Research Article
Electrochemical Analysis Using Nano-Sensor of Stevia Herb as a Healthful Alternative Sugar
Muhammed Mizher Radhi 1, Asmaa Abdulsattar Obaid 2, Wisam Hindawi Hoidy 3*
1 Department of Radiological Techniques, College of Health and Medical Technology/Baghdad, Middle Technical University (MTU), Baghdad, Iraq.
2 Department of Physiotherapy Techniques, College of Health and Medical Technology/Baghdad, Middle Technical University (MTU), Baghdad, Iraq.
3 Department of Chemistry, College of Education, University of Al-Qadisiyah, Al-Qadisiyah City, Iraq.
* Correspond author: E mail: Wisam Hindawi Hoidy: wisam.hoidy@qu.edu.iq
Received: Jul. 4, 2020; Accepted: Apr. 22, 2021; Published: Jun. 2, 2021
Citation: Muhammed Mizher Radhi, Asmaa Abdulsattar Obaid, and Wisam Hindawi Hoidy, Electrochemical Analysis Using Nano-Sensor of Stevia Herb as a Healthful Alternative Sugar. Nano Biomed. Eng., 2021, 13(2): 207-211.
DOI: 10.5101/nbe.v13i2.p207-211.
Abstract
Stevia rebaudiana is a plant herb which has good properties for using as an alternative of the glucose sugar. Electrochemical study was used to calculate the limited safety dose by a cyclic voltammetric technique using nano-sensor as modified glassy carbon electrode (GCE) with carbon nanotubes (CNT) as MWCNT/GCE. The oxidation current peak of stevia compound was appeared in the concentration of 0.4 mM (28 mg/mL), and the reduction current peak appeared at -500 mV, and so it can be concluded that stevia compound is an anti-oxidative sweetener compound at a limited concentration for safety using. Also, the electrochemical properties of the stevia compound were studied in acidic and alkaline pH to accord the oxidative behavior in acidic medium and act as anti-oxidative in alkaline solution.
Keywords: Stevia compound, Cyclic voltammetry, Nano-sensor, KCl solution, PH
Introduction
Stevia is a substitute for sugar extracted from the leaves of the Stevia rebaudiana plant as shown in the structure of the stevia plant in Fig. 1. Its sweet taste comes from stevia glycosides, which are 250 to 300 times sweeter than regular sugar. Researchers found that using stevia sweeteners as an alternative for sugar may benefit diabetics, children, and those wishing to reduce their caloric intake [1, 2]. Through the research and studies in the electrochemistry field, we have found that the use of different chemical compounds in the aqueous electrolytes [3-5], and the others can be used blood medium to finding the electrochemical properties of pharma-compounds [6-10]. Sucrose consumption is a food asset for some health concerns, such as tooth decay, obesity and type 2diabetes. The statistics are alarming. Therefore, to reduce these major and growing problems in society, replacing sugar with low-calorie sweeteners may be effective. The use of these sweetening compounds has increased dangerously. Extracts of stevia ribodiana burtoni leaves are natural, sweet and calorie-free and can also be used as a sugar substitute or as an alternative to artificial sweeteners [11-13]. To test and discover new potential sources of natural antioxidants, the antioxidant ability of laboratory regenerated tissues has been introduced in Stevia rebaudiana, Citrus sinensis and Saccharum officinarum. These species are used in daily life, providing essential secondary metabolites that remove free toxic radicals. Toxic, free radicals in the human body can cause various diseases [14]. An improved analytical method has been developed that can be applied to the quality control of stevioside and rebaudioside content in the dried stevia rebaudiana leaves before treatment; looks for plants with higher productivity in the dieterbin glycoside content in a selective sampling program; or when large numbers of samples are sent to the laboratory for analysis [15,16]. In this study, stevia plant sweeter was used as an alternative of sugar which was proved in the electrochemical analysis using nano-sensor as anti-oxidative reagent.
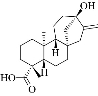
Fig. 1 The chemical structure of stevia.
Experimental
Materials
Stevia powder was purchased from Mitosh World Fzco (Indonesia), carbon nanotubes (purity 99%) were supplied by Fluka Company (Germany), KCl and NaOH from Sinopharm Chemical Reagent Co, Ltd. (SCRC), China, and HCl3 normally from Aldrich compony (England). Deionized water was used for preparing the solutions.
Apparatus instruments
EZstat (potentiostat / glvanostat) sequence (NuVant Systems Inc., USA) was adopted. All parts of the cyclic voltammetry (CV) apparatus contained the glass carbon electrode (GCE) as a working electrode, Ag/AgCl (3 M KCl) as a reference electrode, and the platinum wire (1 mm diameter) as a counter electrode. All three electrodes were connected with the potetio-state which operated by the electro-analytical measuring program by a personal computer. GCE surface was cleaned by polishing with alumina solution and in sonic water route for around 10 min to eliminate any impurities before use in CV cell.
Preparing the modification of glassy carbon electrode with carbon nanotube (CNT/GCE)
The basic mechanical attachment method used to prepare the CNT / GCE working electrode was used for the nano-sensor preparation [16, 17]. The GCE modification method included abrasive application of the multiwall carbon nanotubes (MWCNT) on GCE's clean surface, forming an array of MWCNT as modified working electrode MWCNT / GCE and replacing 0.1 M KCl solution in a cyclic voltammetric cell in 10 mL, then connecting all the electrodes (modified working electrode, reference electrode and counter electrode) to the potentiostat.
Procedure
Three electrodes (modified glassy carbon electrode MWCNT / GCE as working electrode, Ag / AgCl as reference electrode and platinum wire as counter electrode) were immersed in a 10 mL solution of 0.1 M KCl mounted in a voltammetric quartz cell (volume: 15 mL). The three electrodes were connected to potentiostat to determine the results using personal computers via the cyclic voltammogram [18].
Results and Discussion
Effect of different concentrations
Stevia compound study is very important to find the effect of the alternative to the sugar which was studied using the cyclic voltammetric technique by nano-sensor MWCNT/GCE. Fig. 2 illustrated the cyclic voltammogram of stevia solution in KCl electrolyte with two oxidation-reduction current peaks at the potentials of -600 and -500 mV, respectively, which enhanced with increasing the concentration of stevia compound. It was found that oxidation current peaks started to appear at the 0.4 mM of stevia concentration and enhanced through increasing the concentration, and so the stevia compound was anti-oxidative in KCl solution at less than of 0.4 mM because stevia compound had a reduction peak at -500 mV.
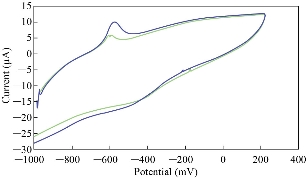
Fig. 2 Cyclic voltammogram of different concentrations of stevia solution in 0.1 M KCl as an electrolyte on modified working electrode MWCNT/GCE and Ag/AgCl as a reference electrode at scan rate 0.1 Vs-1.
Effect of different pH media on the redox peaks of stevia compound
The acidic and alkaline media were selected to find the electrochemical properties of stevia compound in KCl solution by oxidation - reduction current peaks which were illustrated in the cyclic voltammogram. Fig. 3 shows the oxidation - reduction current peaks of stevia compound in different pH, and it was found that the oxidation peak was enhanced in acidic medium at pH = 6, but in alkaline medium at pH = 11 the oxidation peak disappeared and the reduction peak enhanced. Additionally, Fig. 4 illustrates the enhancement of oxidation current peak of stevia compound in high acidic medium (pH = 3) and enhancement of reduction current peak in alkaline medium; hence, it can be concluded that stevia compound acts as an antioxidant reagent in alkaline medium.
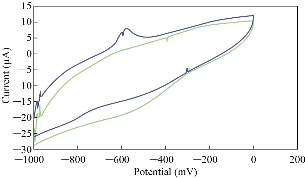
Fig. 3 Cyclic voltammogram of stevia compound in 0.1 M of KCl solution at different pH (blue line at pH = 6 and green line at pH = 11) on a modified electrode MWCNT/GCE and Ag/AgCl as reference electrode at scan rate 0.1 Vs-1.
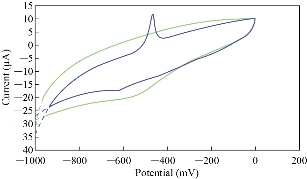
Fig. 4 Cyclic voltammogram of stevia compound in 0.1 M of KCl solution at different pH (blue line at pH = 3 and green line at pH = 10) on a modified electrode MWCNT/GCE and Ag/AgCl as reference electrode at scan rate 0.1 Vs-1.
Effect of different scan rates
Different scan rates on the nano-sensor MWCNT/GCE were studied for the stevia compound in KCl solution to prove the electrochemical reaction which has enhanced consistency of the redox peaks of stevia compound against the increasing the scan rates as shown in Fig. 5 and 6. It means that the diffusion of electrons in the electrolyte with the electro-catalyst of nanoparticles of CNT helps the enhancement of redox current in the voltammetric process [19].
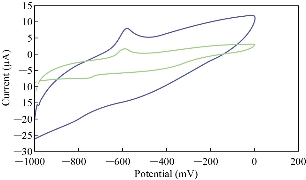
Fig. 5 Cyclic voltammogram of stevia compound in 0.1 M of KCl solution at different scan rates (blue line at 0.1 Vs-1 and green line at 0.01 Vs-1) on a modified electrode MWCNT/GCE and Ag/AgCl as a reference electrode.
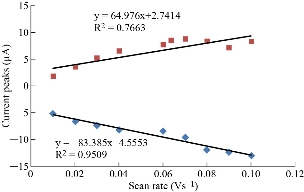
Fig. 6 Relationship between redox current peaks and different scan rates of stevia compound in KCl solution on the modified electrode MWCNT/GCE and Ag/AgCl as reference electrode.
Effect of ascorbic acid (AA) the stevia redox peaks
Ascorbic acid (AA) has alkaline properties in electrolytes such as blood medium [20], so it can be used in the electrochemical study with stevia compound to find the effect of oxidation peak of AA on the redox peaks of stevia in KCl solution as shown in Fig.7. Fig.8 illustrates the relationship between the oxidation-reduction current peaks of stevia with different concentrations of AA. AA solution acts as an anti-oxidative reagent on the stevia compound which enhanced the reduction peak and decreased the oxidation peak as shown in Fig. 8.
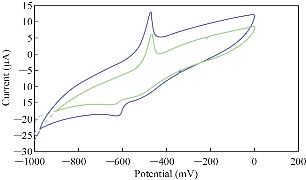
Fig. 7. Cyclic voltammogram of stevia compound in 0.1 M KCl solution at different concentrations of ascorbic acid (blue line at 0.02 mM and green line at 0.2 mM) on the modified electrode MWCNT/GCE and Ag/AgCl as reference electrode at scan rate 0.1 Vs-1.
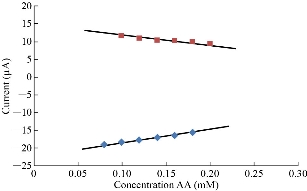
Fig. 8 Relationship between redox current peaks and different scan rates of stevia compound in KCl solution on the modified electrode MWCNT/GCE and Ag/AgCl as the reference electrode.
Reliability and stability study of the modified electrode MWCNT/GCE
Fig. 9 illustrates the cyclic voltammogram of stevia compound in KCl solution at ten times scanning to prove the stability of nanoparticles on the surface of GCE and to find the reliability of the results of the study. A good overlapping of the redox peaks was found which can be dependent on the experiment of the study [21].
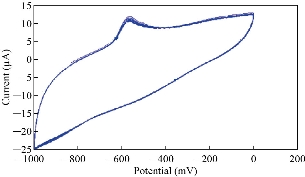
Fig. 9 Cyclic voltammogram of stevia compound in 0.1 M of KCl solution at ten times scanning on the modified electrode MWCNT/GCE and Ag/AgCl as reference electrode at scan rate 0.1 Vs-1.
Scanning electron microscopy (SEM)
Fig. 10 illustrates the scanning electron microscopy of stevia powder. It is found that the morphology of the stevia particles had a spherical shape with microstructural scale.
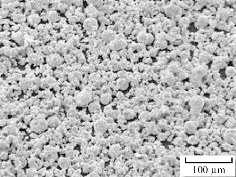
Fig.10 SEM of stevia powder.
Conclusions
Stevia compound has been used as an alternative of sugar in our life. In the present study, they were studied in electrolytes from an electrochemical analysis using cyclic voltammetry method on the nano-sensor MWCNT/GCE, and the electrochemical behavior of stevia in the electrolyte medium was found. The electrochemical conductivity of stevia compound in acidic pH enhanced the oxidation - reduction current peaks, but the redox process in alkaline pH of the stevia was decreased, and so stevia acted as an anti-oxidative in alkaline medium. Stevia compound was affected by ascorbic acid (AA) which acted as anti-oxidative reagent where the oxidation current peak of stevia disappeared in AA, and enhanced the reduction peak. The study proved that using stevia was safe until the concentration of 0.4 mM (28 mg/mL), whereas the oxidation peak of stevia in KCl electrolyte appeared at concentrations of higher than 0.4 mM. With the nanosensor MWCNT/GCE it was discovered that the electrochemical behaviors of the stevia compound in the electrolyte were more accurately determined. One of the most important benefits of using stevia is to reduce the amount of sugar to a higher degree than that of glucose. In addition, stevia bears all the merits that can be attributed to being a plant compound instead of a chemical compounds as is the case of artificial sweeteners.
Conflict of Interests
The authors declare that no competing interest exists.
References
Copyright© Muhammed Mizher Radhi, Asmaa Abdulsattar Obaid, and Wisam Hindawi Hoidy. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.