reagents calcium chloride anhydrous (CaCl2H2O) and sodium hydroxide (NaOH) of 99.9% purity were purchased from Merck (India) and used as received without any special treatment.
Sample preparation
In the present experiment, pure CaO nanoparticles are synthesized by direct precipitation method as it is one of the most versatile, cost effective, simple and conventional method for producing CaO and Ca(OH)2 in comparison to other synthesis methods. To synthesize CaO nanoparticles, 0.1 M solution CaCl2 was prepared in 100 mL distilled water at 40 oC under continuous stirring for 1 h using hot plate magnetic stirrer. Then 50 mL solution of NaOH (0.1 M) prepared in distilled water was added dropwise into the CaCl2 solution. The reaction process was carried out for 2 h at temperature 40 oC and pH = 10.5 which results in white color precipitates. These precipitates were sonicated, filtered and washed repeatedly with ethanol and distilled water which was further dried for 6 h at 60 oC. The end product of this chemical reaction was a precursor of CaO that is Ca(OH)2 which is finally calcinated at 350 oC for 2 h in a microwave oven. The obtained CaO nanoparticles kept in the vacuum desiccator before further characterization. The synthesis of CaO nanoparticles by direct precipitation technique started with the initial precipitation of Ca(OH)2 during the chemical process from the dissolved Ca2+ and OH– ions in the aqueous medium. As the concentration of these ions exceeds a critical value, precipitation of CaO nuclei starts under the calcination process to form solid CaO nanoparticles (Fig. 1). The chemical reaction process and synthesis steps as follows:
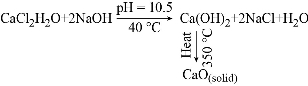
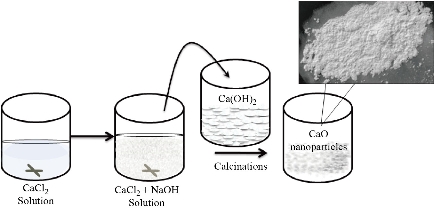
Fig. 1 Schematic illustration of the synthesis steps for CaO nanoparticles.
Characterization of CaO nanoparticles
The obtained CaO nanoparticles were subjected to physical analysis using Perkin Elmer 1600 Fourier transform infrared spectrometer with KBr pellet technique and PANalytical X’pert Pro X-ray diffractometer. The XRD pattern further matches with the standard JCPDS data cards. The morphology and elemental composition of nanoparticles were observed by Jeol JSM-6100 field emission scanning electron microscope equipped with energy dispersive X-ray analyzer. The optical study was carried out by Ultraviolet-visible (UV-vis) diffuse reflectance spectrometer (Schimazdu 2600) between the wavelength ranges of 200 to 800 nm.
Antibacterial activity test
Antibacterial activity test was performed against the gram-negative bacterial strains of E. coli and V. cholerae by agar well diffusion method. The stock cultures were prepared by inoculating the bacterial strains in Muller Hinton agar plates (Himedia, India) followed by the incubation at 37 °C for 24 h. These cultures were maintained at 4 °C until the preparation of the working cultures. The cultures were diluted with fresh Muller–Hinton broth to obtain optical densities of the order of 2.0×106 CFU/mL. Sterile pipettes were used for the transfer of 50 µL molten agar to broth culture (0.5 mL) of test organisms, mixed properly and transferred into sterile dishes. The wells were prepared using sterile cork borer (6 mm) and each well was filled with different concentrations (5 mg/mL to 25 mg/mL) of CaO nanoparticles. The plates were incubated at 37 °C for 24 h. After incubation, the dishes were examined for the zone of inhibition and measured in terms of millimetre of the diameter.
Results and discussion
FTIR characterization of CaO nanoparticles
FTIR spectrum of CaO particles is shown in Fig. 2. The strong peak at 3642 cm–1 is assigned to the O–H free hydroxyl bond from the residual hydroxide or the moisture content in the sample [9]. This peak is sharp well which indicates the formation of pure phase particles. The residual hydroxyl groups further supported by the presence of absorbance bands in the region of ~ 1600 cm–1 and ~ 800 cm–1 [9, 22]. The strong broad band at 1471 cm–1 and a peak at 876 cm–1 indicate the C–O bond arose because of the carbonation of CaO nanoparticles [9]. During calcination, the exposure of highly reactive surface area of CaO nanoparticles to air during leads to CO2 and H2O formation which further adsorbed on the CaO surface in the form of free –OH and carbonate species. This specifies that the surface –OH and the lattice oxygen of CaO nanoparticles make available oxygen which is more liable on the high surface area of the nanoparticles [16]. Two small dips at in the spectra at 1229 cm–1, 1633 cm–1 and 2515 cm–1 attributed to C–O stretch of the CO2 stretching [17]. The characteristic peak at 554 cm–1 has been detected for Ca–O bond stretch. Hence, we can say that FTIR analysis justifies the traces of chemicals used in the synthesis of CaO nanoparticles.
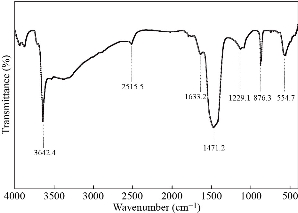
Fig. 2 FTIR spectrum of CaO nanoparticles.
XRD characterization of CaO nanoparticles
Fig. 3 shows the XRD spectrum for the synthesized CaO nanoparticles which is measured over the diffraction angle (2θ) between 20° to 80° using Cu(Kα) source. The presence of highly intense and multiple XRD peaks with broadening nature indicate that the product is well crystallized. On comparing with the standard diffraction pattern (Table 1), five distinguish peaks (Θ) indexed to the cubic (zinc blende) structure (space group Fm3m, JCPDS card No. 82–1690) for CaO nanoparticles [23]. These diffraction peaks at position 2θ = 32.1°, 37.5°, 54.2°, 65.7° and 67.5° belong to (111), (200), (202), (311), and (222) reflection planes respectively. Further, two additional peaks (Δ) at 2θ = 50.5° and 57.7° are also observed that match with (110) and (003) planes of Ca(OH)2 with hexagonal structure (Portlandite, JCPDS card No. 04–0733) [9]. The existence of these impure phase peaks of Ca(OH)2 may be due to the presence of moisture content in the synthesized nanoparticles. This moisture content appears as a result of solution–based synthesis of nanoparticles and their exposure to the environment after synthesis that results to the oxidization of CaO. The interplanar spacing (d-spacing) between different crystal planes of cubic the CaO structure is calculated using Bragg diffraction equation and represented in Table 1. The crystallite size (D) has been calculated for all peaks (Table 1) using Debye–Scherrer’s formula [21]:
![]() ,
,
where k, λ, β and θ are the shape factor (0.81). X-ray wavelength (1.5406 Å), full width at half maximum and Bragg diffraction angle respectively. The average crystallite size is found to be 13.5 nm. Many researchers have been reported different size CaO nanoparticles by different solution methods. Mirghiasi Z, et. al have synthesized CaO and Ca(OH)2 nanoparticles having crystallite size of 40 nm and 41 nm respectively via direct decomposition method [9]. Habte et al. have reported sol-gel synthesized CaO nanoparticles of size 24.51 nm from waste eggshell [18]. Roy et al. synthesized cubic CaO nanoparticles of size 16 nm by microwave irradiation method. However, the present study successfully attains the smallest size CaO nanoparticles [13]. The lattice constant (![]() ) for cubic CaO nanoparticles is calculated from the most intense XRD peak and found to be 4.793 nm. The small variation in the value of lattice constant and d-spacing from that of the bulk value is attributed to the presence of microstrain and defects that occurred during the growth process. These microstrain and dislocation densities have been determined (Table 1) using the relation given by Dhupar et al. [22] with an average value of 2.764×10–3 and 6.32×10–15 line/m2 respectively.
) for cubic CaO nanoparticles is calculated from the most intense XRD peak and found to be 4.793 nm. The small variation in the value of lattice constant and d-spacing from that of the bulk value is attributed to the presence of microstrain and defects that occurred during the growth process. These microstrain and dislocation densities have been determined (Table 1) using the relation given by Dhupar et al. [22] with an average value of 2.764×10–3 and 6.32×10–15 line/m2 respectively.
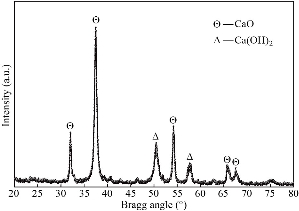
Fig. 3 XRD spectrum of CaO nanoparticles.
Table 1 Structural properties of CaO nanoparticles analyzed from XRD spectrum and values from standard JCPDS data card No. 82–1690
|
2θ (°) |
d-spacing (Å) |
Intensity |
β (°) |
Particle size (nm) |
(hkl) |
Micro stain (×10-3) |
Dislocation density (×1015) line/m-2 |
||
|
Observed |
JCPDS |
Observed |
JCPDS |
(I/I0) |
|||||
|
32.10 |
32.20 |
2.786 |
2.779 |
34 |
0.4909 |
17.6 |
(111) |
4.14 |
3.23 |
|
37.50 |
37.35 |
2.396 |
2.407 |
100 |
0.6949 |
12.6 |
(200) |
3.25 |
6.29 |
|
54.20 |
53.85 |
1.691 |
1.702 |
38 |
0.5679 |
16.4 |
(311) |
3.30 |
3.71 |
|
65.70 |
64.15 |
1.420 |
1.451 |
14 |
0.9151 |
10.8 |
(220) |
1.41 |
8.58 |
|
67.50 |
67.37 |
1.387 |
1.390 |
12 |
0.9857 |
10.1 |
(222) |
1.72 |
9.79 |
FE-SEM characterization of CaO nanoparticles
Fig. 4(a)-(c) shows the surface morphology of the synthesized nanoparticles at three different magnification scales (1 μm, 500 nm and 200 nm). The grown particles are observed to be closely packed in the cluster cum porous arrangements which makes them more active in the catalysis and gas sorbent applications. These particles appeared to be solid, fully developed and randomly scattered with non–uniform cubical dimensions. The size of individual CaO nanoparticles, which are available freely on the surface, has been calculated using inception method (Fig. 4c) and found to vary from 20 nm to 80 nm. The EDS spectrum (Fig. 4(d)) of CaO nanoparticles indicates that the Ca and O elements are present in the sample while the peak of carbon arises due to the coating used in the analysis. The atomic ratio of Ca and O is found to be 48.85 : 51.15 which is inconsistent with the stoichiometry of CaO and slightly rich in oxygen content.

Fig. 4 FE-SEM micrographs at magnification scales (a) 1 μm, (b) 500 nm and (c) 200 nm, and EDS spectra of CaO nanoparticles.
Optical characterization of CaO nanoparticles
UV-vis diffuse reflectance spectra in absorbance mode of the CaO nanoparticles has been recorded for the wavelength of 200 nm to 800 nm (inset of Fig. 5). It is obvious that the absorption edge observed at 360 nm i.e., in the UV-light region and almost negligible absorption in the visible region. The optical band gap of CaO nanoparticles (Fig. 5) was calculated by plotting F(R)2 vs. photon energy (hν) from the Kubelka-Munk function F(R) [20] which is related to the diffuse reflectance (R) as F(R) = (1–R)2/2R. The linear part of this curve is extrapolated to zero on the energy axis to get the value of optical band gap. The value of optical band gap thus obtained to be 3.48 eV which is lower than the value (6.26 eV) for bulk CaO but found compatible with the reported results for CaO nanoparticles by different researches [11, 19]. This low value of optical band gap may be associated with the presence of Ca(OH)2 phase in the CaO nanoparticles which provides defect states between the energy gap and reduces the band gap.
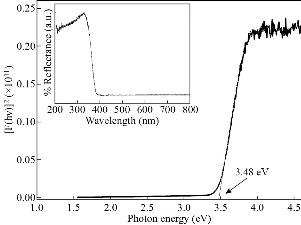
Fig. 5 The estimation of optical band gap by Kubelka–Munk plot of F(R)2 vs. hν and the inset shows UV-vis diffuse reflectance spectra of CaO nanoparticles.
Antibacterial Activity of CaO nanoparticles
The antibacterial activities of CaO nanoparticles, at different concentrations of 5, 10, 15, 20 and 25 mg/mL, against both bacterial strains (E. coli and V. cholera), have been shown in Fig. 6. It clearly demonstrates that the antibacterial activity of the CaO nanoparticles could be effective against the pathogenic bacteria by increasing the concentration of nanoparticles. The antibacterial results have indicated that the degree of the zone of inhibition is more against bacterial strain E. coli (28 ± 0.15 mm) when compared to the V. cholerae (25 ± 0.10 mm) at all concentrations of nanoparticles. The trend of antibacterial activity of the CaO nanoparticles for these gram-negative bacteria is almost the same, which agrees well with the previous reports [15,23]. The creation of an inhibition zone evidently states the mechanism of the biocidal properties of nanoparticles that lead to the disruption of the bacterial membrane [23]. Since nanoparticles exhibit large surface area so they can tightly attach to the surface of the bacterial cells and penetrate easily into the membrane [24]. This would result in the leakage of intracellular components of the cell that kills the bacterial cell. The range of inhibition depends on the concentration of nanoparticles and increases with the increase in concentration.
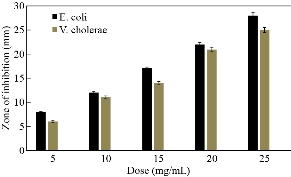
Fig. 6 Antibacterial activity of CaO nanoparticles against E. coli and V. cholerae
Conclusions
In conclusion, we have successfully synthesized CaO nanoparticles by direct precipitation technique at the optimized value of temperature (40 oC) and pH (10.5) within a period of 2 h. The synthesized nanoparticles are characterized for different features using FTIR, XRD, FE-SEM, EDS and diffuse reflectance spectroscopy. FTIR analysis evidenced the presence of prime O–H and Ca–O stretching modes around 3642 cm–1 and 554 cm−1 respectively, attributed to the presence of the desired phase which is confirmed by XRD results. XRD investigation revealed the existence of cubic zinc blende structure of CaO nanoparticles of size 13.5 nm along with some content of hexagonal Ca(OH)2 as a separate phase. FE-SEM micrographs exposed the presence of agglomerated cubical nanoparticles. The peaks in the EDX spectra representing Ca and O elements only are demonstrating the successful formation of CaO nanoparticles. A low optical bandgap value of 3.48 eV for CaO nanoparticles is associated with the existence of Ca(OH)2 phase. The synthesized cubical CaO nanoparticles showed good antibacterial activity against gram-negative bacteria strains of E. coli and V. cholera, which is well demonstrated by the well diffusion method. The synthesized CaO nanoparticles having unique structural, optical and antibacterial response can be exploited in near future for eco-friendly agents for many biomedical applications and targeted drug delivery systems.
Acknowledgments
We are very much thankful to SAIF, Chandigarh and Maharishi Markandeshwar (Deemed to be University) - Mullana for various instrumentation and test facilities.
Conflict of Interests
The authors declare that no competing interest exists.
References
Copyright© Suresh Kumar, Vandana Sharma, Jatindra Kumar Pradhan, Sanjay Kumar Sharma, Prem Singh, and Jatinder Kumar Sharma. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.