Research Article
Zn, Cu and Mn Determination by Metals Oxide Nanoparticles Mix Ions Selective Carbon Past Electrode Industrialization and Cyclic Voltammetry Application Study
Emad Salaam Abood *
Department of Medical Physics, Hilla University College, Babylon, Iraq.
* Corresponding author. E-mail: dr.emadsalaama@hilla-unc.edu.iq
Received: Feb. 7, 2021; Accepted: Jul. 21, 2021; Published: Sep. 4, 2021
Citation: Emad Salaam Abood, Zn, Cu and Mn Determination by Metals Oxide Nanoparticles Mix Ions Selective Carbon Past Electrode Industrialization and Cyclic Voltammetry Application Study. Nano Biomed. Eng., 2021, 13(3): 249-256.
DOI: 10.5101/nbe.v13i3.p249-256.
Abstract
A new modified ion selective electrode with carbon and metal oxide nanoparticles to increase sensitivity and selectivity to measure the trace concentration of heavy elements in solutions and pharmaceutical compounds.by preparing the Nano-oxides of ZnO, CuO and MnO2 using a hydrothermal method. The temperature and pH of the electrode studied to select the best conditions. Where the voltmogram recorded a single signal in relation to the oxidation and reduction process of the zinc element by a reversable mechanism rever to one electron transmission and a quasi-reversable signal for the copper element, and irreversible signal for the manganese element resulting from the transmission of two electrons. The thermodynamic and kinetic factors were also studied and finally the standard mixture of the elements salt was detection to electrode sensitivity mutation the result show the new electrode can distinguish between the signals produced by the oxidation and reduction process of the three elements as the same time with three deferent peaks for oxidation and two peaks of redaction.
Keywords: Cyclic voltammetry; carbon past electrode, ZnO nanoparticles, CuO nanoparticles, MnO2 nanoparticles, Hydrothermal method
Introduction
Drug analysis plays important roles in drug quality control, and has great impact on public health. Therefore, a simple, sensitive and accurate method for the determination of active ingredient is very important [1]. Zinc is a nutrient that people need to stay healthy [2]. Zinc is found in cells throughout the body; it helps the immune system fight off invading bacteria and viruses; however, the change in the concentration may result in many diseases [3]. Thus, a quantitative determination of zinc concentration is significant for developing immune physiological and pharmacological research and life sciences. Copper is an essential trace element that is vital to the health of all living beings (humans, plants, animals and microorganisms). In humans, copper is essential for the proper functioning of organs and metabolic processes. The human body has complex homeostatic mechanisms which attempt to ensure a constant supply of available copper, while eliminating excess copper whenever it occurs. However, like all essential elements and nutrients, too much or too little nutritional ingestion of copper can result in corresponding conditions of copper excess or deficiency in the body, each of which has its own unique set of adverse health effects. Copper deficiency and toxicity can be either of genetic or non-genetic origin [4]. Copper is found in cells throughout the body; it helps our bodies make red blood cells and keeps nerve cells and our immune systems healthy. It also helps to form collagen, a key part of bones and connective tissues. Copper may also act as an antioxidant, reducing free radicals that can damage cells and DNA. Copper helps the body absorb irons. Our bodies need copper to make energy [5-7]. Manganese (MnO2 NPs) has intrigued material science researches extensively due to its wide range of applications. They are widely used in energy storage devices, catalysts, adsorbent, sensors and imaging, therapeutic activity, etc. Since they hold a lot of distinguished potentials, a robust protocol for cheap, stable, biocompatible and eco-friendly MnO2 NPs is necessary. Thus, owing to their peculiar character, they could be utilized for various purposes depending on the mode of action and applications. Hence, this review has summarized conventional methods, such as hydrothermal, sol–gel, oxidation–reduction used for the generation of MnO2 NPs. Likewise, morphological characterization by various spectroscopic techniques also outlined [8]. The electro chemical methods using chemically modified electrode have been widely used in sensitive and selective analytical methods for the detection of the trace amounts of biologically important compounds [9]. Ion-selective membrane electrodes (ISEs) have become very attractive sensing platforms for environmental solution analysis. This review mainly presents recent advances in polymeric-based ISEs relevant to solution research and primarily focused on alkali and alkali earth-metal cations, ammonium ions, halide anions and certain oxyanions involved in biogeochemical cycles [10]. ISEs have the potential to be the icon of decentralized ion chemical information for water research as in the case of wearable ISE sensors. The modern development of robust ISEs (mainly in all-solid-state format) has allowed an easy implementation either into submersible or non-submersible probes that maintain, to an acceptable degree, the required analytical performance [11]. A perspective on the current requirements of ISEs in terms of analytical performance and engineering construction is provided initially and is followed by recent contributions listed according to the sampling methodology. Importantly, there is a plenty of room for improvement and new approaches; and it should be stressed that the recent progress in water research using ISEs has been owing to multidisciplinary efforts. Facing this challenge is very exciting and the development of ISE platforms that enable working in real conditions is quite plausible [12]. Electrode surface can be modified with metal nanoparticles and such surfaces have found numerous applications in the field of bio electrochemistry, particularly in biosensors. It has also been observed that nanoparticles can act as conduction centers facilitating the transfer of electrons. In addition, they provide large catalytic surface area. Many kinds of nanoparticles, including metal nanoparticles, oxide nanoparticles, semiconductor nanoparticles and even composite nanoparticles have been widely used in electrochemical sensors and bio sensors [13]. Zinc is electro active component that can be determined electrochemically [14]; however, it is very difficult to distinguish its response signals at bare electrodes because of their similar potential and interference from each other. Therefore, it is very important to develop a modified electrode to resolve the voltammetric response from each other. To the best of our knowledge, no study has been reported so far on the simultaneous determination of Zn, Cu and Mn by using carbon paste electrode modified with (Zn, Cu and Mn) oxide nanoparticles. In this research, we report the preparation and application of a carbon paste electrode modified with (Zn, Cu and Mn) oxide nanoparticles for determination of (Zn, Cu and Mn) ions without any additional modification such as addition of electron transfer mediator or specific reagent for the first time.
Experimental
Apparatus and chemicals
The electrochemical measurements were performed with an Auto Lab Potentiostat (Digi- Ivy 2113 Texas, USA) controlled by the General-Purpose Electrochemical System Software. The ZnO, CuO and MnO2 nanoparticle mixing carbon paste electrode was used as the working electrode. A calomel electrode and a platinum (Pt) wire were employed as the reference electrode and counter electrode, respectively, and all potentials were reported with respect to the former. A Metrohm pH/ion meter was used for pH measurement. All solutions were freshly prepared with DW. All reagents were of analytical grade from Merck; graphite powder and paraffin oil both from Merck were used as received. High dilution of acids and bases was in the pH range of 2.0-8.0. All solution was deoxygenated with pure nitrogen gas for about 20 min prior to each electrochemical experiment (Fig. 1).
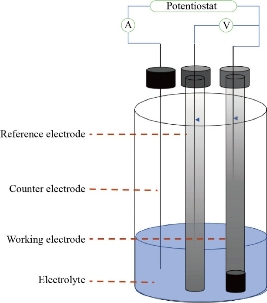
Fig. 1 Cyclic voltammetry cell.
Preparation of metals oxide nanoparticle by hydro-thermal method
ZnO nanoparticles were synthesized by hydrothermal method. Typically, 0.1 mole of ZnCl2 was dissolved in 300 mL of distilled water, and then a solution containing 0.2 mole of NaOH was slowly added with stirring. After 8 h, a white precipitate was obtained. The resulting precipitate was washed with distilled water and dissolved again in water to get solution, which was mixed with 50 mL of polyvinylpyrrolidone (PVP) (13 g of PVP dissolved in water) solution during a continuous stirring to get a homogeneous solution. The homogeneous solution was transferred into autoclave and heated at 160 °C for 8 h. Then ZnO powder was obtained and dried at 100 °C after it had been washed with distilled water. TEM INSPECT S50, was used to determine the morphology of the synthesized ZnO sample. The morphology of the prepared ZnO was studied by Scanning electron microscopy (SEM) [15]. CuO nanoparticles were synthesized by hydrothermal method. Typically, 0.1 mole of CuCl2 was dissolved in 200 mL of distilled water, and then a solution containing 0.2 mole of NaOH was slowly added with stirring. After 8 h, a dark brown precipitate was obtained. The resulting precipitate was washed with distilled water and dissolved again in water to get solution A, which was mixed with 50 mL of poly vinyl Polyline (PVP) solution B (13 g of PVP dissolved in water) solution during a continuous stirring to get a homogeneous solution. The homogeneous solution was transferred into autoclave and heated at 160 °C for 8 h. Then, CuO powder was obtained and dried at 100 °C after it had been washed with distilled water. The morphology of the synthesized CuO nanoparticle sample was studied by transmission electron microscopy (TEM) by using TEM Inspect S50 [16]. MnO2 nanoparticles were synthesized by hydrothermal method. Typically, 0.1 mole of MnCl2 was dissolved in 150 mL of distilled water, and then a solution containing 0.2 mole of NaOH was slowly added with stirring. After 8 h, a dark grey precipitate was obtained. The resulting precipitate was washed with distilled water and dissolved again in water to get solution, which was mixed with 50 mL of poly vinyl Polyline (PVP) solution (13 g of PVP dissolved in water) solution during a continuous stirring to get a homogeneous solution. The homogeneous solution was transferred into autoclave and heated at 160 °C for 8 h. Then, MnO2 powder was obtained and dried at 100 °C after it had been washed with distilled water [17].
Preparation of oxide nanoparticles (ZnO, CuO and MnO2) electrode
The ZnO, CuO and MnO2 nanoparticles mix carbon past electrode (working electrode) modified carbon paste electrodes were prepared by mixing 0.1 g of ZnO, 0.5g of CuO and 0.9g nanoparticles, with 0.9 g of graphite powder and 0.7 mL of paraffin oil respectively with a mortar and pestle until a uniform paste was obtained. The paste was packed into the end of a glass tube (cross-section area 0.44 mm2 and length 10 cm) and isolated by fiber for three equal arias with small hole. The electrical contact was provided by inserting a copper wire into the carbon paste. Prior to the experiment, the surface of the carbon paste was polished with fine paper. The unmodified carbon paste electrode was prepared in the same way without adding element nanoparticles to the paste (Fig. 2).
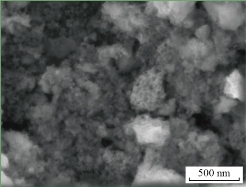
Fig. 2 SEM image of ZnO nanoparticles.
Result and Discussion
Fig. 2 refers to The SEM image that showed the agglomerated ZnO nanoparticles had an average size equal to 200 nm. The TEM image showed the agglomerated CuO nanoparticles had an average size of less than 100 nm as shown in Fig. 3. Fig. 4 refers to the SEM image that shows the agglomerated MnO2 nanoparticles had an average size less than to 100 nm. The TEM and SEM images refer to the hydrothermal process that has succeeded in preparing nano-oxide, which is one of the most common and widely used methods for preparing nanoparticles. The morphology for metal oxide nanoparticles reveals homogenous shape and small particle size with increased surface area led to high sensitively and selectivity for ion under study. In another study, we experiment the difference between metal oxides and metal oxide nanoparticles as the same ion with different condition for electrode.
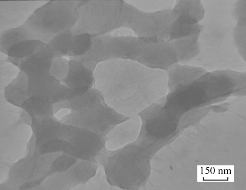
Fig. 3 TEM image of CuO nanoparticles.
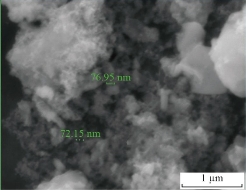
Fig. 4 Scanning electron microscopy (SEM) image of MnO2 nanoparticles.
Fig. 5 ZnO, CuO and MnO2 nanoparticles mix carbon past electrode (working electrode).
Cyclic voltammetry study of Zn, Cu and Mn
Fig. 6 depicts the cyclic voltammetric response for the electrochemical oxidation-reduction of 0.1 mol ZnSO4 at ZnO nanoparticle carbon paste electrode. The anodic peak potential for the oxidation of Zn+2 and the reduction peak were about -1.261 V and -0.920 V, respectively. The result clearly indicated that the combination of graphite powder with ZnO nanoparticle improved zinc oxidation signal. The effect of scan rate on the electro-oxidation of zinc at the ZnO nanoparticle carbon paste electrode was investigated by cyclic voltammetry (CV) (Fig. 7). A plot of peak current (Ip) versus the square root of scan rate (V1/2) in the range of 0.1-5 V/s was linear. This suggested that, at sufficient overpotential, the process was diffusion rather than surface control [18].
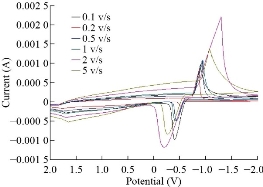
Fig. 6 Cyclic voltammograms of ZnO nanoparticles electrode in the presence of 0.1 M of ZnSO4 solution at different scan rates of 0.1, 0.2, 0.5. 1.2 and 5 V/s, respectively.
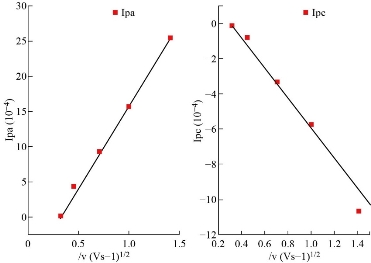
Fig.7 Ipoxd and Ipred peaks of reduction versus ʋ1/2 for Zn+2.
Temperature dependence study on the electro-oxidation of ZnO nanoparticle electrode
A study of the electro-oxidation on electrodes was carried out with CV in the temperature range from 15 °C to 30 °C with calomel electrode; the best condition of 18-25 °C was used for calomel electrode. The variation of the cyclic voltammogram (j-E) curves allowed the determination of activation energy in a wide interval of potentials where characteristic oxidation peaks appeared. Stationary current intensity measurements allowed the determination of the activation energy in pure kinetic region conditions. Temperature dependence of the charge-transfer resistance and the rate were constantly associated with the surface coverage by a diffusion intermediate during the electro-oxidation process, which was evaluated and discussed, suggesting that a complex mechanism took place for the electro-oxidation free of strong interactions with the ZnO nanoparticle electrode surface [19]. Result referred to the working electrode tacked the clear signal with Nernst equation (Eq. (1)) at 15-30 °C [20]:
ln D = ln Do − (Ea/RT), (1)
where D is the diffusion coefficient, T is the temperature, and K is the kelvin. From the slop between ln D and 1/Tk shown in Fig. 4, the free energy was calculated and equal to Ea = 68.94 KJ/mol. This result revealed the exothermic reaction accrued when potential was provided on the working electrode, by Equation (2) [21]:
∆G = − nFR(∆Ep), (2)
where ∆G is the free energy, F is the Faraday constant, R is the gas constant, and Ep is the difference in potential. The free Gibbs energy was determined as of 58.7 KJ/mol, which referred to the spontaneous reaction. From last result, the constant of reaction Kp was calculated by used Nicholson equation (Eq. (3)) [22]:
K = Ko exp(−αF/RT)(E − Eo). (3)
1.43×10-5 V/s for the quasi-reversible reaction of cyclic voltammtry referred to the first order reaction. Table 1 shows the kinetic and thermodynamic parameter for the ZnO nanoparticle carbon paste electrode.
Table 1 Result of voltammograms of ZnO nanoparticle electrode in the presence of 0.1 M of ZnSO4 solution at different scan rate of 0.1, 0.2, 0.5, 1, 2 and 5 V/s, respectively
|
Ipa/Ipc |
Fpred A/(V/s)1/2(10-4) |
Ipred A (10-4) |
Epc |
FpOX A/(V/s)1/2(10-4) |
Ipox A (10-4) |
Epa (V) |
√ʋ (V/s)1/2 |
ʋ (V/s) |
|
0.804 |
-0.235 |
-0.199 |
-0.422 |
0.437 |
0.160 |
-0.642 |
0.316 |
0.1 |
|
1.990 |
-0.472 |
-1.503 |
-0.402 |
8.725 |
2.992 |
-0.780 |
0.447 |
0.2 |
|
3.242 |
-3.595 |
-2.220 |
-0.336 |
12.051 |
7.198 |
-0.835 |
0.707 |
0.5 |
|
3.903 |
-3.738 |
-3.638 |
-0.299 |
14.590 |
14.590 |
-0.849 |
1.000 |
1.0 |
|
2.445 |
-6.499 |
-9.559 |
-0.163 |
17.700 |
23.380 |
-1.198 |
1.410 |
2.0 |
Calibration plots and determination of zinc in ZnO nanoparticle carbon paste electrode
Cyclic voltammetry was used to obtain the analytical features of the method such as linear ranges of the calibration plots and to detect the limits for Zn2+. Capability of separating the electrochemical response of Zn2+ by ZnO nanoparticle modified electrode was studied. Therefore, CV was used for the simultaneous determination of species based on its superior elimination of the capacitive background current. Analytical experiments were carried out by varying concentrations of ZnSO4 pure stock solution with pH 7.0 by using ZnO nanoparticle electrode. the calibration curve by CV obtained at 2-20 ppm, clearly revealing the response of the ZnO nanoparticle to Zn. (Hasanl A– Z VITAL, Germany) that contained vitamins A, C and E, folic acid, lutein, calcium, phosphorus, copper, iron and zinc with composition percentages of 40, 10, 20, 10, 5, 5, 5 and 5%, respectively. Equation (4) was used to prepare the measurement solutions:
w.t = (ppm×M.wt of Zn/M.wt of drug)/(10-6×V). (4)
All results pointed out that ZnO electrode was more sensitive than colorimetric and atomic analyzer for determination of trace amount in drug component. Fig. 8 depicts the cyclic voltammetric response for the electrochemical oxidation-reduction of 0.1 M CuSO4 at CuO nanoparticle carbon paste electrode. The anodic peak potential for the oxidation of Cu2+ was about −0.615 V, and the reduction peak was about 1.110 V, but no peak appeared on the carbon paste electrode. In other words, the result clearly indicated that the combination of graphite powder and CuO nanoparticle improved the copper oxidation signal. A study of the electro-oxidation on electrodes has been investigated by cyclic voltammetry, in the temperature range between 15-35 ºC with calomel electrode (18–25) oC the best condition was used electrode, the variation of the j-E curves allows the determination of activation energy in a wide interval of potentials where characteristic oxidation peaks appear in Fig. 9. The same as last methods was used to studied the effected of temperature for ZnO and CuO nanoparticle carbon paste electrode used to mentation of temperature over the MnO2 nanoparticle carbon paste electrode. The two mechanism of cyclic voltammetry appeared in voltammograms, Quesi and irreversible cyclic voltammetry and the kinetic and thermodynamic parameters calculated, after understanding the transported of two electrons by see that the curve shifting to the lift and slop of curve is also increase this phenomena can be understood the reaction tack place on the electrode surface, the temperature of which is growing and the solvent is maintained at the temperature Fig. 10.
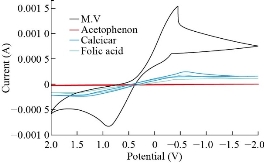
Fig. 8 Cyclic voltammograms by CuO nanoparticle electrode for varies solutions.
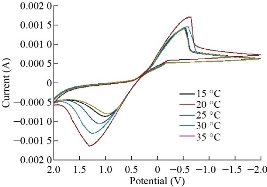
Fig. 9 Effect of temperature on the cyclic voltammograms on CuO nanoparticle electrode with different temperatures of 15, 20, 25, 30 and 35 oC, respectably.
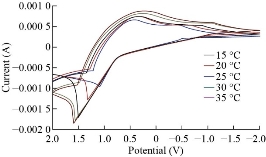
Fig. 10 Effect of temperature on the cyclic voltammograms on MnO2 nanoparticles electrode with different temperatures of 15, 20, 25, 30 and 35 oC, respectably.
Working electrode with mixing ions
Mixing solution containing 0.1 M of ZnSO4, CuSO4 and MnSO4 in electrical cell with platinum wire and calomel reference electrode and (Zn, Cu, Mn) oxide nanoparticles carbon paste electrode to completed a tringle current of cyclic voltammetry a wide peak for oxidation reduction cyclicvoltamogram including the electrons transfer when used deferent potential on work electrode fig 11 show the cyclicvoltamograms for signal responses. The idea for metal oxide mixes past electrode found for the purpose of loss time in measurement and reduce costs, electrode with homework preparing it is very simple and easy, with best specifications when working alone, the result refers to highest signal response for the specific ion solution, but in mix ion solution when applied potential the redox prosses get at the same time so that cyclovoltammograms refer to broadband when electrons transferring.
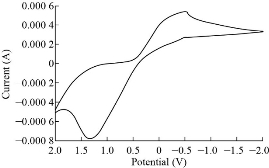
Fig. 11 Cyclic voltamograms for mixing electrode.
Conclusions
Nano metals oxide is a highly sensitive and selective vehicle that senses the small changes in the current passing through its solution when it becomes an electrode. Therefore, it was used to prepare the electrode and to study its physical and chemical properties. It was applied to measuring the small concentrations of some drugs containing metals; the study showed it could not be controlled in shielding and must be work ion selective electrode for one ion only to prevent the interaction redox prosses when applying potential on the reaction cell. The relationship Ipa/Ipc was nonlinear, which reveals more than one electron transported at the same time.
Conflict of Interests
The authors declare that no competing interest exists.
References
Copyright© Emad Salaam Abood. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.